Degradation Behavior of Poly(Lactide-Co-Glycolide) Monolayers Investigated by Langmuir Technique: Accelerating Effect
Abstract
1. Introduction
2. Results and Discussions
2.1. Synthesis of Copolymers
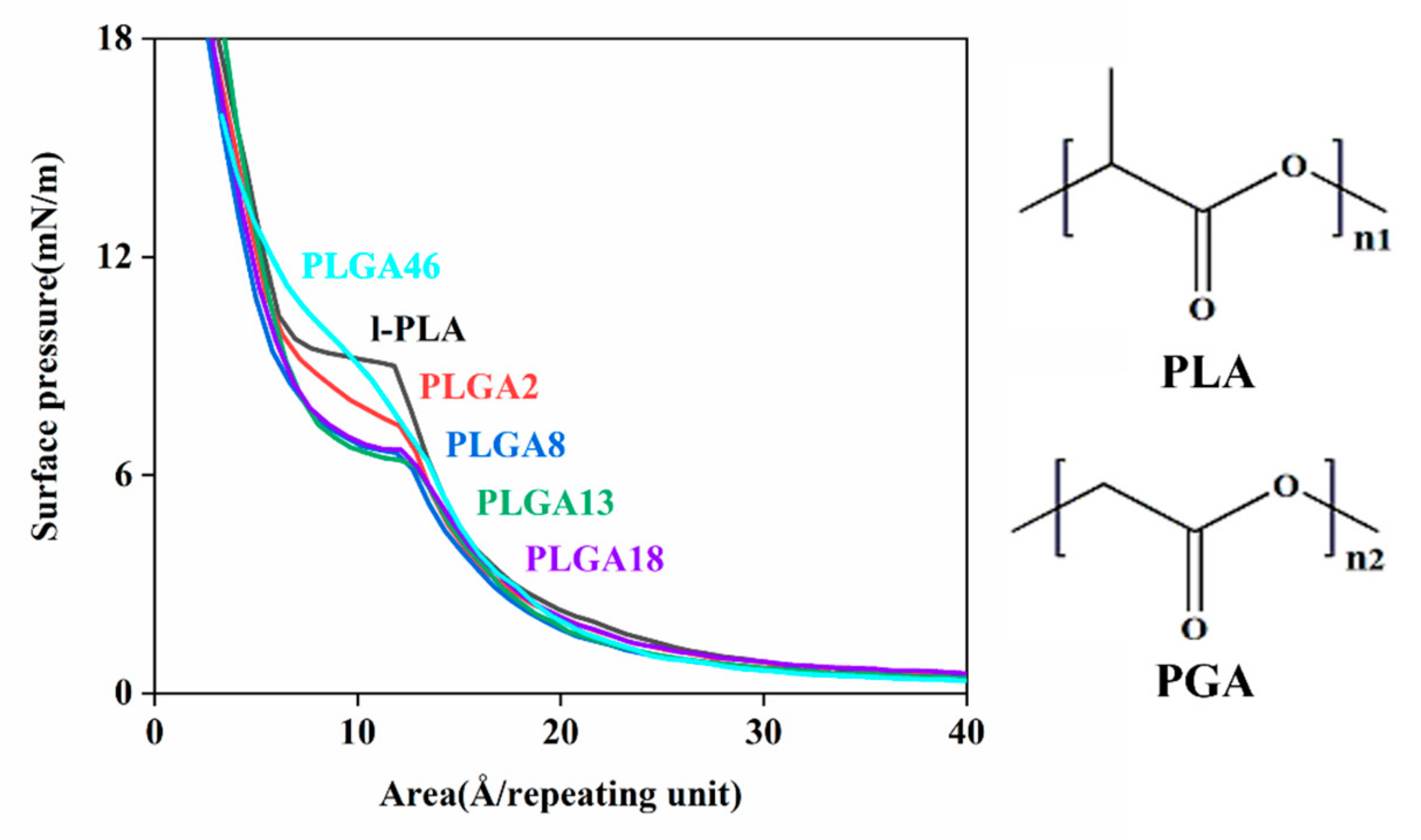
2.2. Surface Pressure–Area Isotherms
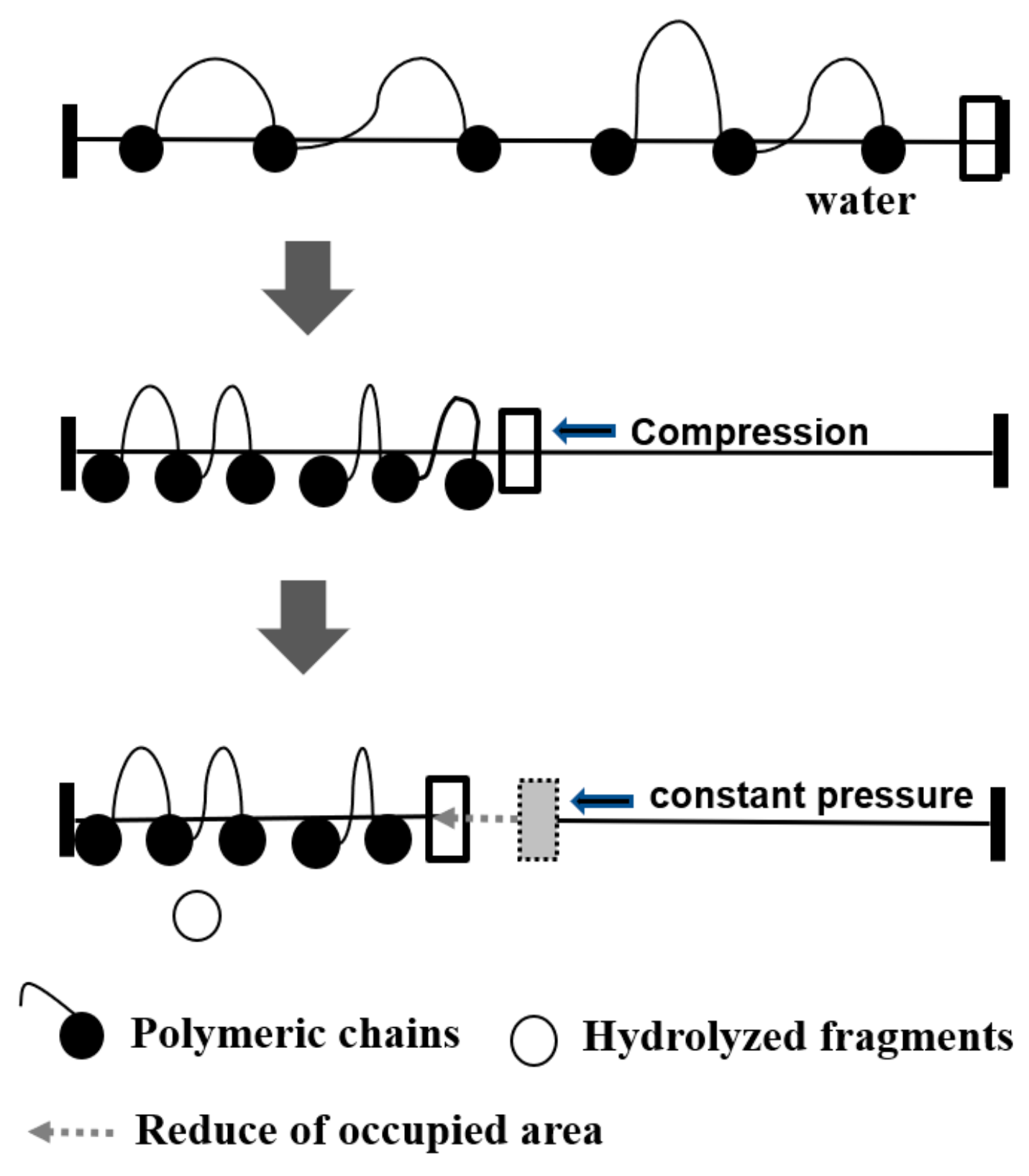
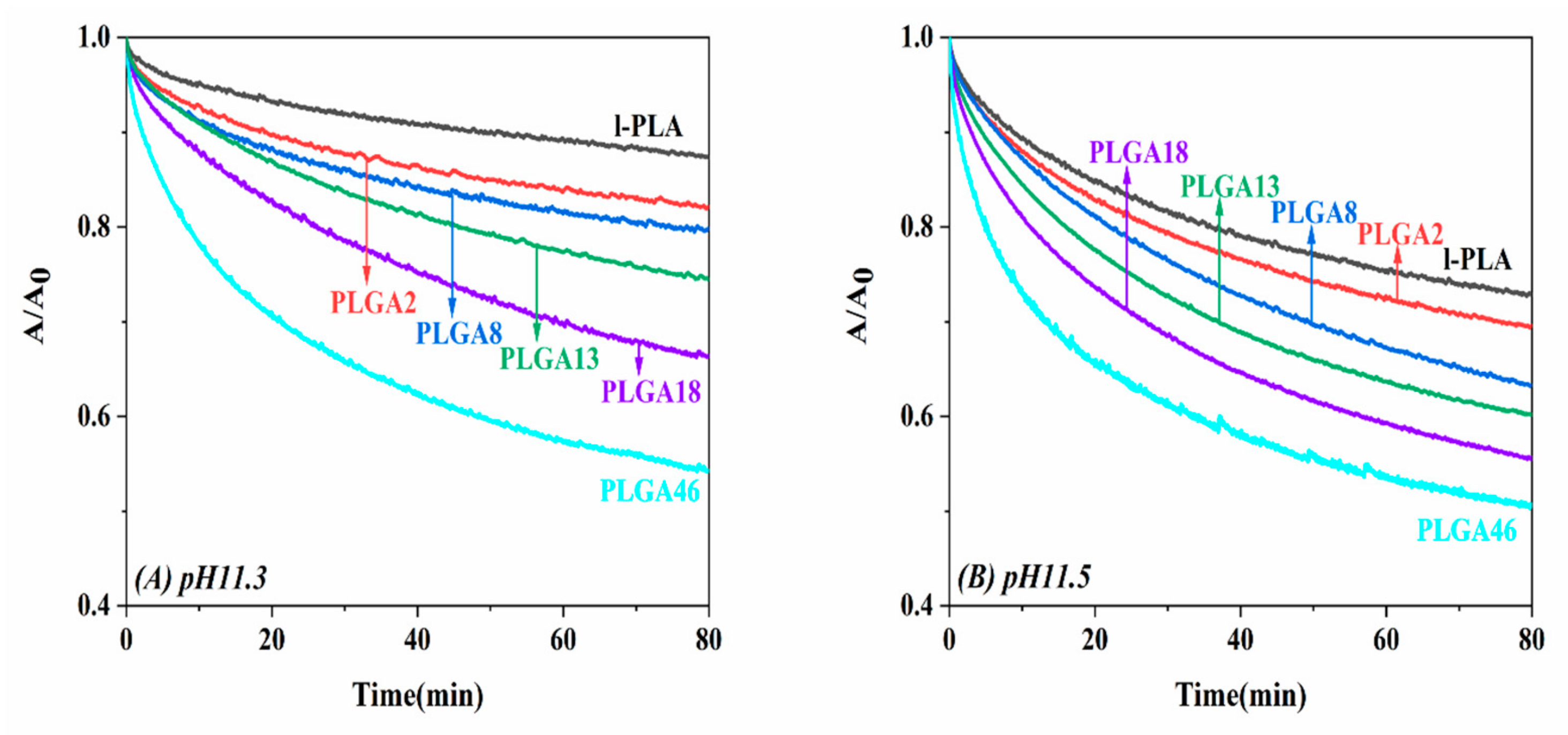
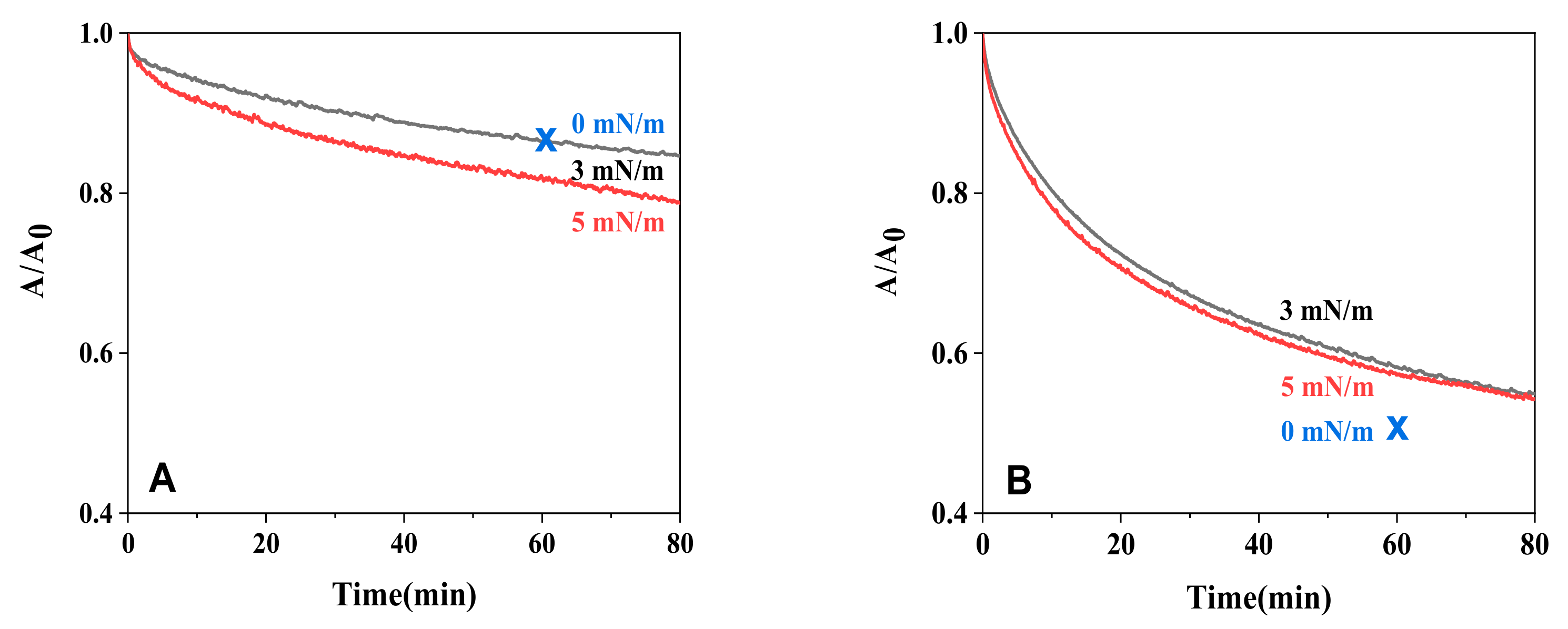
2.3. Alkaline Hydrolysis of Monolayers
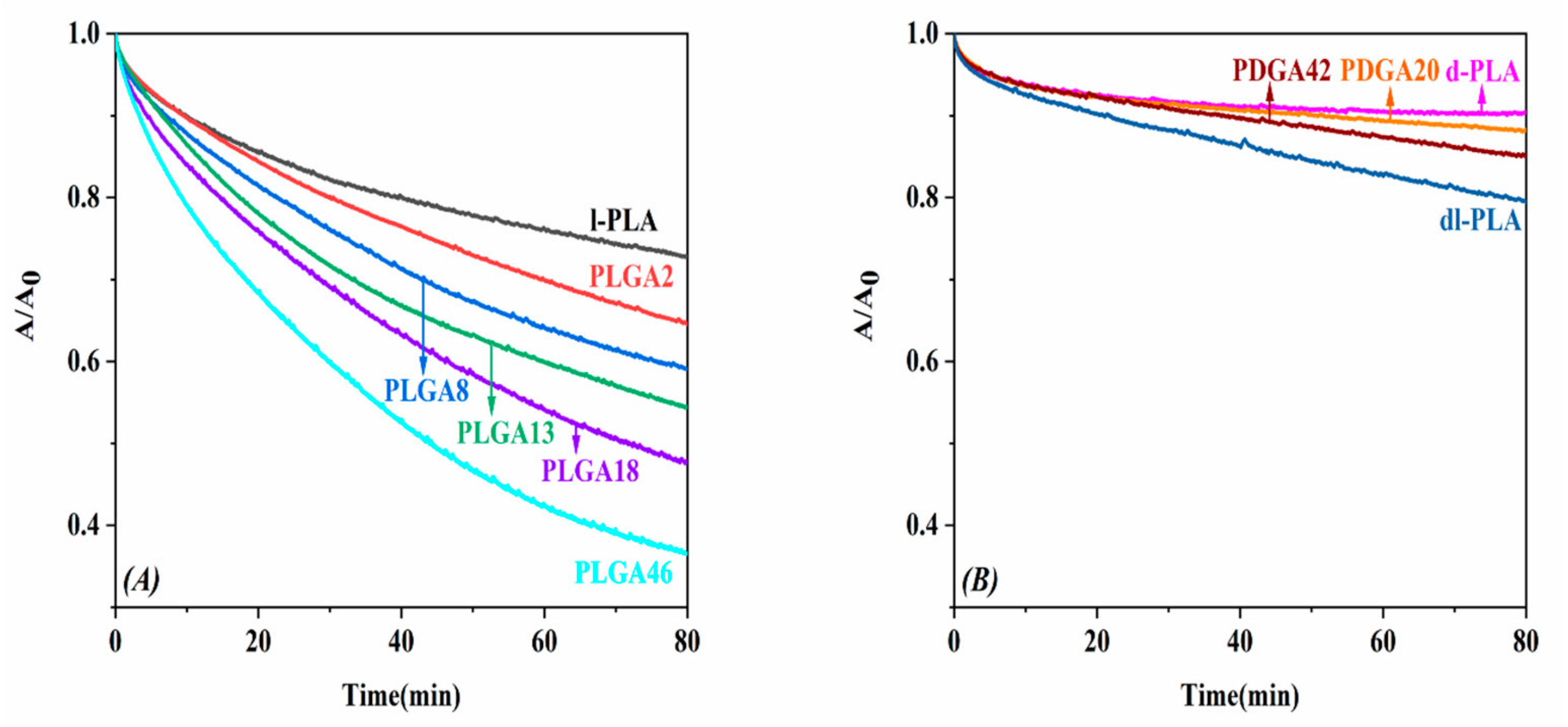
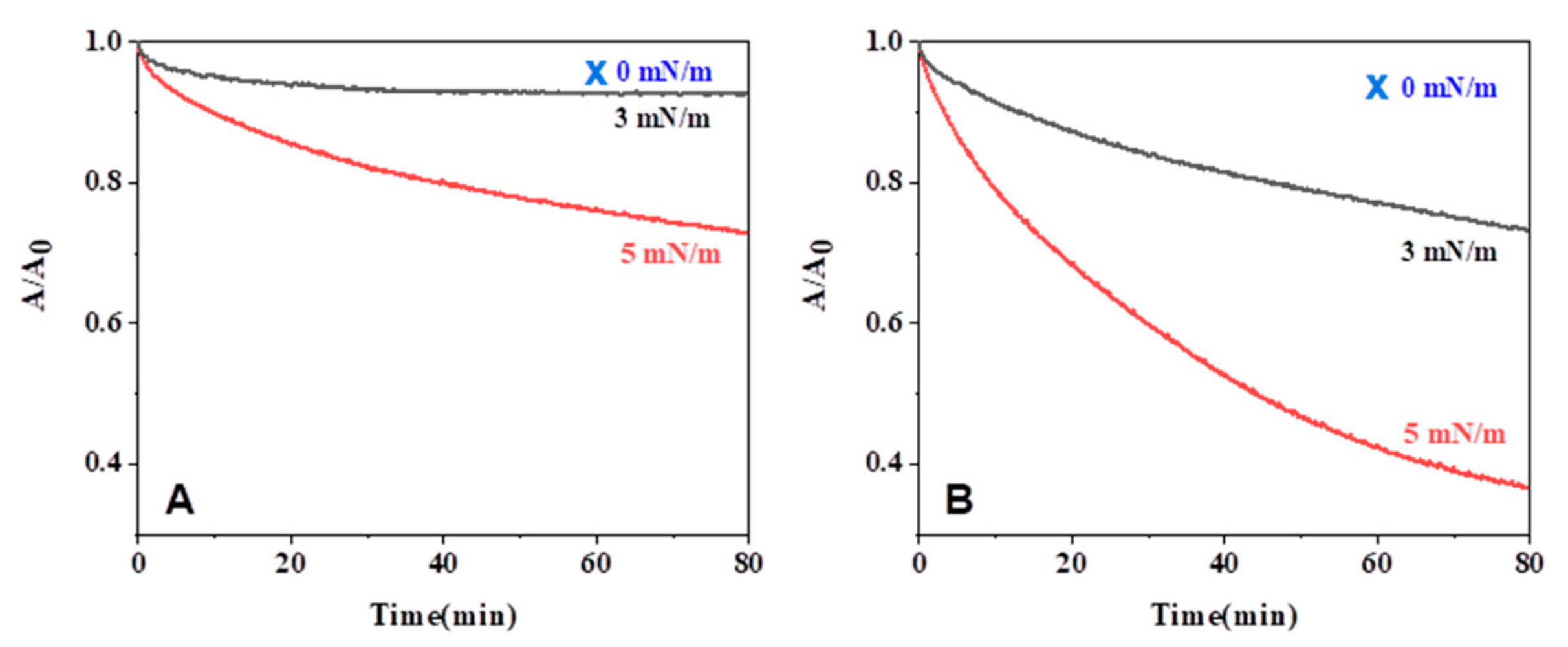
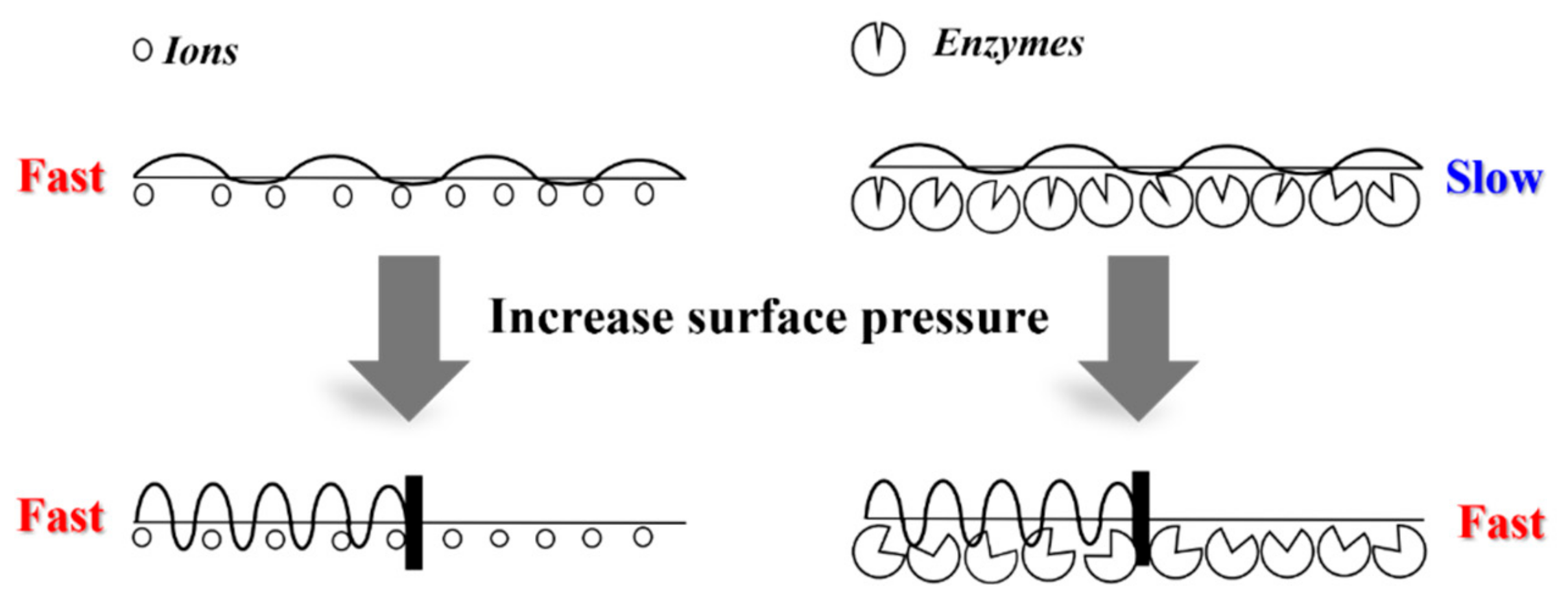
2.4. Enzymatic Degradation of Monolayers
3. Experimental
3.1. Materials
3.2. Synthesis of PLGA
3.3. Characterization of PLGA Copolymer
3.4. Degradation Behavior of Monolayers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Van der Walle, G.A.M.; De Koning, G.J.M.; Weusthuis, R.A.; Eggink, G. Properties, Modifications and Applications of Biopolyesters. In Biopolyesters; Babel, W., Steinbüchel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 263–291. ISBN 978-3-540-40021-9. [Google