The Residue and Dietary Risk Assessment of Spirotetramat and Its Four Metabolites in Cabbage Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
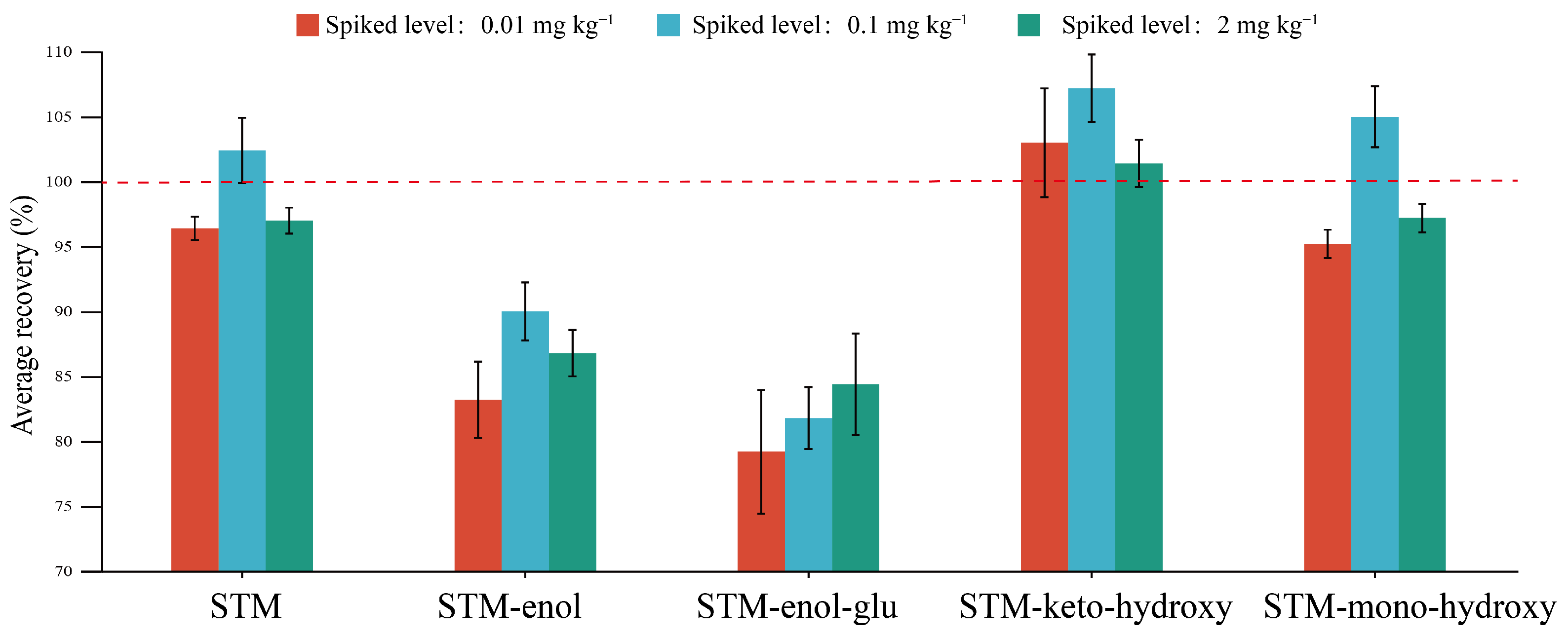
2.1. Method Validation
2.2. The Terminal Residues
2.3. Dietary Risk Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Field Trials and Sampling
3.3. Sample Preparations
3.4. UHPLC–MS/MS Analysis
3.5. Method Validation
3.6. Definition of STM Residue
3.7. Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Franzke, A.; Lysak, M.A.; Al-Shehbaz, I.A.; Koch, M.A.; Mummenhoff, K. Cabbage family affairs: The evolutionary history of Brassicaceae. Trends Plant Sci. 2011, 16, 108–116. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#compare (accessed on 5 November 2022).
