Author Contributions
Conceptualization, M.E. and D.J.; methodology, M.E. and D.J.; software, M.E.; validation, M.E.; formal analysis, M.E. and J.C.; investigation, M.E., J.C., M.J. and D.J.; resources, D.J.; data curation, J.C. and M.J.; writing—original draft preparation, M.E.; writing—review and editing, D.J. and M.E.; visualization, M.E.; supervision, D.J.; project administration, D.J.; funding acquisition, D.J. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Mean spectra from five brands of isoniazid tablets collected in (left): Centurion, South Africa and (right): Durham, NC, USA using handheld spectrometers (900–1700 nm) and using background (C), a borosilicate glass vial with a Teflon insert.
Figure 1.
Mean spectra from five brands of isoniazid tablets collected in (left): Centurion, South Africa and (right): Durham, NC, USA using handheld spectrometers (900–1700 nm) and using background (C), a borosilicate glass vial with a Teflon insert.
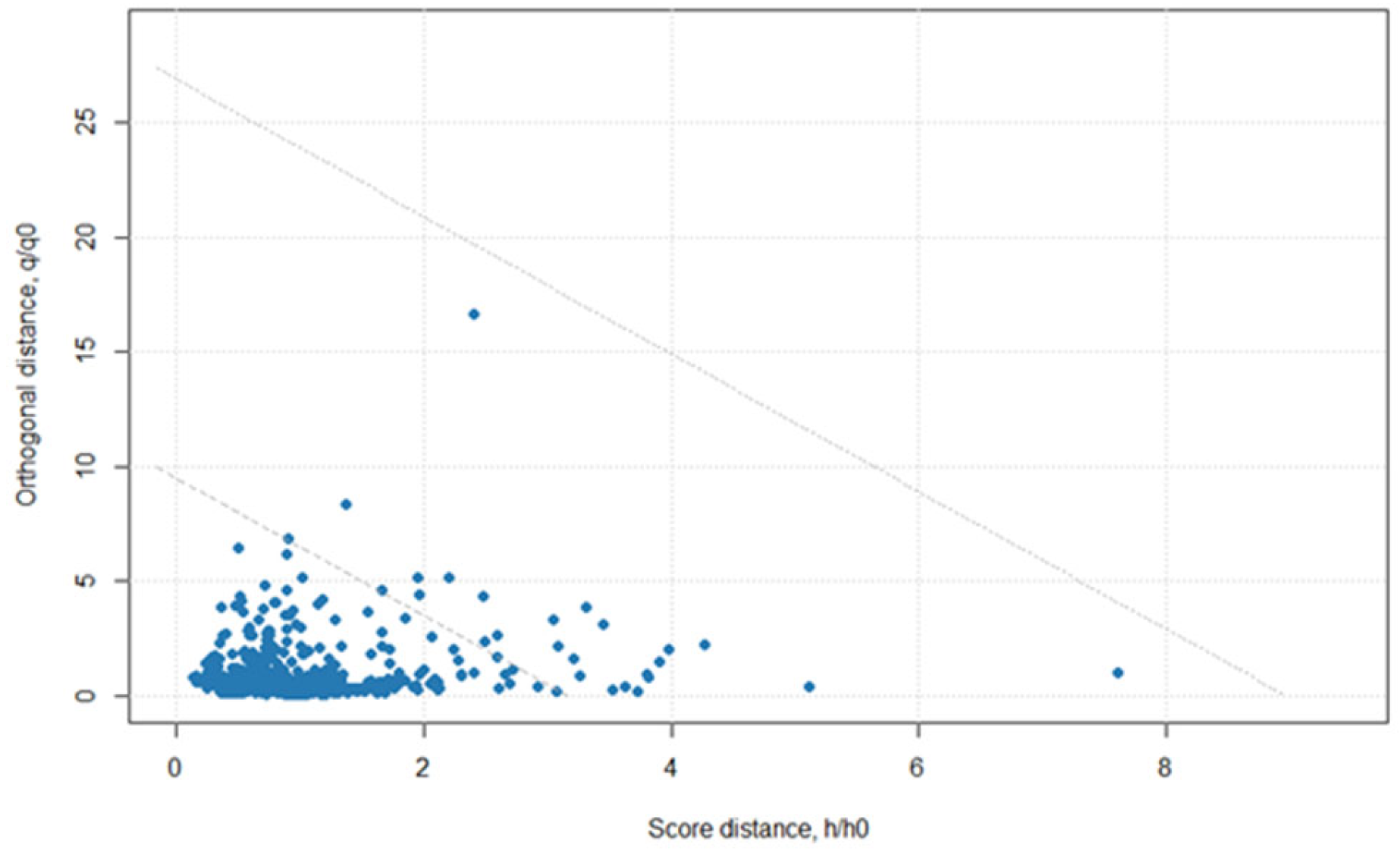
Figure 2.