Scholar]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Im, D.; Gavande, V.; Lee, H.; Iwata, T.; Lee, W.K. Compatibility and hydrolytic behaviors of polylactide isomer/poly(butylene succinate) mixtures by the Langmuir technique. Polym. Degrad. Stab. 2021, 186, 109517. [Google Scholar] [CrossRef]
- Lee, W.K.; Nowak, R.W.; Gardella, J.A. Hydrolytic degradation of polyester blend monolayers at the air/water interface: Effects of a slowly degrading component. Langmuir 2002, 18, 2309–2312. [Google Scholar] [CrossRef]
- Lee, W.K.; Iwata, T.; Gardella, J.A. Hydrolytic behavior of enantiomeric poly(lactide) mixed monolayer films at the air/water interface: Stereocomplexation effects. Langmuir 2005, 21, 11180–11184. [Google Scholar] [CrossRef]
- Gavande, V.; Im, D.; Jin, Y.; Lim, K.T.; Lee, W.K. 3D bio polybutylene succinate electrospun nanofiber scaffolds for biomimetic structure. Mol. Cryst. Liq. Cryst. 2020, 706, 55–61. [Google Scholar] [CrossRef]
- Ha, C.S.; Gardella, J.A. Surface chemistry of biodegradable polymers for drug delivery systems. Chem. Rev. 2005, 105, 4205–4232. [Google Scholar] [CrossRef]
