Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria
Abstract
1. Introduction
2. Results
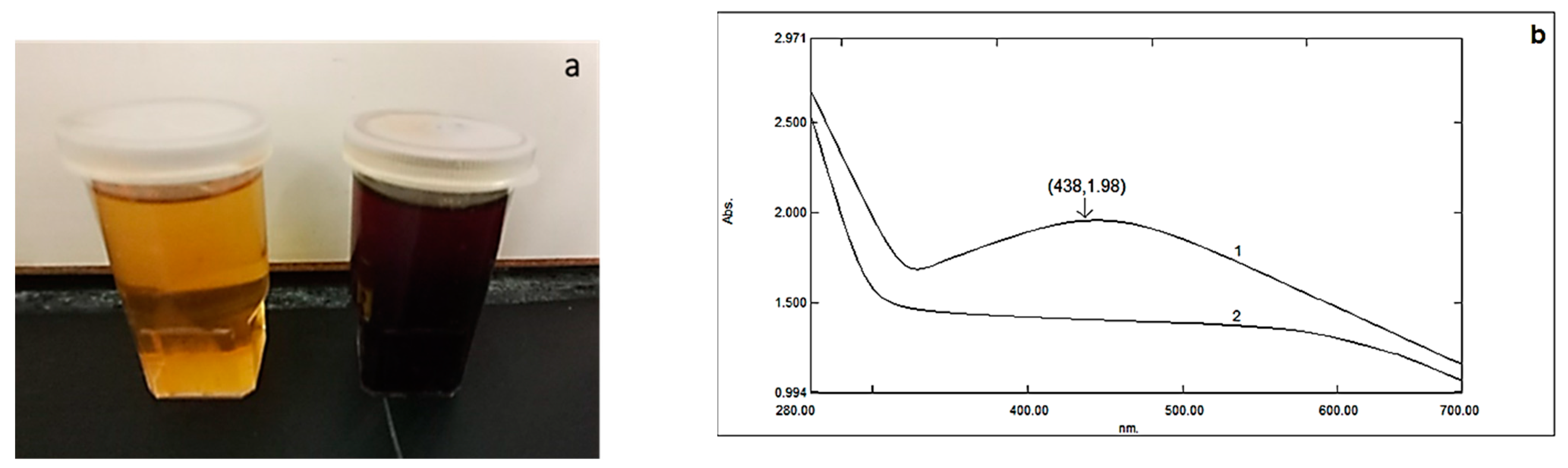
2.1. Silver Nanoparticles (AgNPs) and Biochar
2.2. Particle Size and Zeta Potential
2.3. XRD Spectra Analysis
2.4. ATR-IR Spectra Analysis
2.5. Antimicrobial Activity of Silver Nanoparticles
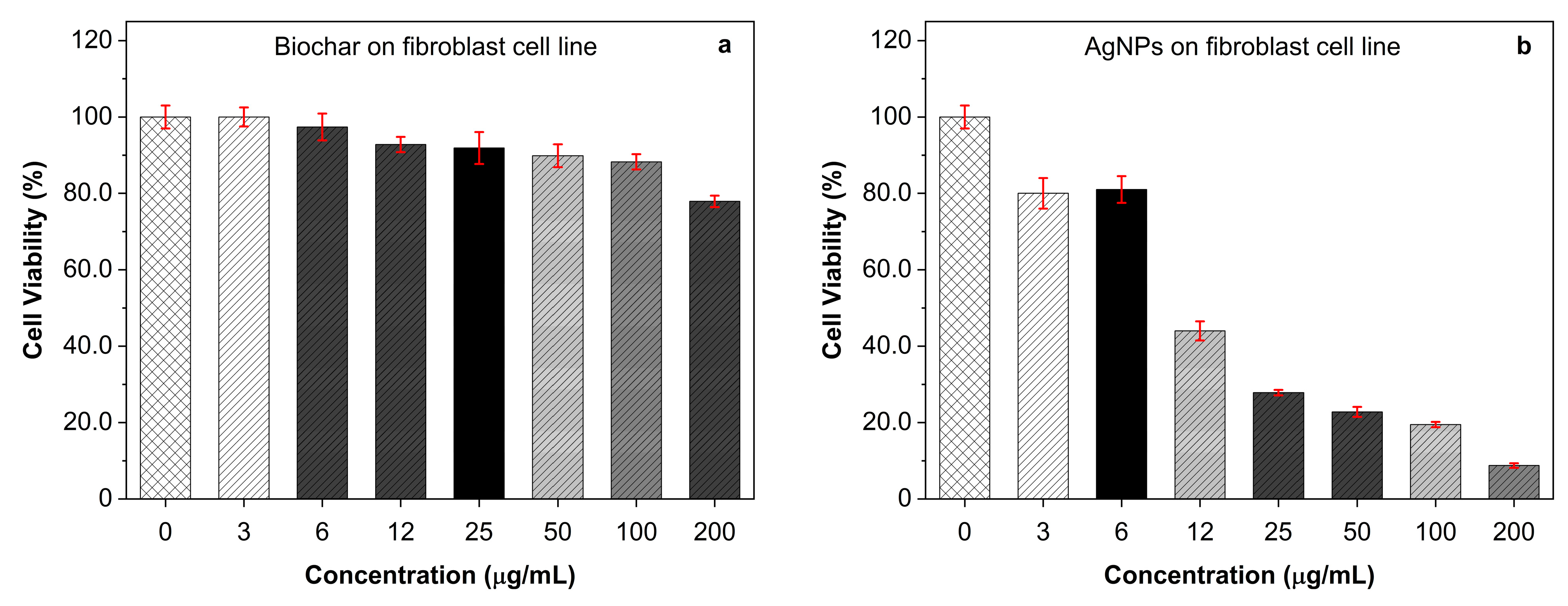
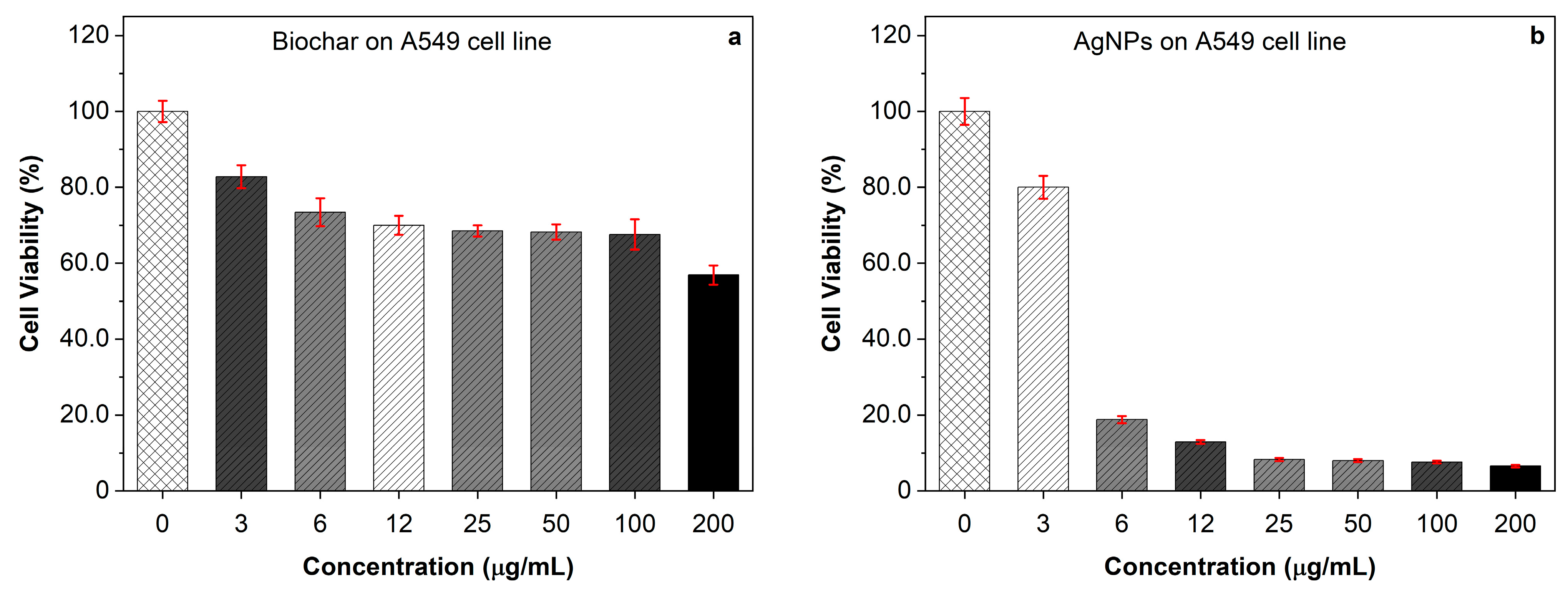
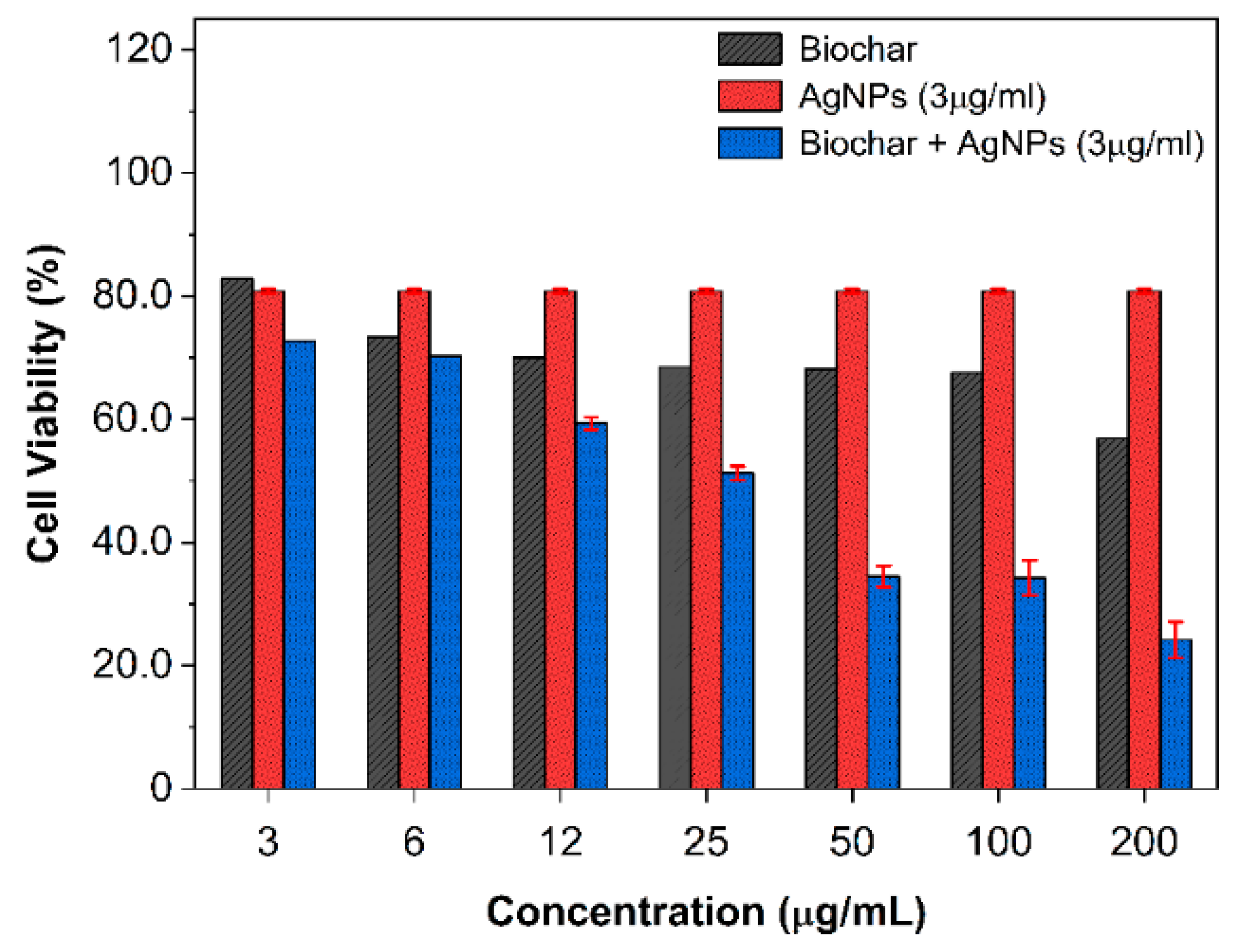
2.6. Cytotoxicity Effect of AgNPs and Biochar on A549 Cells
2.7. Effect of AgNPs and Biochar on the Expression of Gene Regulation
2.7.1. Pro- and Anti-Apoptotic Genes in A549 Cells
2.7.2. The Expression of IL-1β, IL-6, and TNF-α in the A549 Cell Line
3. Discussion
4. Material and Methods
4.1. Fungal Strain
4.2. Bacterial Strains and Reagents
4.3. Raw Materials for Biochar and Processing Conditions
4.4. Date Seeds’ (DSs) Physicochemical Characteristics
4.5. AgNPs Biosynthesis
4.5.1. AgNPs Characterization
4.5.2. The Size Distribution of the Particles and Their Zeta Potential
4.6. The Antibacterial Activity of Silver Nanoparticles, Biochar, and Their Combination
4.6.1. MIC Determination
4.6.2. Cancer Cell Lines’ Culture
4.6.3. Cell Harvesting and Counting
4.6.4. Cytotoxicity Assay
4.6.5. Gene Expression Level Assay
4.6.6. Ribonucleic Acid (RNA) Extraction and Analysis
4.6.7. Complementary Deoxyribonucleic Acid (cDNA) Synthesis
4.6.8. Relative Quantitative RT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Ombaka, L.M.; McGettrick, J.D.; Oseghe, E.O.; Al-Madanat, O.; Rieck genannt Best, F.; Msagati, T.A.M.; Davies, M.L.; Bredow, T.; Bahnemann, D.W. Photocatalytic H2 production and degradation of aqueous 2-chlorophenol over B/N-graphene-coated Cu0/TiO2: A DFT, experimental and mechanistic investigation. J. Environ. Manag. 2022, 311, 114822. [Google Scholar] [CrossRef]