Residuals from a principal component analysis using isoniazid tablet data collected at both locations, Centurion, South Africa, and Durham, North Carolina, USA, with a handheld spectrometer (900–1700 nm).
Figure 2.
Residuals from a principal component analysis using isoniazid tablet data collected at both locations, Centurion, South Africa, and Durham, North Carolina, USA, with a handheld spectrometer (900–1700 nm).
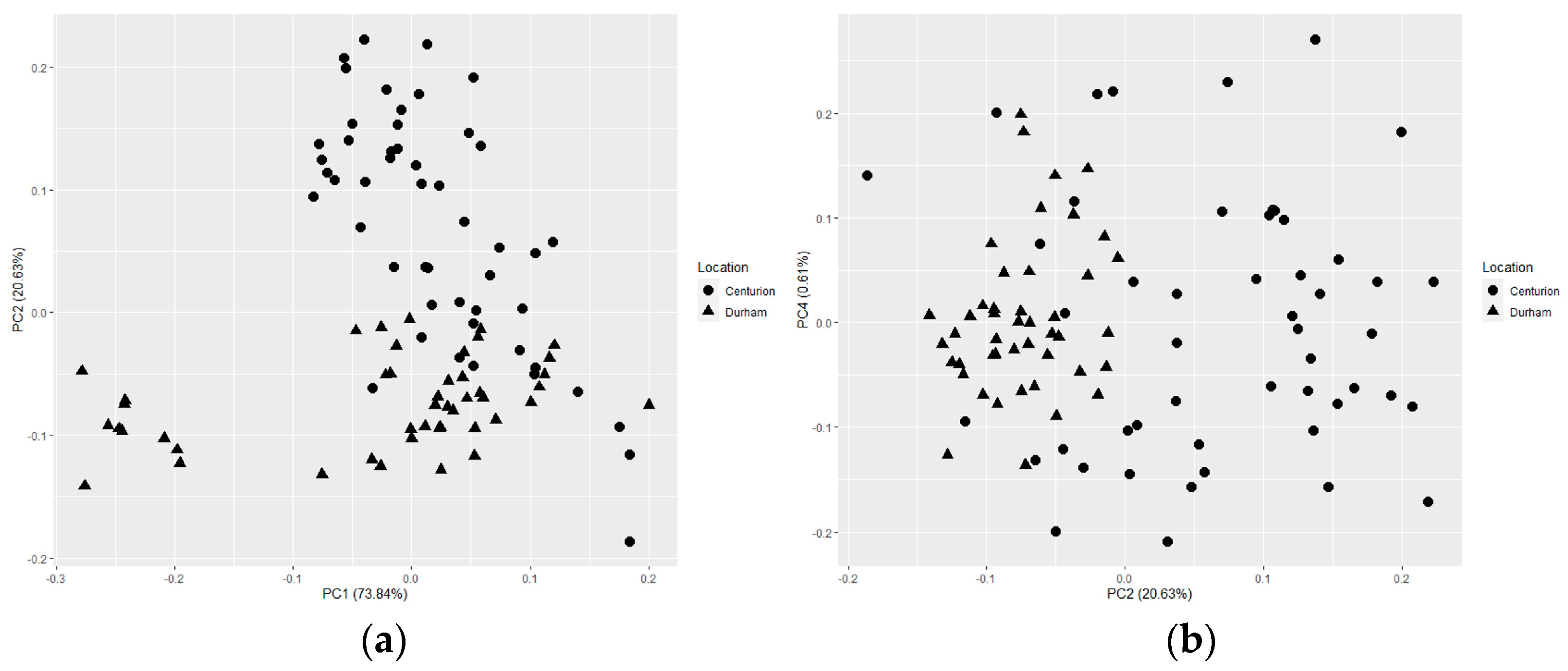
Figure 3.