- Fredericks, R.J.; Melveger, A.J.; Dolegiewitz, L.J. Morphological and structural changes in a copolymer of glycolide and lactide occurring as a result of hydrolysis. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 57–66. [Google Scholar] [CrossRef]
- Im, D.; Gavande, V.; Park, E.; Kim, D.; Lee, W.-K. Synthesis and selective enzymatic degradation of polylactide/poly (butylene succinate) mixtures. Mol. Cryst. Liq. Cryst. 2022, 758, 64–70. [Google Scholar] [CrossRef]
- Gavande, V.; Kim, G.; Kim, B.; Jin, Y.; Jang, S.-H.; Chun, J.H.; Lee, W.-K. Effect of molecular weight on stereocomplexation of enantiomeric polylactide mixtures and their degradation behaviors by Langmuir technique. Mol. Cryst. Liq. Cryst. 2022, 742, 133–138. [Google Scholar] [CrossRef]
- Kwon, Y.; Gavande, V.; Kim, S.; Im, D.; Lee, W.-K. Renewable triblock copolymers of polylactide and polyether polyol: Degradation behaviors of their monolayers and films. Mol. Cryst. Liq. Cryst. 2023, 1–9. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Murcia Valderrama, M.A.; Van Putten, R.J.; Gruter, G.J.M. PLGA Barrier Materials from CO2. The influence of Lactide Co-monomer on Glycolic Acid Polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Grijpma, D.W.; Nijenhuis, A.J.; Pennings, A.J. Synthesis and hydrolytic degradation behaviour of high-molecular-weight l-lactide and glycolide copolymers. Polymer 1990, 31, 2201–2206. [Google Scholar] [CrossRef]
- Erbetta, C.D.A.C.; Alves, R.J.; Resende, J.M.; Freitas, R.F.S.; de Sousa, R.G. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Qian, H.; Wohl, A.R.; Crow, J.T.; Macosko, C.W.; Hoye, T.R. A Strategy for Control of “Random” Copolymerization of Lactide and Glycolide: Application to Synthesis of PEG-b-PLGA Block Polymers Having Narrow Dispersity. Macromolecules 2011, 44, 7132–7140. [Google Scholar] [CrossRef]
- Lee, W.K.; Gardella, J.A. Hydrolytic kinetics of biodegradable polyester monolayers. Langmuir 2000, 16, 3401–3406. [Google Scholar] [CrossRef]
- Machatschek, R.; Schulz, B.; Lendlein, A. The influence of pH on the molecular degradation mechanism of PLGA. MRS Adv. 2018, 3, 3883–3889. [Google Scholar] [CrossRef]