- Alsalka, Y.; Al-Madanat, O.; Hakki, A.; Bahnemann, D.W. Boosting the H2 production efficiency via photocatalytic organic reforming: The role of additional hole scavenging system. Catalysts 2021, 11, 1423. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Curti, M.; Günnemann, C.; AlSalka, Y.; Dillert, R.; Bahnemann, D.W. TiO2 photocatalysis: Impact of the platinum loading method on reductive and oxidative half-reactions. Catal. Today 2021, 380, 3–15. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Alsalka, Y.; Dillert, R.; Bahnemann, D.W. Photocatalytic H2 production from naphthalene by various TiO2 photocatalysts: Impact of pt loading and formation of intermediates. Catalysts 2021, 11, 107. [Google Scholar] [CrossRef]
- Satalkar, P.; Elger, B.S.; Shaw, D.M. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci. Eng. Ethics 2016, 22, 1255–1276. [Google Scholar] [CrossRef] [PubMed]
- Khleifat, K.; Alqaraleh, M.; Allimoun, M.; Alfarrayeh, I.; Khatib, R.; Qaralleh, H.; Alsarayreh, A.; Al Qaisi, Y.; Hajleh, M.A. The Ability of Rhizopus stolonifer MR11 to Biosynthesize Silver Nanoparticles in Response to Various Culture Media Components and Optimization of Process Parameters Required at Each Stage of Biosynthesis. J. Ecol. Eng. 2022, 23, 8. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; AL-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosm. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; AL-Samydai, A.; Al-Dujaili, E.A. Nano, micro particulate and cosmetic delivery systems of polylactic acid: A mini review. J. Cosm. Dermatol. 2020, 19, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Khleifat, K.M.; Abboud, M.M.; Al-Mustafa, A.H. Effect of Vitreoscilla hemoglobin gene (vgb) and metabolic inhibitors on cadmium uptake by the heterologous host Enterobacter aerogenes. Process Biochem. 2006, 41, 930–934. [Google Scholar] [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef]