Score plots from a singular value decomposition-based principal component analysis of all samples (N = 482) collected in Centurion, South Africa and Durham, North Carolina, USA from five isoniazid tablet manufacturing sources. (a) Principal components (PC) 1 vs. PC 2, (b) PC 1 vs. PC 3, (c) PC 2 vs. PC 3, and (d) PC 2 vs. PC 4.
Figure 3.
Score plots from a singular value decomposition-based principal component analysis of all samples (N = 482) collected in Centurion, South Africa and Durham, North Carolina, USA from five isoniazid tablet manufacturing sources. (a) Principal components (PC) 1 vs. PC 2, (b) PC 1 vs. PC 3, (c) PC 2 vs. PC 3, and (d) PC 2 vs. PC 4.
Figure 4.
Score plots from a singular value decomposition based principal component analysis of brand ‘A’ samples used for the proceeding qualitative classification model’s reference and positive controls. Principal component (PC) 1 vs. PC 2 is shown in (a), while PC 2 vs. PC 4 is shown in (b).
Figure 4.
Score plots from a singular value decomposition based principal component analysis of brand ‘A’ samples used for the proceeding qualitative classification model’s reference and positive controls. Principal component (PC) 1 vs. PC 2 is shown in (a), while PC 2 vs. PC 4 is shown in (b).
Figure 5.
Principal component analysis loading vectors for principal components (PC) 1–4, with PC 1 shown in (a) and PCs 2–4 shown in (b). Explained variance per PC is shown as a percentage in parenthesis ().
Figure 5.
Principal component analysis loading vectors for principal components (PC) 1–4, with PC 1 shown in (a) and PCs 2–4 shown in (b). Explained variance per PC is shown as a percentage in parenthesis ().
Figure 6.
(a) Handheld near-infrared spectrometer setup for scanning tablets. A borosilicate glass vial with a 1.6 mm thick Teflon insert positioned directly over the spectrometer’s scanning window with a tablet placed over the opening for sample collection. (b) Three background setups for scanning tablets with a portable near-infrared spectrometer. (A): A borosilicate glass vial with a Teflon insert containing a 2 mm opening and a second Teflon top to cover the opening, (B): a Teflon square large enough to cover the scanning window, (C): a borosilicate glass vial with a Teflon insert and no hole.
Figure 6.
(a) Handheld near-infrared spectrometer setup for scanning tablets. A borosilicate glass vial with a 1.6 mm thick Teflon insert positioned directly over the spectrometer’s scanning window with a tablet placed over the opening for sample collection. (b) Three background setups for scanning tablets with a portable near-infrared spectrometer. (A): A borosilicate glass vial with a Teflon insert containing a 2 mm opening and a second Teflon top to cover the opening, (B): a Teflon square large enough to cover the scanning window, (C): a borosilicate glass vial with a Teflon insert and no hole.
Table 1.
Explained variance from a principal component analysis using portable spectrometers with data collection from five brands of isoniazid tablets scanned in both Centurion, South Africa and Durham, North Carolina, USA.
Table 1.
Explained variance from a principal component analysis using portable spectrometers with data collection from five brands of isoniazid tablets scanned in both Centurion, South Africa and Durham, North Carolina, USA.
| Explained Variance | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 |
|---|
| Proportional | 75.8 | 17.3 | 3.6 | 1.7 | 0.6 |
| Cumulative | 75.8 | 93.2 | 96.7 | 98.4 | 99.0 |
Table 2.
Notable spectra peaks found in principal component analysis loading vectors and correlated to reference spectra collected on a benchtop spectrometer (350–2500 nm).
Table 2.