- Rudin, A.; Choi, P. Chapter 2—Basic Principles of Polymer Molecular Weights. In The Elements of Polymer Science & Engineering, 3rd ed.; Rudin, A., Choi, P., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 63–87. ISBN 978-0-12-382178-2. [Google Scholar]
- Machatschek, R.; Schulz, B.; Lendlein, A. Langmuir Monolayers as Tools to Study Biodegradable Polymer Implant Materials. Macromol. Rapid Commun. 2019, 40, 1800611. [Google Scholar] [CrossRef]
- Ivanova, T.; Panaiotov, I.; Boury, F.; Proust, J.E.; Benoit, J.P.; Verger, R. Hydrolysis kinetics of poly(D,L-lactide) monolayers spread on basic or acidic aqueous subphases. Colloids Surf. B Biointerfaces 1997, 8, 217–225. [Google Scholar] [CrossRef]
- Jo, N.J.; Iwata, T.; Lim, K.T.; Jung, S.H.; Lee, W.K. Degradation behaviors of polyester monolayers at the air/water interface: Alkaline and enzymatic degradations. Polym. Degrad. Stab. 2007, 92, 1199–1203. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Tezuka, Y. Alkaline and enzymatic degradation of L-lactide copolymers, 1: Amorphous-made films of L-lactide copolymers with D-lactide, glycolide, and ε-caprolactone. Macromol. Biosci. 2005, 5, 135–148. [Google Scholar] [CrossRef]