- Zhang, X.F.; Gurunathan, S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int. J. Nanomed. 2016, 11, 3655–3675. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Talapko, J.; Matijević, T.; Juzbašić, M.; Antolović-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.G.; Scheper, T. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Aqueous Extract with the Evaluation of Its Antibacterial Activity against Clinical and Food Pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef]
- Lara, H.H. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- El Hanandeh, A.; Mahdi, Z.; Imtiaz, M.S. Modelling of the adsorption of Pb, Cu and Ni ions from single and multi-component aqueous solutions by date seed derived biochar: Comparison of six machine learning approaches. Environ. Res. 2021, 192, 110338. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Ibrahim, E.H.; Kilnay, M. Majra Honey Abrogated the Normal and Cancer Cells Proliferation Inhibition by Juniperus procera Extract and Extract/Honey Generated AgNPs. Anticancer Agents Med. Chem. 2020, 20, 970–981. [Google Scholar] [CrossRef]
- Palem, R.R.; Shimoga, G.; Kang, T.J.; Lee, S.H. Fabrication of multifunctional Guar gum-silver nanocomposite hydrogels for biomedical and environmental applications. Int. J. Biol. Macromol. 2020, 159, 474–486. [Google Scholar] [CrossRef]
- Kumar, N.; Chamoli, P.; Misra, M.; Manoj, M.K.; Sharma, A. Advanced metal and carbon nanostructures for medical, drug delivery and bio-imaging applications. Nanoscale 2022, 14, 3987–4017. [Google Scholar] [CrossRef] [PubMed]
- Kvolik, S.; Dobrosevic, B.; Marczi, S.; Prlic, L.; Glavas-obrovac, L. Different apoptosis ratios and gene expressions in two human cell lines after sevoflurane anaesthesia. Acta Anaesthesiol. Scand. 2009, 53, 1192–1199. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Eltaweil, A.S. Green synthesis of bimetallic Ag/ZnO@Biohar nanocomposite for photocatalytic degradation of tetracycline, antibacterial and antioxidant activities. Sci. Rep. 2022, 12, 7316. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Narasimha, G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf. B Biointerfaces 2010, 81, 430–433. [Google Scholar] [CrossRef]