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance spirotetramat. EFSA J. 2013, 11, 3243. [Google Scholar] [CrossRef]
- Vang, L.E.; Opperman, C.H.; Schwarz, M.R.; Davis, E.L. Spirotetramat causes an arrest of nematode juvenile development. Nematology 2016, 18, 121–131. [Google Scholar] [CrossRef]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thielert, W. Biological profile of spirotetramat (Movento®)—A new two-way systemic (ambimobile) insecticide against sucking pest species. Bayer Crop. J. 2008, 61, 245–278. [Google Scholar]
- Pesticides, A.; Authority, V.M. Evaluation of the New Active SPIROTETRAMAT in the Product MOVENTO 240 SC INSECTICIDE; APVMA: Kingston, Australia, 2009; pp. 9–10. [Google Scholar]
- Salazar-López, N.-J.; Aldana-Madrid, M.-L.; Silveira-Gramont, M.-I.; Aguiar, J.-L. Spirotetramat—An alternative for the control of parasitic sucking insects and its fate in the environment. In Insecticide Resistance; IntechOpen: London, UK, 2016; pp. 41–55. [Google Scholar]
- China Pesticide Information Network. Available online: http://www.chinapesticide.org.cn/zwb/dataCenter (accessed on 26 April 2022).
- Ambrus, A.; Yang, Y.Z. Global harmonization of maximum residue limits for pesticides. J. Agric. Food Chem. 2016, 64, 30–35. [Google Scholar] [CrossRef]
- Winter, C.K.; Jara, E.A. Pesticide food safety standards as companions to tolerances and maximum residue limits. J. Integr. Agric. 2015, 14, 2358–2364. [Google Scholar] [CrossRef]
- Cui, S.; Li, Z.; Cheng, G.; Li, R.; Wang, Y.; Zhang, X.; Zhao, F. Determination of sulfoxaflor, pyrifluquinazon and spirotetramat residues in fruits and vegetables by UPLC-MS/MS. Shipin Kexue/Food Sci. 2018, 39, 302–308. [Google Scholar]
- Mohapatra, S.; Deepa, M.; Lekha, S.; Nethravathi, B.; Radhika, B.; Gourishanker, S. Residue dynamics of spirotetramat and imidacloprid in/on mango and soil. Bull. Environ. Contam. Toxicol. 2012, 89, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvi, S.; Sathiyanarayanan, S.; Ramesh, A. Determination of spirotetramat and imidacloprid residues in cotton seed, lint, oil and soil by HPLC UV method and their dissipation in cotton plant. Pestic. Res. J. 2010, 22, 168–173. [Google Scholar]
- Kumar, B.; Kumaran, N.; Kuttalam, S. Determination of harvest time residues of spirotetramat on cotton using HPLC. J. Plant Prot. Environ. 2009, 6, 1–5. [Google Scholar]
- Li, S.; Liu, X.; Dong, F.; Xu, J.; Xu, H.; Hu, M.; Zheng, Y. Chemometric-assisted QuEChERS extraction method for the residual analysis of thiacloprid, spirotetramat and spirotetramat’s four metabolites in pepper: Application of their dissipation patterns. Food Chem. 2016, 192, 893–899. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Dong, F.; Li, W.; Liu, X.; Li, Y.; Kong, Z.; Zhu, Y.; Liu, N.; Zheng, Y. The fate of spirotetramat and its metabolite spirotetramat-enol in apple samples during apple cider processing. Food Control 2013, 34, 283–290. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kumar, S.; Prakash, G. Residue evaluation of imidacloprid, spirotetramat, and spirotetramat-enol in/on grapes (Vitis vinifera L.) and soil. Environ. Monit. Assess. 2015, 187, 632. [Google Scholar] [CrossRef]
