Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. BBB Hinders AD Treatment
2.1. Current Therapy Strategy
2.2. The Impact of BBB on Treatment of AD
2.3. New Strategies for Treating AD through BBB
2.3.1. Route of Administration for Treating AD
2.3.2. Potential Agents for AD Treatment
2.3.3. Nanoparticle Technology for Treating AD
3. Core NPs
3.1. NPs
3.1.1. Polymeric NPs
3.1.2. Lipid-Based NPs
3.1.3. Inorganic NPs
3.2. Synthesis of Core NPs
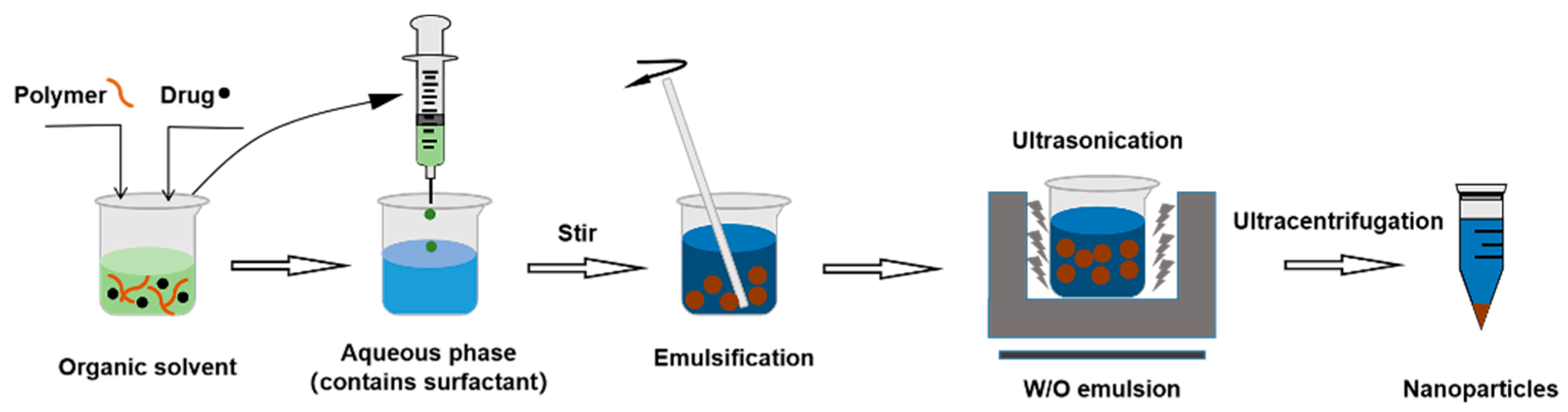
3.2.1. Single Emulsification–Solvent Evaporation Method
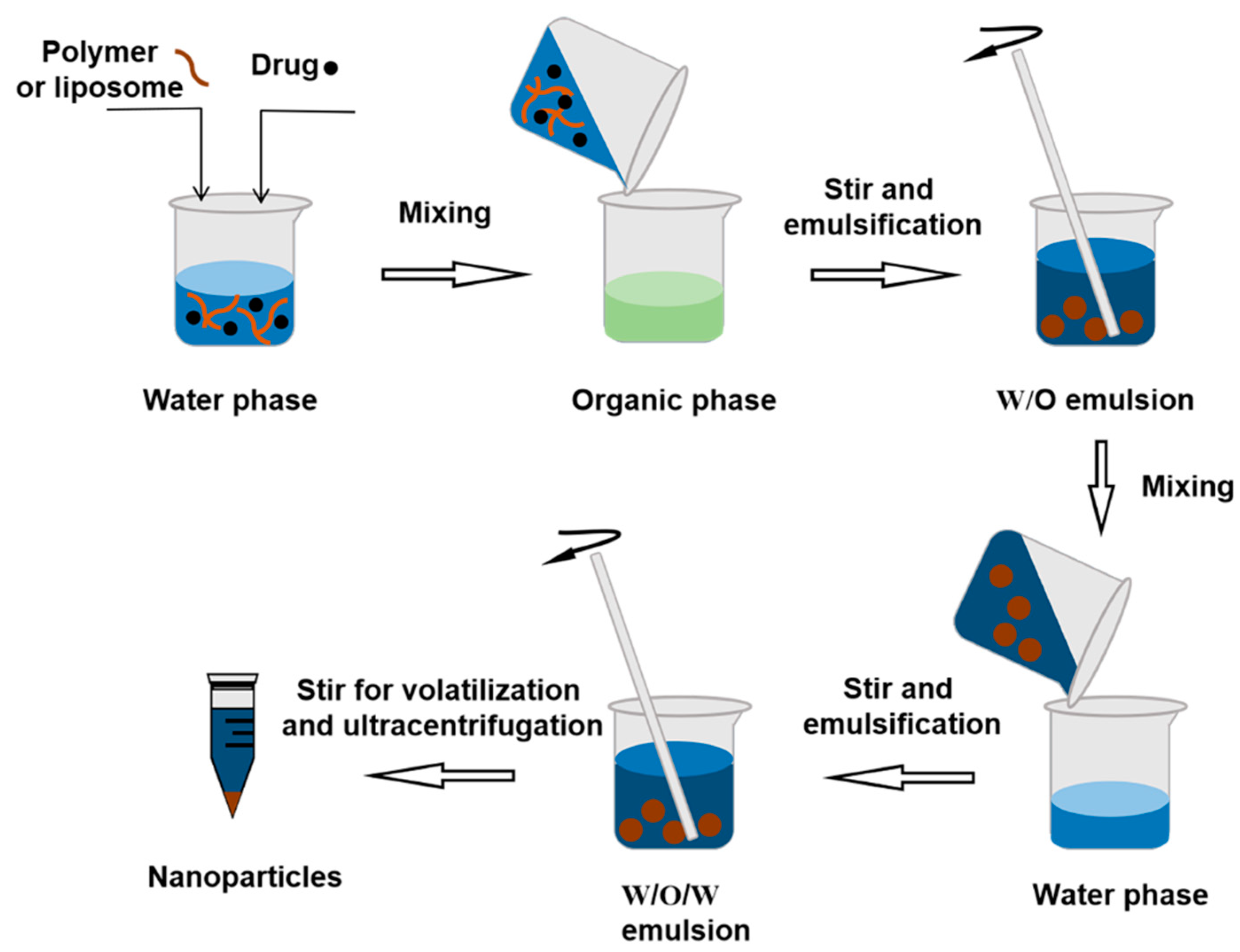
3.2.2. Double Emulsion Method
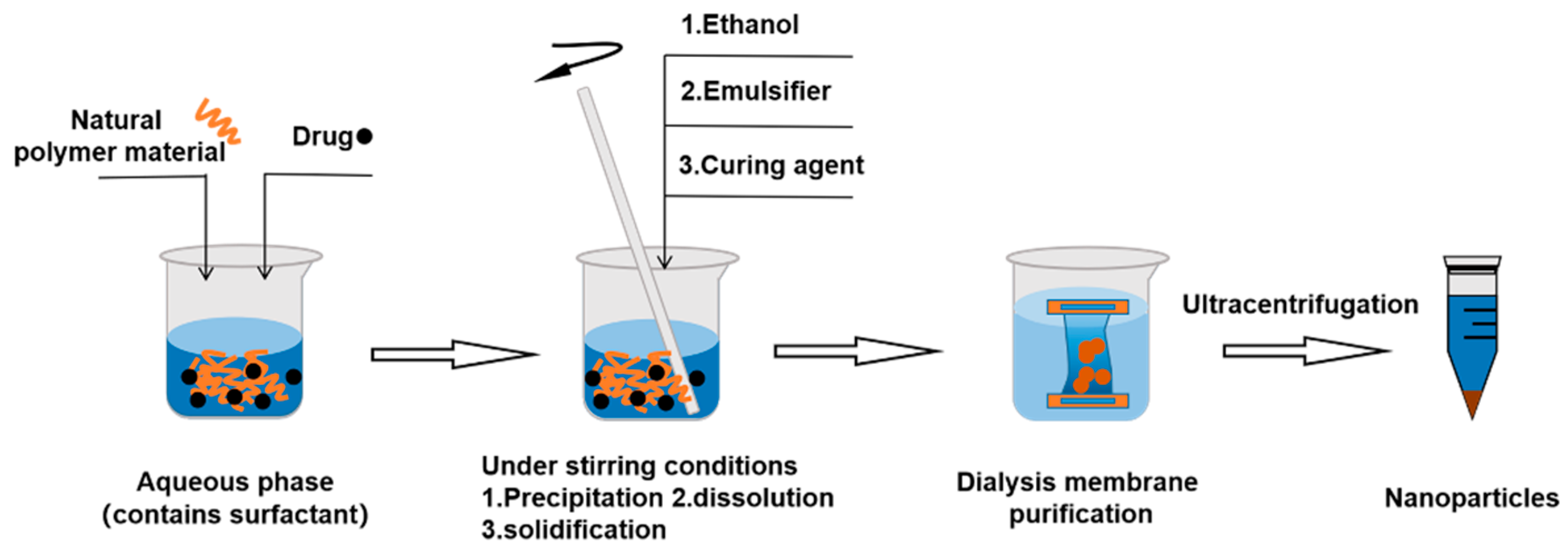
3.2.3. Nanoprecipitation Method
3.2.4. Salting out Emulsification–Diffusion Method
3.2.5. Supercritical Fluid Method
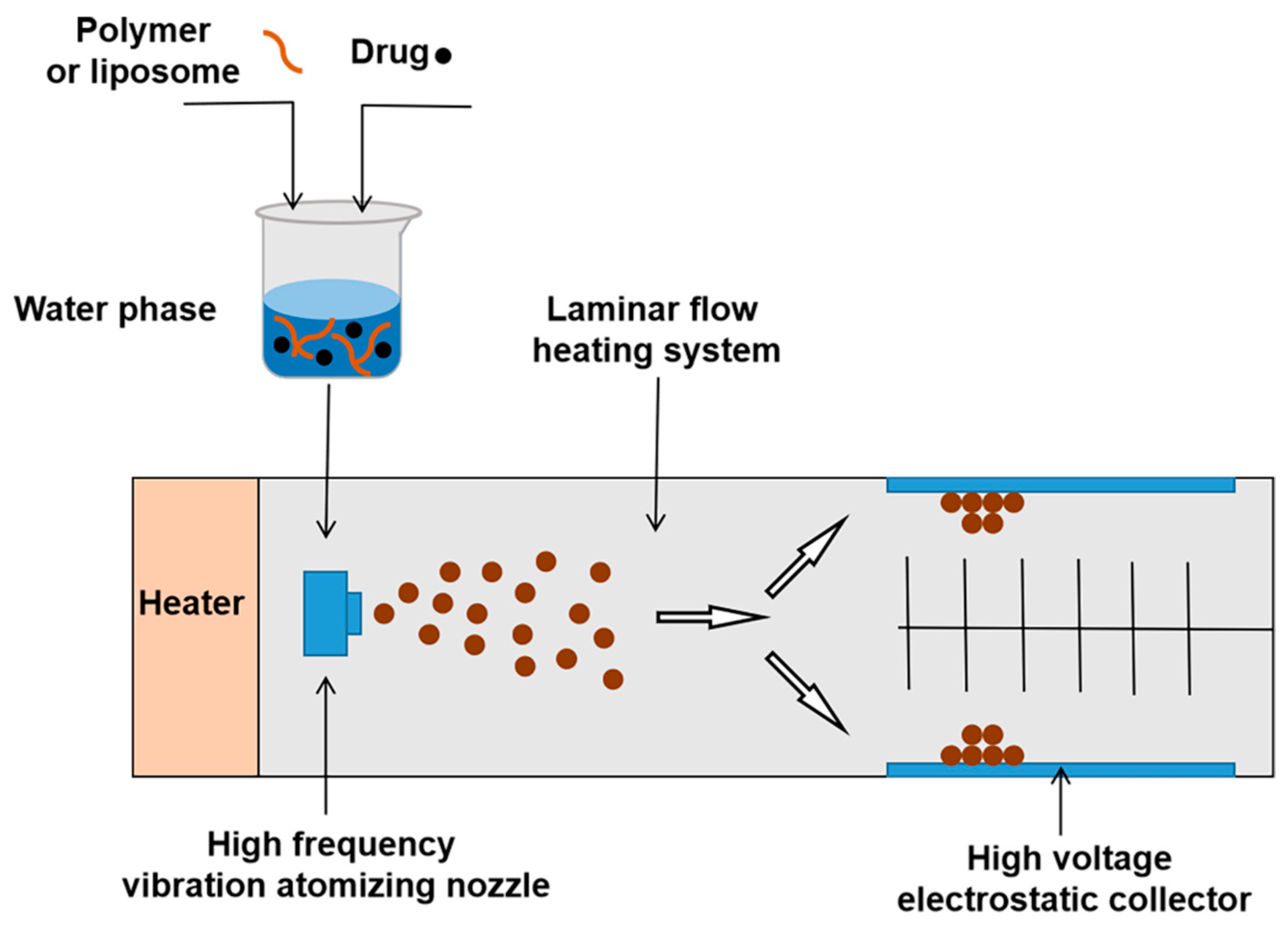
3.2.6. Spray Drying Method
3.2.7. Solvothermal Method
3.2.8. Sol–Gel Method
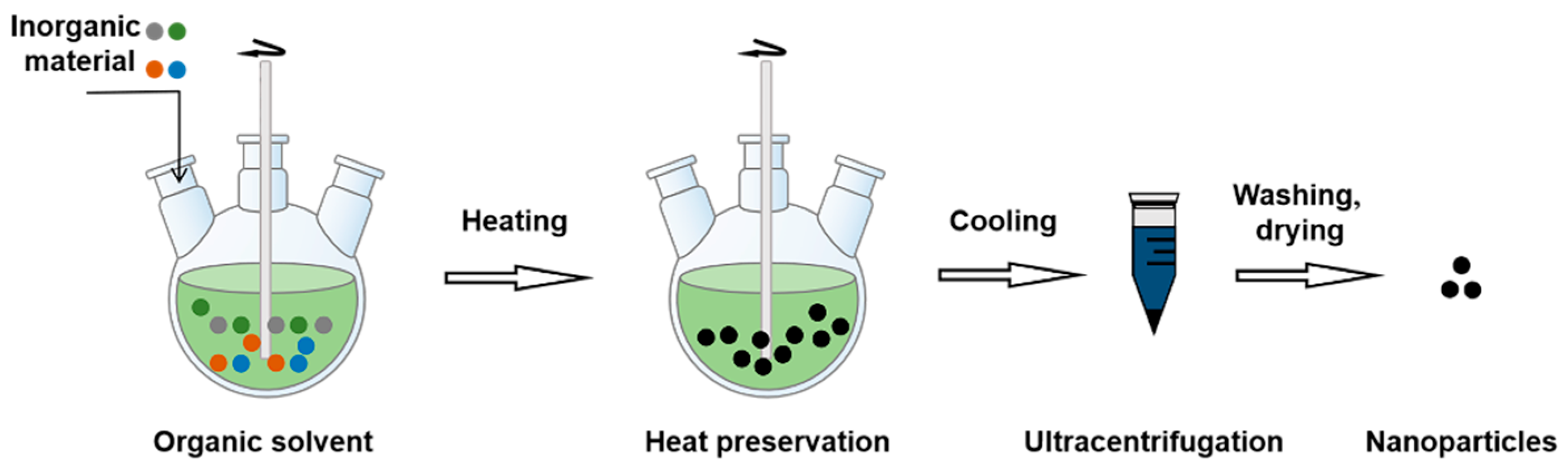
3.2.9. Thermal Decomposition
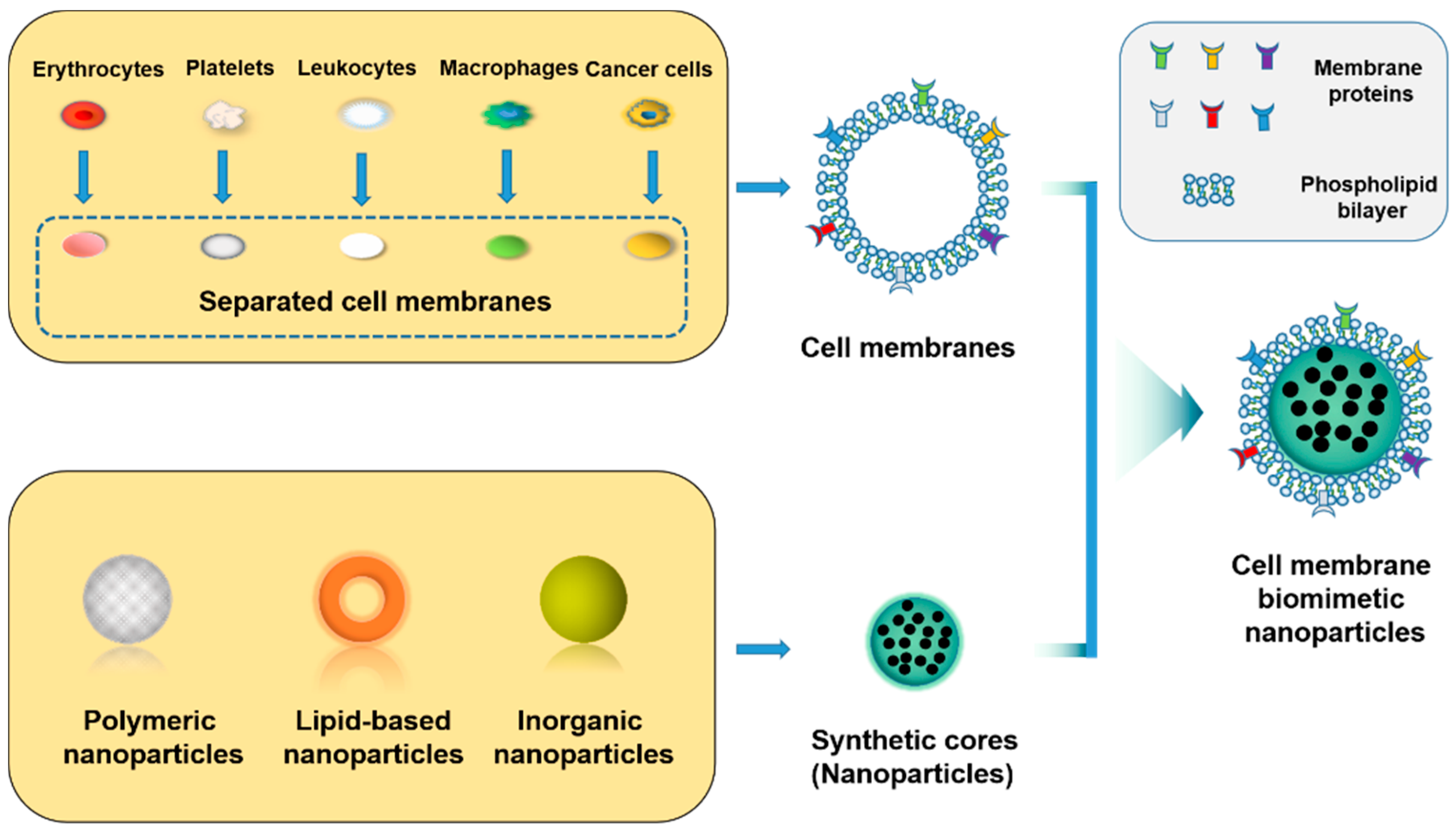
4. Cell Membrane
4.1. Source Cell
4.1.1. Erythrocyte
4.1.2. Platelet
4.1.3. Leukocyte
4.1.4. Macrophages