- Al-limoun, M.; Qaralleh, N.H.; Khleifat, M.K.; Al-Anber, M.; Al-Tarawneh, A.; Al-sharafa, K.; Kailani, M.H.; Zaitoun, M.A.; Matar, S.A.; Al-soub, T. Culture Media Composition and Reduction Potential Optimization of Mycelia-free Filtrate for the Biosynthesis of Silver Nanoparticles Using the Fungus Tritirachium oryzae W5H. Curr. Nanosci. 2020, 16, 757–769. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- Chau, Y.F.; Yeh, H.H.; Tsai, D.P. Surface Plasmon Resonances Effects on Different Patterns of Solid-silver and Silver-shell Nanocylindrical Pairs. J. Electromagn. Waves Appl. 2010, 24, 1005–1014. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Louhelainen, J.K.; Kłosińska, T.; Drożdżek, M.; Alén, R. Characterization of alkali-extracted wood by FTIR-ATR spectroscopy. Biomass Convers. Biorefin. 2018, 8, 847–855. [Google Scholar] [CrossRef]
- Osorio-Echavarría, J.; Osorio-Echavarría, J.; Ossa-Orozco, C.P.; Gómez-Vanegas, N.A. Synthesis of silver nanoparticles using white-rot fungus Anamorphous Bjerkandera sp. R1: Influence of silver nitrate concentration and fungus growth time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef]
- Yıldızlı, G.; Coral, G.; Ayaz, F. Biochar as a Biocompatible Mild Anti-Inflammatory Supplement for Animal Feed and Agricultural Fields. Chem. Biodivers. 2021, 18, e2001002. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Mohuiddin, I.; Ece, F.; McConkey, D.J.; Smythe, W.R. Histone deacetylase inhibitor downregulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am. J. Respir. Cell Mol. Biol. 2001, 25, 562–568. [Google Scholar] [CrossRef]
- Xu, H.W.; Huang, Y.J.; Xie, Z.Y.; Lin, L.; Guo, Y.C.; Zhuang, Z.R.; Lin, X.P.; Zhou, W.; Li, M.; Huang, H.H.; et al. The expression of cytoglobin as a prognostic factor in gliomas: A retrospective analysis of 88 patients. BMC Cancer 2013, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Combination Effect of Silver Nanoparticles and Histone Deacetylases Inhibitor in Human Alveolar Basal Epithelial Cells. Molecules 2018, 23, 2046. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Ivashkiv, L.B. Inhibition of IL-6 and IL-10 Signaling and Stat Activation by Inflammatory and Stress Pathways. J. Immunol. 2000, 165, 5227–5237. [Google Scholar] [CrossRef]
- Yang, L.; Guo, P.; Wang, P.; Wang, W.; Liu, J. IL-6/ERK signaling pathway participates in type I IFN-programmed, unconventional M2-like macrophage polarization. Sci. Rep. 2023, 13, 1827. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Ahmed, R. Effects of biochar on uptake, acquisition and translocation of silver nanoparticles in rice (Oryza sativa L.) in relation to growth, photosynthetic traits and nutrients displacement. Environ. Pollut. 2019, 250, 728–736. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Zia-ur-Rehman, M.; Rizwan, M.; Rinklebe, J. Biochar-induced immobilization and transformation of silver-nanoparticles affect growth, intracellular-radicles generation and nutrients assimilation by reducing oxidative stress in maize. J. Hazard. Mater. 2020, 390, 121976. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Sharaf, E.F.; Al-limoun, M.O. Biodegradation of 2-Chlorobenzoic Acid by Enterobacter cloacae: Growth Kinetics and Effect of Growth Conditions. Bioremediation J. 2015, 19, 207–217. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and Characterization of Biochar Sorbents Produced from Malt Spent Rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Mohawesh, O.; Coolong, T.; Aliedeh, M.; Alqaraleh, S. Greenhouse evaluation of biochar to enhance soil properties and plant growth performance under arid environment. Bulg. J. Agric. Sci. 2018, 24, 1012–1019. [Google Scholar] [CrossRef]
- Greenberg, I.; Kaiser, M.; Polifka, S.; Wiedner, K.; Glaser, B.; Ludwig, B. The effect of biochar with biogas digestate or mineral fertilizer on fertility, aggregation and organic carbon content of a sandy soil: Results of a temperate field experiment. J. Plant Nutr. Soil Sci. 2019, 182, 824–835. [Google Scholar] [CrossRef]