Notable spectra peaks found in principal component analysis loading vectors and correlated to reference spectra collected on a benchtop spectrometer (350–2500 nm).
| PC | Position (nm) | Reference Standard | Associated Brand Notes |
|---|
| 1 | 1294 ψ | Mannitol | Slightly noticeable in brand D, n/p in others |
| 1 | 1429 | PEG in Castor Oil | No definitive peak in brand C, n/p in others |
| 1 | 1562 ψ | Lactose | Definitive peak in brand C, n/p in others |
| 1 | 1643 Υ | Isoniazid | Sharp peak found at end of all samples |
| 1 | 1673 Υ | Isoniazid | Sharp peak found at end of all samples |
| 2 | 979 * | Water | Moisture in tablets |
| 2 | 1193 | Mannitol | Slightly noticeable in brand D, n/p in others |
| 2 | 1440 * | Water | Moisture in tablets |
| 2 | 1635 ψ | EDTA | Present in brands A and E. Not seen, but may be masked by isoniazid peak found at 1643 nm |
| 2 | 1690 | Mannitol/EDTA | Difficult to determine due to isoniazid peak at 1673 nm |
| 3 | 1024 | Unknown | Not noticeable in any mean spectra |
| 3 | 1092 | Unknown | Not noticeable in any mean spectra |
| 3 | 1189 | Soluble/Corn Starch | Possibly masked by strong isoniazid peak at 1150 nm |
| 3 | 1272 | Magnesium Stearate | Not noticeable in brands A and B |
| 3 | 1391 | Stearic Acid | Possibly overtaken by large water peak at 1440 nm |
| 3 | 1510 | Mannitol | Not noticeable in brand D (n/p) in others, but location is between water and isoniazid related peaks |
| 3 | 1607 | EDTA | Not present in brands A and E, appears to be between two strong isoniazid peaks |
| 4 | 982 * | Water | Moisture in tablets |
| 4 | 1065 Υ | Isoniazid | Peak found in all tablets |
| 4 | 1148 Υ | Isoniazid | Present in all mean spectra |
| 4 | 1243 ψ | Lactose | Noticeable peak in brand C, n/p in others |
| 4 | 1335 | Unknown | Not noticeable in any mean spectra |
| 4 | 1405 | Talc | Possibly overtaken by large water peak at 1440 nm |
| 4 | 1561 Υ | Isoniazid | Noticeable in all mean spectra |
| 4 | 1659 Υ | Isoniazid | Noticeable in all mean spectra |
Table 3.
Summary of classification results for the brand ‘A’ method using three different backgrounds, (a) a borosilicate glass vial with a Teflon insert with a 2 mm hole cut in the center and a second Teflon insert to cover the opening, (b) a single piece of Teflon, and (c) a borosilicate glass vial with a Teflon insert that had no center hole cut.
Table 3.
Summary of classification results for the brand ‘A’ method using three different backgrounds, (a) a borosilicate glass vial with a Teflon insert with a 2 mm hole cut in the center and a second Teflon insert to cover the opening, (b) a single piece of Teflon, and (c) a borosilicate glass vial with a Teflon insert that had no center hole cut.
| Background | Sensitivity | Specificity | Accuracy |
|---|
| A | 1.0 | 0.29 | 40.87% |
| B | 1.0 | 0.72 | 77.80% |
| C | 1.0 | 1.0 | 100% |
Table 4.
Classification results for a qualitative brand ‘A’ method. Mahalanobis distance (MD) values (mean ± standard deviation) were calculated from the centroid of the reference dataset (pooled from both sensors) based on five principal component scores and residuals (99.02% explained variance).
Table 4.
Classification results for a qualitative brand ‘A’ method. Mahalanobis distance (MD) values (mean ± standard deviation) were calculated from the centroid of the reference dataset (pooled from both sensors) based on five principal component scores and residuals (99.02% explained variance).
| Data | Brand | Durham MD | Durham Correct ID | S. Africa MD | S. Africa Correct ID |
|---|
| Ref | A | 0.897 ± 0.21 | 32/32 (100) | 1.01 ± 0.30 | 35/35 (100) |
| PC | A | 0.896 ± 0.22 | 14/14 (100) | 1.271 ± 0.49 | 15/15 (100) |
| NC 1 | B | 19.332 ± 4.99 | 69/69 (100) | 9.356 ± 3.11 | 60/60 (100) |
| NC 2 | C | 54.608 ± 5.69 | 51/51 (100) | 64.891 ± 7.32 | 50/50 (100) |
| NC 3 | D | 260.616 ± 18.37 | 27/27 (100) | 272.61 ± 46.56 | 30/30 (100) |
| NC 4 | E | 26.994 ± 3.1 | 69/69 (100) | 32.63 ± 6.45 | 30/30 (100) |
Table 5.