- Singh, B.; Mandal, K.; Sahoo, S.K.; Bhardwaj, U.; Battu, R.S. Development and validation of an HPLC method for determination of spirotetramat and spirotetramat cis enol in various vegetables and soil. J. AOAC Int. 2013, 96, 670–675. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Wang, S.; Yu, Y.; Lu, P.; Hu, D.; Yang, Z. Dissipation, residues and risk assessment of spirotetramat and its four metabolites in citrus and soil under field conditions by LC-MS/MS. Biomed. Chromatogr. 2018, 32, e4153. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, W.; Cheng, X.; Hu, J. Residues, fate and risk assessment of spirotetramat and its four metabolites in pineapple under field conditions. Int. J. Environ. Anal. Chem. 2020, 100, 900–911. [Google Scholar] [CrossRef]
- Faraji, M.; Noorbakhsh, R.; Shafieyan, H.; Ramezani, M. Determination of acetamiprid, imidacloprid, and spirotetramat and their relevant metabolites in pistachio using modified QuEChERS combined with liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 240, 634–641. [Google Scholar] [CrossRef]
- Lan, F.; Yao, J.; Zhou, X.; Liu, C.; Li, X.; Lu, Z.; Jiang, W.; Wang, Z. Residue of spirotetramat, diafenthiuron and their metabolites in fruits and vegetables by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Pestic. Sci. 2019, 21, 219–226. [Google Scholar]
- Zhu, Y.; Liu, X.; Xu, J.; Dong, F.; Liang, X.; Li, M.; Duan, L.; Zheng, Y. Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1299, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Spirotetramat. 2013. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report13/5.32_SPIROTETRAMAT__234_.pdf (accessed on 26 April 2022).
- Liu, H.C.; Lin, D.L.; McCurdy, H.H. Matrix Effects in the Liquid Chromatography-Tandem Mass Spectrometry Method of Analysis. Forensic Sci. Rev. 2013, 25, 65–78. [Google Scholar] [PubMed]
- Hayama, T. Matrix Effects in Mass Spectrometry Analysis. Anal. Sci. 2020, 36, 1151. [Google Scholar] [CrossRef] [PubMed]
- Codex Online Databases. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=234 (accessed on 26 April 2022).
- EU Pesticide Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 26 April 2022).
- United States Pesticide MRLs. Available online: https://bcglobal.bryantchristie.com/db#/pesticides/results?q=eyJmaWx0ZXJzIjp7ImZVU1NlY3Rpb24xOFJlZ2lvbmFsIjowLCJmRmFjaWxpdHlVc2UiOjIsImZJbXBvcnRUb2xlcmFuY2UiOjAsInR5cGVTb3J0T3JkZXIiOlsxNSwyLDFdfSwiYm9keSI6W3siaXRlbVR5cGVJRCI6MTUsImxpc3QiOls0MzY2XX0seyJpdGVtVHlwZUlEIjoyLCJsaXN0IjpbNDU4XX0seyJpdGVtVHlwZUlEIjoxLCJsaXN0IjpbMjFdfV19&isSimpleViewProp=true (accessed on 26 April 2022).
- The Japen Food Chemical Research Foundation. Available online: http://db.ffcr.or.jp/front/ (accessed on 26 April 2022).
- Australian Goverment. Available online: https://www.legislation.gov.au/Details/F2023C00438 (accessed on 26 April 2022).
- World Health Organization. Template for the Evaluation of Acute Exposure (IESTI). Available online: https://cdn.who.int/media/docs/default-source/food-safety/gems-food/guidance-iesti-2014.pdf?sfvrsn=9b24629a_2 (accessed on 26 April 2022).
- European Commission. Guidance Document on Analytical Qualitycontrol and Method Validation Procedures for Pesticides Residues Andanalysis in Food and Feed. 2021. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 26 April 2022).
- GB2763-2021; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2021.