- Ryner, M.; Albertsson, A.C. Resorbable and highly elastic block copolymers from 1,5-dioxepan-2-one and L-lactide with controlled tensile properties and hydrophilicity. Biomacromolecules 2002, 3, 601–608. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Flanagan, D.R.; Linhardt, R.J. Importance of distinct water environments in the hydrolysis of poly (DL-lactide-co-glycolide). Macromolecules 1994, 27, 743–748. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.; Fan, Z.; Li, S.; Kasperczyk, J.; Dobrzyński, P. Enzyme-catalyzed degradation of biodegradable polymers derived from trimethylene carbonate and glycolide by lipases from candida antarctica and hog pancreas. J. Biomater. Sci. Polym. Ed. 2012, 23, 1355–1368. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, Q.; Yan, N.; Deng, X.; Yang, X. In vitro hydrolytic and enzymatic degradation of nestlike-patterned electrospun poly(D,L -lactide-co-glycolide) scaffolds. J. Biomed. Mater. Res. Part A 2010, 95, 755–765. [Google Scholar] [CrossRef]
- Tsuji, H.; Muramatsu, H. Blends of aliphatic polyesters: V. Non-enzymatic and enzymatic hydrolysis of blends from hydrophobic poly(L-lactide) and hydrophilic poly(vinyl alcohol). Polym. Degrad. Stab. 2001, 71, 403–413. [Google Scholar] [CrossRef]
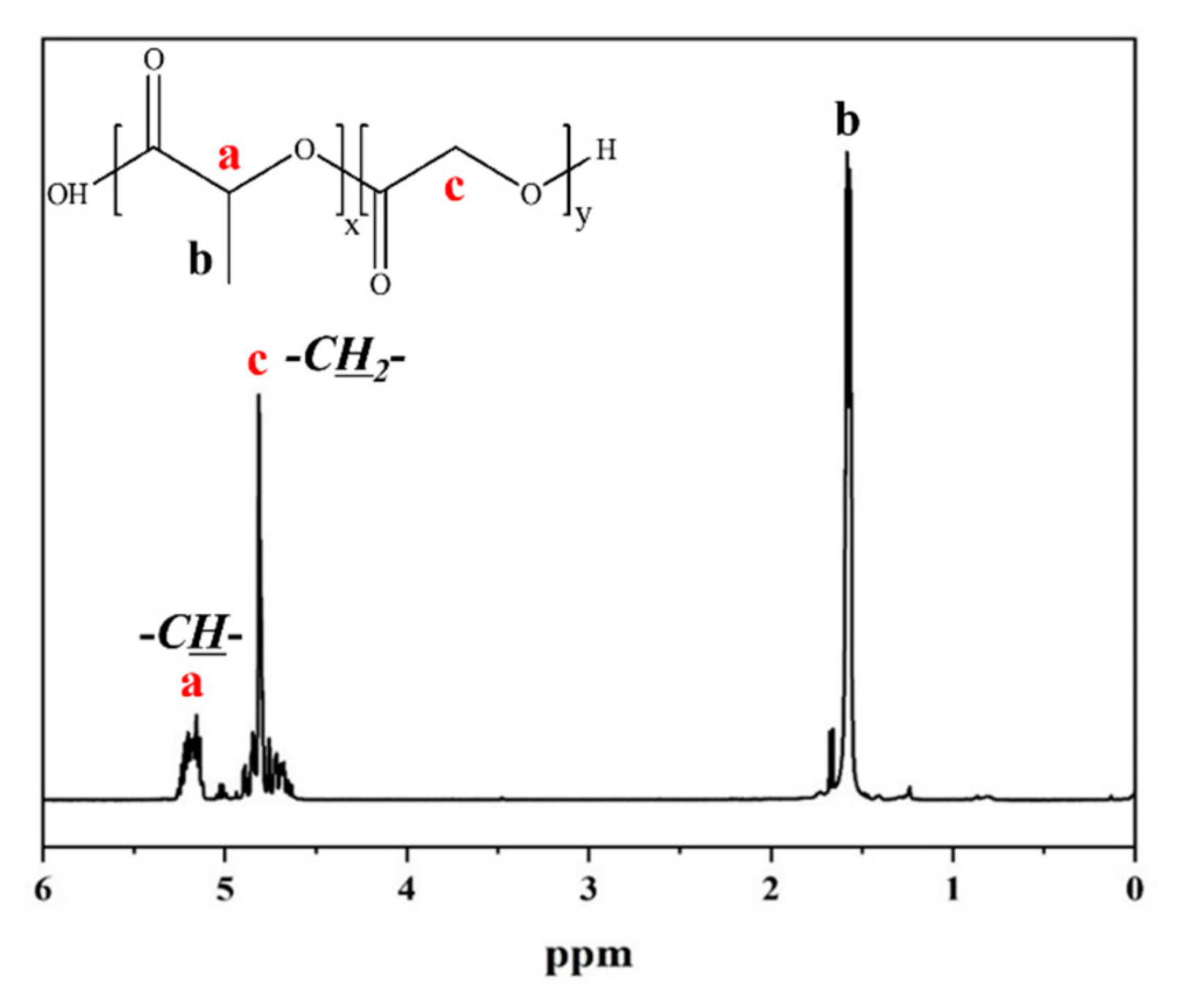
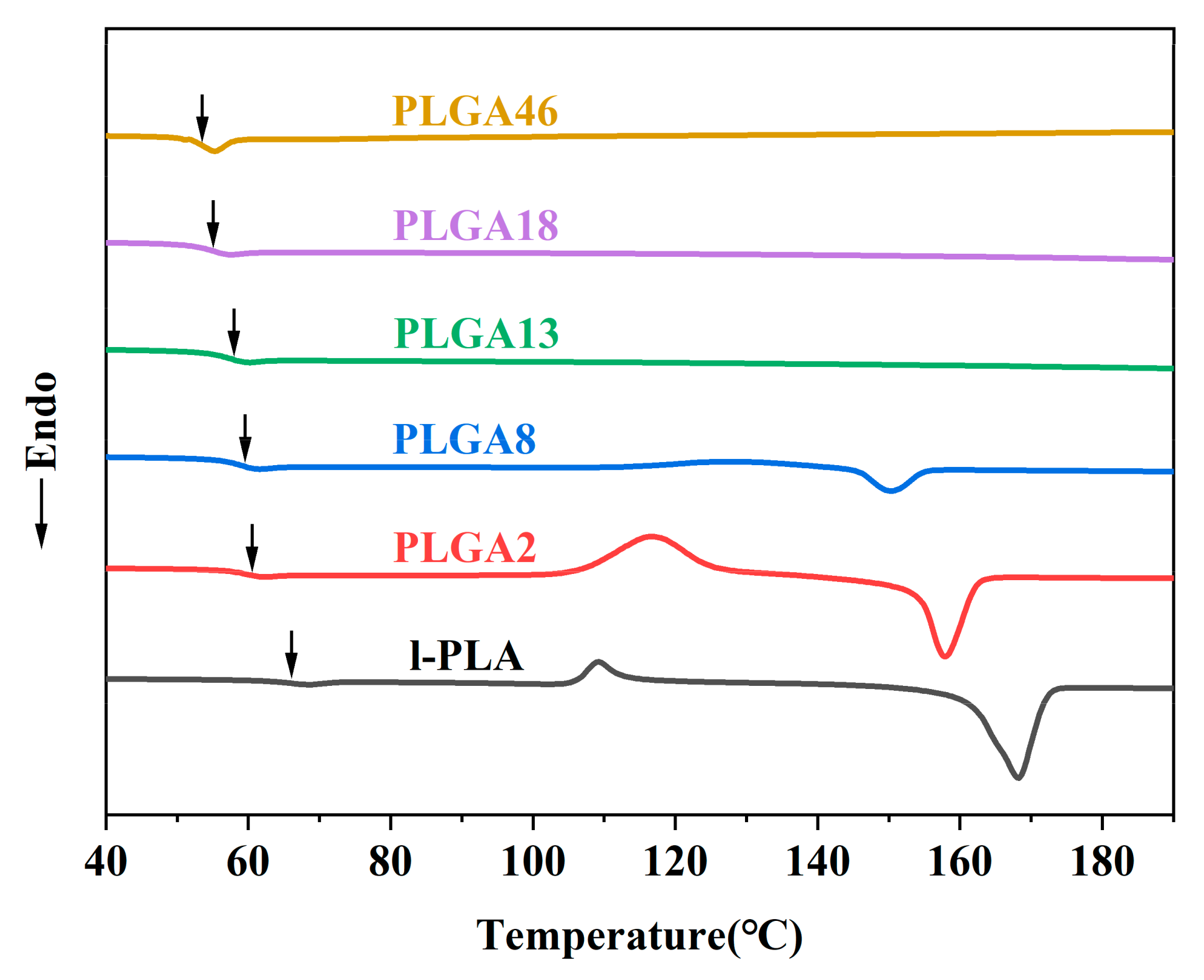

| Sample Code | In Feed (mol%) | Product (mol% by NMR) | ||
|---|---|---|---|---|
| l-LA | GA | LA | GA | |
| l-PLA | 100 | 0 | 100 | 0 |
| PLGA2 | 95 | 5 | 98 | 2 |
| PLGA8 | 90 | 10 | 92 | 8 |
| PLGA13 | 85 | 15 | 87 | 13 |
| PLGA18 | 80 | 20 | 82 | 18 |
| PLGA46 * | 70 | 30 | 54 | 46 |
| PDGA20 ** | 80 | 20 | 80 | 20 |
| PDGA42 *, ** | 70 | 30 | 58 | 42 |
| Mn | PDI | Tg | Tm | Crystallinity (%) | |
|---|---|---|---|---|---|
| l-PLA | 28K | 1.6 | 64.2 | 169.6 | 44.7 |
| PLGA2 | 27K | 2.3 | 60.5 | 158.2 | 4.4 |
| PLGA8 | 27K | 2.3 | 59.3 | 150.3 | 2.8 |
| PLGA13 | 26K | 2.4 | 58.0 | - | |
| PLGA18 | 16K | 3.8 | 54.9 | - | |
| PLGA46 | 23K | 2.1 | 53.6 | - | |
| PDGA20 | 28K | 2.2 | 55.0 | - | |