- Sutcu, M.; Erdogmus, E.; Gencel, O.; Gholampour, A.; Atan, E.; Ozbakkaloglu, T. Recycling of bottom ash and fly ash wastes in eco-friendly clay brick production. J. Clean. Prod. 2019, 233, 753–764. [Google Scholar] [CrossRef]
- Gurunathan, S.; Lee, K.J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D.W. Photocatalytic H2 evolution from oxalic acid: Effect of cocatalysts and carbon dioxide radical anion on the surface charge transfer mechanisms. ACS Appl. Energy Mater. 2020, 3, 6678–6691. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Alsalka, Y.; Curti, M.; Dillert, R.; Bahnemann, D.W. Mechanistic insights into hydrogen evolution by photocatalytic reforming of naphthalene. ACS Catal. 2020, 10, 7398–7412. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Hakki, A. TiO2-based photocatalytic hydrogen production: How to transfer it to an applicable approach? Appl. Catal. A Gen. 2023, 662, 119287. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Tarawneh, K.A.; Ali Wedyan, M.; Al-Tarawneh, A.A.; Al Sharafa, K. Growth kinetics and toxicity of Enterobacter cloacae grown on linear alkylbenzene sulfonate as sole carbon source. Curr. Microbiol. 2008, 57, 364–370. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential Oil of Centaurea damascena Aerial Parts, Antibacterial and Synergistic Effect. J. Essent. Oil-Bear. Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Qaralleh, H.; Khleifat, K.M.; Al-Limoun, M.O.; Alzedaneen, F.Y.; Al-Tawarah, N. Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025016. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawarah, N.M.; Qaralleh, H.; Khlaifat, A.M.; Nofal, M.N.; Alqaraleh, M.; Khleifat, K.M.; Al-limoun, M.O.; Al Shhab, M.A. Anticancer and Antibacterial Properties of Verthemia Iphionides Essential Oil /Silver Nanoparticles. Biomed. Pharmacol. J. 2020, 13, 1175–1184. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Kasabri, V. The Antiglycation Effect of Monomethyl Branched Chained Fatty Acid and Phytochemical Compounds and their Synergistic Effect on Obesity Related Colorectal Cancer Cell Panel. Romanian J. Diabetes Nutr. Metab. Dis. 2019, 26, 361–369. [Google Scholar] [CrossRef]