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Cabrera, L.C.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Review of the existing maximum residue levels for spirotetramat according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, e05960. [Google Scholar] [CrossRef]
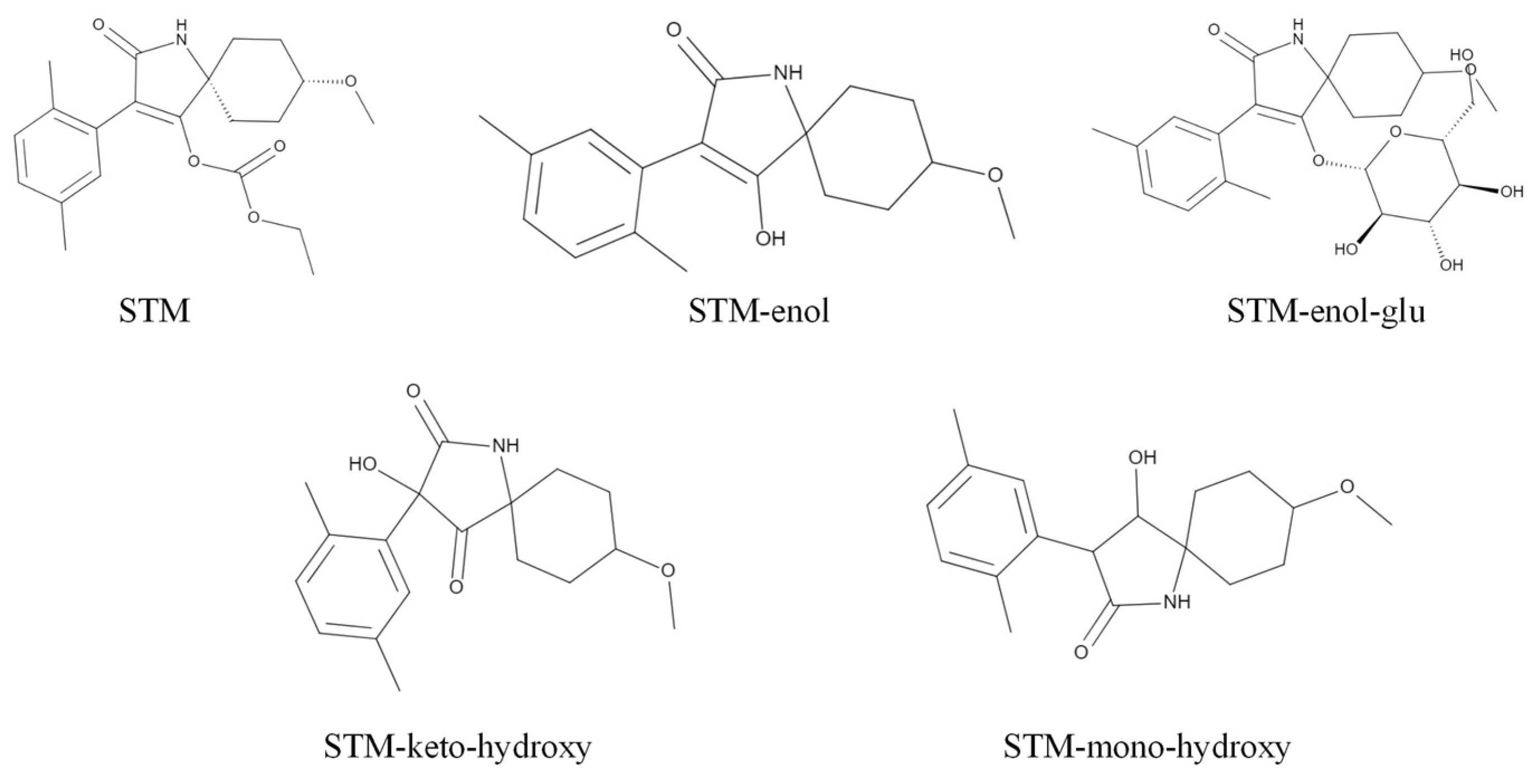

| Compound | Retention Time (Rt, min) | Production (m z−1) | Declustering Potential (DP, V) | Collision Energy (CE, V) |
|---|---|---|---|---|
| STM | 2.91 | 374.20 > 330.1 (quantitation) | 66 | 47 |
| 374.20 > 216.1 (confirmation) | 21 | |||
| STM-enol | 2.65 | 302.30 > 270.2 (confirmation) | 60 | 40 |
| 302.30 > 216.0 (quantitation) | 30 | |||
| STM-enol-glu | 2.10 | 464.40 > 302.2 (confirmation) | 67 | 20 |
| 464.40 > 216.0 (quantitation) | 40 | |||
| STM-keto-hydroxy | 2.71 | 318.20 > 214.0 (quantitation) | 40 | 20 |
| 318.20 > 268.1 (confirmation) | 20 | |||
| STM-mono-hydroxy | 2.49 | 304.30 > 254.1 (confirmation) | 60 | 20 |
| 304.30 > 211.1 (quantitation) | 20 |
| Compounds | Matrix | Calibration Curve | R2 | Matrix Effect (%) |
|---|---|---|---|---|
| STM | Acetonitrile | y = 4.089 × 106x + 355.0 | 0.9998 | - |
| Cabbage | y = 4.292 × 106x + 389.9 | 0.9998 | 5.0 | |
| STM-enol | Acetonitrile | y = 9.598 × 107x + 1.015 × 105 | 0.9979 | - |
| Cabbage | y = 4.865 × 107x − 4125 | 0.9979 | −29.2 | |
| STM-enol-glu | Acetonitrile | y = 1.627 × 107x + 3.406 × 104 | 0.9916 | - |
| Cabbage | y = 1.023 × 107x + −813.7 | 0.9909 | −49.3 | |
| STM-keto-hydroxy | Acetonitrile | y = 2.822 × 107x + 2.228 × 104 | 0.9989 | - |
| Cabbage | y = 1.962 × 107x + 3.421 × 104 | 0.9994 | −37.1 | |
| STM-mono-hydroxy | Acetonitrile | y = 1.322 × 107x + 2.637 × 104 | 0.9953 | - |
| Cabbage | y = 9.614 × 106x + 1474 | 0.9981 | −29.9 |
| Locations | Pre-Harvest Interval (Days) | Mean Residues (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|
| STM | STM-enol | STM-enol-glu | STM-keto-hydroxy | STM-mono-hydroxy | Total Residues | ||
| Shanxi | 7 | 0.039, 0.108 | 0.019, 0.035 | <0.010, <0.010 | 0.081, 0.14 | <0.010, <0.010 | 0.18, 0.33 |