4.1.5. Cancer Cells
4.1.6. Membrane Hybridization
4.1.7. Other Cells
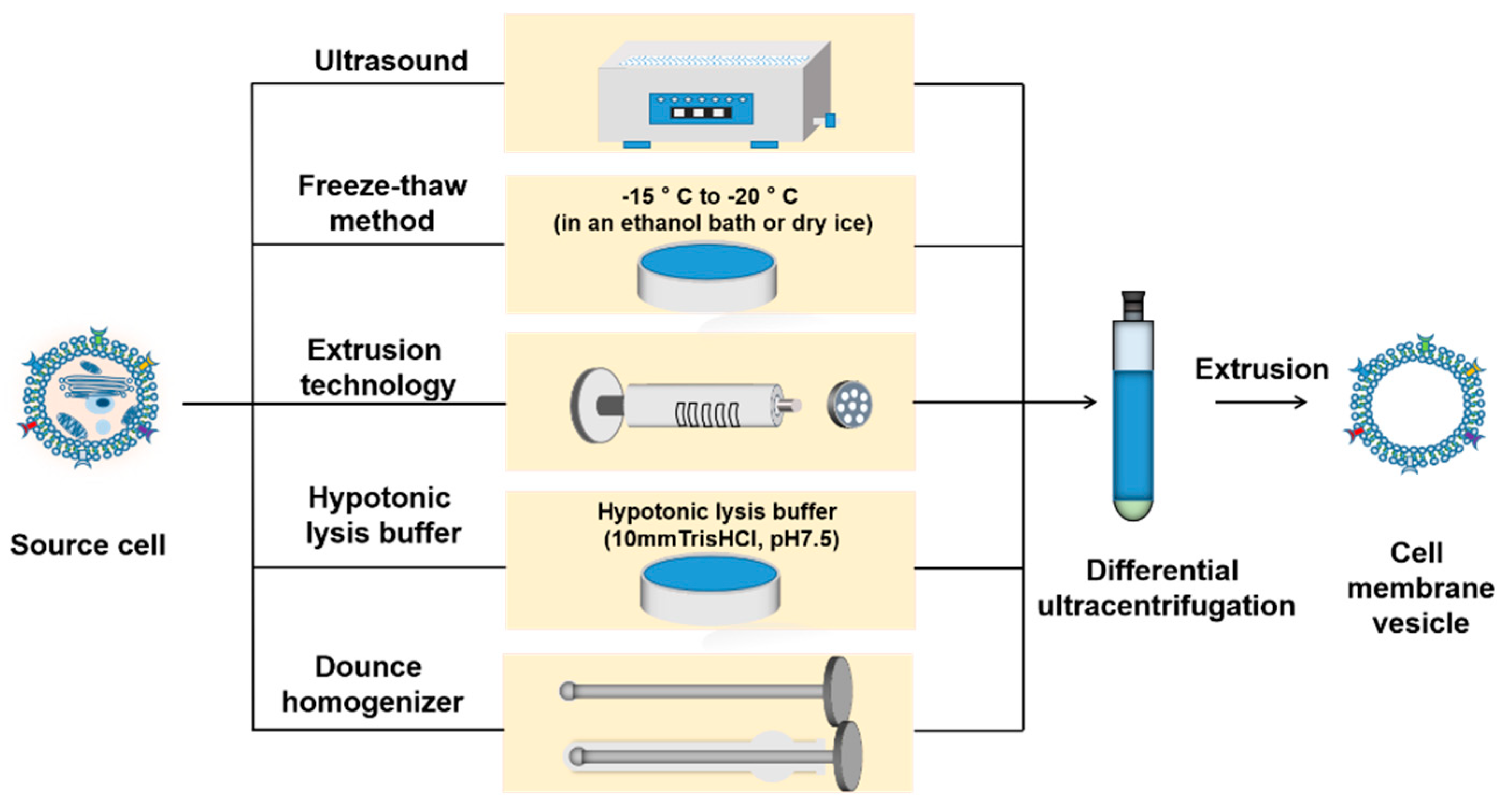
4.2. Isolation of Cell Membrane
4.2.1. Ultrasound
4.2.2. Freeze–Thaw
4.2.3. Extrusion
4.2.4. Hypotonicity
4.2.5. Dounce Homogenizer
4.3. Fusion of Membrane Vesicles and NPs
4.3.1. Co-Extrusion
4.3.2. Ultrasound
4.3.3. Microfluidic Electroporation
4.3.4. Other Coating Methods
5. Targeting Peptides
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bukhari, S.N.A. Nanotherapeutics for Alzheimer’s disease with preclinical evaluation and clinical trials: Challenges, promises and limitations. Curr. Drug Deliv. 2022, 19, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; Hout, A.V.D.; Handels, R.; Bos, I.; Flier, W.M.V.D.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef]
- WHO. Dementia; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Todd, S.; Barr, S.; Roberts, M.; Passmore, A.P. Survival in dementia and predictors of mortality: A review. Int. J. Geriatr. Psychiatry 2013, 28, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Leng, F.D.; Xiong, Q.; Zhou, J.; Du, A.L.; Zhu, F.Q.; Kou, X.W.; Sun, W.; Chen, L.Z.; Wang, H.L.; et