- Karna, S.K.L.; Ahmad, F.; Lone, B.A.; Pokharel, Y.R. Knockdown of PTOV1 and PIN1 exhibit common phenotypic anti-cancer effects in MDA-MB-231 cells. PLoS ONE 2019, 14, e0211658. [Google Scholar] [CrossRef]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green Synthesis of Silver Nanoparticles Using Annona muricata Extract as an Inducer of Apoptosis in Cancer Cells and Inhibitor for NLRP3 Inflammasome via Enhanced Autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Hageleit, M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol. Lett. 2001, 23, 275–282. [Google Scholar] [CrossRef]



| DS Biochar Parameters | Pyrolysis Temperature (550 °C) |
|---|---|
| pH | 7.9 ± 0.15 |
| Porosity (%) | 73 ± 4.0 |
| Organic matter (%) | 98.56 ± 3.95 |
| Biochar yield (%) | 25.2 ± 3.2 |
| Water holding capacity (% WHC) | 59.7 ± 4.25 |
| AgNPs | Biochar | AgNPs + Biochar | ||||
|---|---|---|---|---|---|---|
| Micro-Organism | Inhibition Zone (mm) | MIC µg/mL | Inhibition Zone (mm) | MIC µg/mL | Inhibition Zone (mm) | MIC µg/mL |
| B. cereus (ATCC 11778) | 20.5 ± 0.5 | 6.38 | 0.0 | Nd | 24.5 ± 0.5 | 2.13 |
| L. monocytogene (ATCC 7644) | 18.5 ± 0.5 | 6.38 | 0.0 | Nd | 20.5 ± 0.0 | 2.13 |
| P. aeruginosa | 12.5 ± 0.5 | 19.15 | 0.0 | Nd | 14.5 ± 0.4 | 6.38 |
| S. typhi (ATCC 14028) | 15.5 ± 0.5 | 6.38 | 0.0 | Nd | 19.5 ± 0.6 | 2.13 |
| S. aureus | 18.5 ± 0.5 | 6.38 | 0.0 | Nd | 21.5 ± 0.5 | 2.13 |
| E. coli | 14.0 ± 0.0 | 19.15 | 0.0 | Nd | 16.5 ± 0.0 | 6.38 |
| P. aeruginosa ATCC 10145 | 12.3 ± 0.0 | 19.15 | 0.0 | Nd | 16.5 ± 0.5 | 6.38 |
| E. coli ATCC 25922 | 13.5 ± 0.6 | 19.15 | 0.0 | Nd | 15.0 ± 0.6 | 6.38 |
| S. epidermidis | 17.5 ± 0.7 | 6.38 | 0.0 | Nd | 19.5 ± 0.0 | 2.13 |