| 10 | 0.043, 0.073 | <0.010, <0.010 | <0.010, <0.010 | 0.13, 0.11 | <0.010, <0.010 | 0.22, 0.24 | |
| 14 | 0.023, 0.077 | <0.010, <0.010 | <0.010, <0.010 | 0.049, 0.051 | <0.010, <0.010 | 0.11, 0.17 | |
| Liaoning | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Beijing | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.011, <0.010 | <0.010, <0.010 | 0.052, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Shandong | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Henan | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Anhui | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.033, 0.032 | <0.010, <0.010 | 0.078, 0.078 |
| 10 | <0.010, <0.010 | 0.01, 0.02 | <0.010, <0.010 | 0.032, 0.028 | <0.010, <0.010 | 0.08, 0.087 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.012, 0.014 | <0.010, <0.010 | 0.055, 0.057 | |
| Shanghai | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Hunan | 7 | 0.018, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.058, <0.050 |
| 10 | 0.021, 0.039 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.061, 0.079 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Guangxi | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.012, 0.016 | <0.010, <0.010 | 0.054, 0.058 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Guizhou | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, 0.053 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Hainan | 7 | 0.010, 0.013 | <0.010, <0.010 | <0.010, <0.010 | 0.028, 0.023 | <0.010, <0.010 | 0.073, 0.069 |
| 10 | 0.008, 0.012 | <0.010, <0.010 | <0.010, <0.010 | 0.028, 0.03 | <0.010, <0.010 | 0.073, 0.077 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.053, 0.065 | |
| Guangdong | 7 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | 0.01, 0.014 | <0.010, <0.010 | 0.052, 0.056 |
| 10 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| 14 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.010, <0.010 | <0.050, <0.050 | |
| Crops | Food Classification | Fi (kg) | Residue (mg kg−1) | Sources | NEDI (mg) | ADI (mg) | Risk Quotient (RQc, %) |
|---|---|---|---|---|---|---|---|
| Potato | Tubers | 0.0495 | 0.8 | China, MRL | 3.960 × 10−2 | 0.05 | 1.26 |