al. Factors associated with Alzheimer’s disease patients’ caregiving status and family caregiving burden in China. Front. Aging Neurosci. 2022, 14, 865933. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.L.; Samant, N.P. Current druggable targets for therapeutic control of Alzheimer’s disease. Contemp. Clin. Trials. 2021, 109, 106549. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. BBB-genomics: Creating new openings for brain-drug targeting. Drug Discov. Today 2001, 6, 381–383. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X.Y. Drug delivery to the brain across the blood–brain barrier using nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef]
- Tripathi, P.; Shukla, P.; Bieberich, E. Theranostic applications of nanomaterials in Alzheimer’s disease: A multifunctional approach. Curr. Pharm. Des. 2022, 28, 116–132. [Google Scholar] [CrossRef]
- Chai, Z.L.; Hu, X.F.; Lu, W.Y. Cell membrane-coated nanoparticles for tumor-targeted drug delivery. Sci. China Mater. 2017, 60, 504–510. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wang, X.B.; Hu, D.H.; Wang, P.; Liu, Q.H.; Zhang, X.J.; Jiang, J.Y.; Liu, X.; Sheng, Z.H.; Liu, B.; et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2018, 13, 386–398. [Google Scholar] [CrossRef]
- Wang, H.J.; Liu, Y.; He, R.Q.; Xu, D.L.; Zang, J.; Weeranoppanant, N.; Dong, H.Q.; Li, Y.Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2019, 8, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, Y.J.; Yang, Z.P.; Zhang, D.Y.; Lu, Y.Q.; Zheng, M.; Xue, X.; Geng, J.; Chung, R.; Shi, B.Y. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv. Mater. 2018, 30, 1803717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zhao, M.; Gao, Y.L.; Cheng, X.; Liu, X.Y.; Tang, S.K.; Peng, Y.B.; Wang, N.; Hu, D.D.; Peng, H.S.; et al. Biomimetic erythrocytes engineered drug delivery for cancer therapy. Chem. Eng. J. 2021, 433, 133498. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline. Alzheimers Dement. 2021, 7, e12179. [Google Scholar]
- Melnikova, I. Therapies for Alzheimer’s disease. Nat. Rev. Drug Discov. 2007, 6, 341–342. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Nomura, I.; Sakuma, K.; Okuya, M.; Ikuta, T.; Iwata, N. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2018, 17, 1053–1061. [Google Scholar] [CrossRef]