| E. coli 0157:H7 (ATCC 43888) | 14.5 ± 0.6 | 19.15 | 0.0 | Nd | 17.0 ± 0.6 | 6.38 |
| Gene Name or Symbol | Primer Sequence |
|---|---|
| Cyclin D1 | Forward: 5′-ACC TGA GGA GCC CCA ACA A-3′ Reverse: 5′-TCT GCT CCT GGC AGG CC-3′ |
| IL-6 | Forward: 5′-GGTACATCCTCGACGGCATCT-3′; Reverse: 5′-GT GCCTCTTTGCTGCTTTCAC-3′ |
| IL-1β | Forward: 5′-ATGGCAACTGTTCCTGAACTCAAC-3′ Reverse: 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′ |
| TNF-α | Forward: 5′-ATGAGCACAGAAAGCATGATCC-3′ Reverse: 5′-TCACAGAGCAATGACTCCAAAGTAG-3′ |
| BCL-2 | Forward: 5′-AAG CCG GCG ACG ACT TCT-3′ Reverse: 5′-GGT GCC GGT TCA GGT ACT-3′ |
| caspase 3 | Forward: 5′-AGCAAACCTCAGGGAAACATT-3′, Reverse: 5′-CTCAGAAGCACACAAACAAAACT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Hajleh, M.N.; Al-limoun, M.; Al-Tarawneh, A.; Hijazin, T.J.; Alqaraleh, M.; Khleifat, K.; Al-Madanat, O.Y.; Qaisi, Y.A.; AlSarayreh, A.; Al-Samydai, A.; et al. Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria. Molecules 2023, 28, 4757. https://doi.org/10.3390/molecules28124757
Abu Hajleh MN, Al-limoun M, Al-Tarawneh A, Hijazin TJ, Alqaraleh M, Khleifat K, Al-Madanat OY, Qaisi YA, AlSarayreh A, Al-Samydai A, et al. Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria. Molecules. 2023; 28(12):4757. https://doi.org/10.3390/molecules28124757
Chicago/Turabian StyleAbu Hajleh, Maha N., Muhamad Al-limoun, Amjad Al-Tarawneh, Tahani J. Hijazin, Moath Alqaraleh, Khaled Khleifat, Osama Y. Al-Madanat, Yaseen Al Qaisi, Ahmad AlSarayreh, Ali Al-Samydai, and et al. 2023. "Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria" Molecules 28, no. 12: 4757. https://doi.org/10.3390/molecules28124757
APA StyleAbu Hajleh, M. N., Al-limoun, M., Al-Tarawneh, A., Hijazin, T. J., Alqaraleh, M., Khleifat, K., Al-Madanat, O. Y., Qaisi, Y. A., AlSarayreh, A., Al-Samydai, A., Qaralleh, H., & Al-Dujaili, E. A. S. (2023). Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria. Molecules, 28(12), 4757. https://doi.org/10.3390/molecules28124757