| Celery | Dark vegetables | 0.0915 | 4 | China, MRL | 3.660 × 10−1 | 11.62 | |
| Peach | Fruits | 0.0457 | 3 | China, MRL | 1.371 × 10−1 | 4.35 | |
| Tea | Salt | 0.012 | 0.1 | Australia, MRL | 1.200 × 10−3 | 0.04 | |
| Cabbage | Light vegetables | 0.1837 | 0.051 | STMR1 (PHI = 7) | 9.369 × 10−3 | 0.30 | |
| 0.050 | STMR2 (PHI = 14) | 9.185 × 10−3 | 0.29 | ||||
| 0.050 | STMR3 (PHI = 21) | 9.185 × 10−3 | 0.29 | ||||
| Total | 0.3824 | 5.533 × 10−1 (PHI = 7) | 17.56 (PHI = 7) | ||||
| 5.531 × 10−1 (PHI = 14) | 17.56 (PHI = 14) | ||||||
| 5.531 × 10−1 (PHI = 21) | 17.56 (PHI = 21) |
| Age | Weight (kg) | Food Consumption (kg d−1) | NESTI (mg (kg bw)−1) | RQa (%) |
|---|---|---|---|---|
| 2~10 | 12.3~22.9 | 0.0201~0.0343 | 4.94 × 10−4~5.39 × 10−4 | 0.049~0.054 |
| 11~17 | 34.0~46.9 | 0.0381~0.0440 | 3.10 × 10−4~3.70 × 10−4 | 0.031~0.037 |
| 18~59 | 52.1~64.9 | 0.0448~0.0515 | 2.62 × 10−4~2.84 × 10−4 | 0.026~0.028 |
| ≥60 | 51.0~61.5 | 0.0380~0.0472 | 2.53 × 10−4~2.46 × 10−4 | 0.025~0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Li, J.; Ren, P.; Qi, Y.; Qin, S. The Residue and Dietary Risk Assessment of Spirotetramat and Its Four Metabolites in Cabbage Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2023, 28, 4763. https://doi.org/10.3390/molecules28124763
Cao J, Li J, Ren P, Qi Y, Qin S. The Residue and Dietary Risk Assessment of Spirotetramat and Its Four Metabolites in Cabbage Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2023; 28(12):4763. https://doi.org/10.3390/molecules28124763
Chicago/Turabian StyleCao, Junli, Jindong Li, Pengcheng Ren, Yanli Qi, and Shu Qin. 2023. "The Residue and Dietary Risk Assessment of Spirotetramat and Its Four Metabolites in Cabbage Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry" Molecules 28, no. 12: 4763. https://doi.org/10.3390/molecules28124763
APA StyleCao, J., Li, J., Ren, P., Qi, Y., & Qin, S. (2023). The Residue and Dietary Risk Assessment of Spirotetramat and Its Four Metabolites in Cabbage Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 28(12), 4763. https://doi.org/10.3390/molecules28124763