Bias testing for sensor-to-sensor comparison of two handheld spectrometers (900–1700 nm) scanning tablets in Centurion, South Africa and Durham, North Carolina, USA. Results were calculated from resulting Mahalanobis distance results per sample at each location.
Table 5.
Bias testing for sensor-to-sensor comparison of two handheld spectrometers (900–1700 nm) scanning tablets in Centurion, South Africa and Durham, North Carolina, USA. Results were calculated from resulting Mahalanobis distance results per sample at each location.
| Preprocessing | SEMCenturion | d.f. | t-Critical | t-Calculated | Bias Significant |
|---|
| Raw | 0.0556 | 95 | 1.661 | 3.29 | Yes |
| Baseline | 0.0346 | 95 | 1.661 | 2.97 | Yes |
| MSC | 0.0462 | 95 | 1.661 | 2.88 | Yes |
Table 6.
Summary of lots and tablets of isoniazid scanned in Durham, North Carolina, United States and Centurion, South Africa using a handheld spectrometer (900–1700 nm).
Table 6.
Summary of lots and tablets of isoniazid scanned in Durham, North Carolina, United States and Centurion, South Africa using a handheld spectrometer (900–1700 nm).
| Brand | Centurion, South Africa | Durham, NC, USA |
|---|
| | # Lots | # Tablets | # Lots | # Tablets |
|---|
| A | 5 (2) | 50 (20) | 8 (2) | 46 (10) |
| B | 6 (3) | 60 (30) | 9 (3) | 69 (15) |
| C | 5 (3) | 50 (30) | 9 (3) | 51 (15) |
| D | 3 (3) | 30 (30) | 9 (3) | 27 (15) |
| E | 3 (3) | 30 (30) | 9 (3) | 69 (15) |
| Total | 22 (14) | 220 (140) | 44 (14) | 262 (70) |
Table 7.
List of excipients used in each brand, provided through certificates of analysis obtained through the five manufacturers blinded as A–E.
Table 7.
List of excipients used in each brand, provided through certificates of analysis obtained through the five manufacturers blinded as A–E.
| Excipient | A | B | C | D | E |
|---|
| calcium hydrogen phosphate | Y | | | | |
| colloidal anhydrous silica/colloidal silicon dioxide | Y | Y | Y | Y | Y |
| croscarmellose sodium | | | | Y | |
| crospovidone | | Y | | | Y |
| hydrogenated castor oil | | | Y | | |
| lactose monohydrate | | | Y | | |
| magnesium stearate | Y | Y | | | Y |
| maize starch | Y | Y | | | Y |
| mannitol | | | | Y | |
| microcrystalline cellulose | | Y | Y | Y | Y |
| povidone | | | | Y | |
| pregelatinized starch | | | | Y | |
| purified talc | Y | | | | |
| sodium edetate | Y | | | | |
| stearic acid | | | | Y | Y |
Table 8.
Sample breakdown of the reference, positive, and negative controls used in establishing a qualitative two-location screening method for brand A.
Table 8.
Sample breakdown of the reference, positive, and negative controls used in establishing a qualitative two-location screening method for brand A.
| Brand | Type | Centurion, South Africa # Tablets | Durham, NC, USA # Tablets | Total # Tablets |
|---|
| A | Ref | 35 | 32 | 67 |
| A | PC | 15 | 14 | 29 |
| B | NC 1 | 60 | 69 | 129 |
| C | NC 2 | 50 | 51 | 101 |
| D | NC 3 | 30 | 27 | 57 |
| E | NC 4 | 30 | 69 | 99 |