| PDGA42 | - | - | 49.83 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Gavande, V.; Shaikh, V.; Lee, W.-K. Degradation Behavior of Poly(Lactide-Co-Glycolide) Monolayers Investigated by Langmuir Technique: Accelerating Effect. Molecules 2023, 28, 4810. https://doi.org/10.3390/molecules28124810
Kim G, Gavande V, Shaikh V, Lee W-K. Degradation Behavior of Poly(Lactide-Co-Glycolide) Monolayers Investigated by Langmuir Technique: Accelerating Effect. Molecules. 2023; 28(12):4810. https://doi.org/10.3390/molecules28124810
Chicago/Turabian StyleKim, Gayeon, Vishal Gavande, Vasi Shaikh, and Won-Ki Lee. 2023. "Degradation Behavior of Poly(Lactide-Co-Glycolide) Monolayers Investigated by Langmuir Technique: Accelerating Effect" Molecules 28, no. 12: 4810. https://doi.org/10.3390/molecules28124810
APA StyleKim, G., Gavande, V., Shaikh, V., & Lee, W.-K. (2023). Degradation Behavior of Poly(Lactide-Co-Glycolide) Monolayers Investigated by Langmuir Technique: Accelerating Effect. Molecules, 28(12), 4810. https://doi.org/10.3390/molecules28124810