- McKeage, K. Memantine: A review of its use in moderate to severe Alzheimer’s disease. CNS Drugs 2009, 23, 881–897. [Google Scholar] [CrossRef]
- Atri, A.; Hendrix, S.B.; Pejović, V.; Hofbauer, R.K.; Edwards, J.; Molinuevo, J.L.; Graham, S.M. Cumulative, additive benefits of memantine-donepezil combination over component monotherapies in moderate to severe Alzheimer’s dementia: A pooled area under the curve analysis. Alzheimer’s Res. Ther. 2015, 7, 28. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Mahase, E. Aducanumab: European agency rejects Alzheimer’s drug over efficacy and safety concerns. The BMJ 2021, 375, n3127. [Google Scholar] [CrossRef]
- Cano, A.; Turowski, P.; Ettcheto, M.; Duskey, J.T.; Tosi, G.; Sánchez-López, E.; García, M.L.; Camins, A.; Souto, E.B.; Ruiz, A.; et al. Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of Alzheimer’s disease: From current to future challenges. J. Nanobiotechnology 2021, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Liebner, S. Structure and function of the blood-brain barrier (BBB). Handb. Exp. Pharmacol. 2020, 273, 3–31. [Google Scholar]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.A.; Noorani, B.; Alqahtani, F.; Bhalerao, A.; Raut, S.; Sivandzade, F.; Cucullo, L. Understanding the brain uptake and permeability of small molecules through the BBB: A technical overview. J. Cereb. Blood Flow Metab. 2021, 41, 1797–1820. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Zhao, J.J.; Ren, T.J.; Yang, M.B.; Zhang, Y.F.; Wang, Q.W.; Zuo, Z. Reduced systemic exposure and brain uptake of donepezil in rats with scopolamine-induced cognitive impairment. Xenobiotica 2019, 50, 389–400. [Google Scholar] [CrossRef]
- Karasova, J.Z.; Soukup, O.; Korabecny, J.; Hroch, M.; Krejciova, M.; Hrabinova, M.; Misik, J.; Novotny, L.; Hepnarova, V.; Kuca, K. Tacrine and its 7-methoxy derivate; time-change concentration in plasma and brain tissue and basic toxicological profile in rats. Drug Chem. Toxicol. 2019, 44, 207–214. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gorain, B.; Mohananaidu, K.; Sengupta, P.; Mandal, U.K.; Choudhury, H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Wang, L.; Li, G.; Gao, J.; Wang, Y. Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv. 2021, 28, 380–389. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer’s disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. T echnol. 2020, 61, 102214. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed 2018, 13, 705–718. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Xu, J.; Song, X.; Huang, H.; Feng, Y.; Fu, C. Rhynchophylline loaded-mPEG-PLGA nanoparticles coated with Tween-80 for preliminary study in Alzheimer’s disease. Int. J. Nanomed. 2020, 15, 1149–1160. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A review: Halogenated compounds from marine actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef]
- Vio, V.; Marchant, M.J.; Araya, E.; Kogan, M.J. Metal nanoparticles for the treatment and diagnosis of neurodegenerative brain diseases. Curr. Pharm. Des. 2017, 23, 1916–1926. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Joy, R.; George, J.; John, F. Brief outlook on polymeric nanoparticles, micelles, niosomes, hydrogels and liposomes: Preparative methods and action. ChemistrySelect 2022, 7, e202104045. [Google Scholar] [CrossRef]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug. Deliv. 2019, 17, 23–32. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Breton, A.L.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.Y.; Fu, M.F.; Zhai, G.X. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; Silva, M.C.D.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Khan, I.; Gothwal, A.; Sharma, A.K.; Kesharwani, P.; Gupta, L.; Lyer, A.K.; Gupta, U. PLGA nanoparticles and their versatile role in anticancer drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 159–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chu, X.; Gong, W.; Zheng, J.; Xie, X.; Wang, Y.; Yang, M.; Li, Z.; Gao, C.; Yang, Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J. Nanobiotechnology 2020, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.Y.; Maruf, A.; Wang, J.X.; Xu, J.X.; Liao, X.L.; Wu, W.; Wang, G.X. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen Biomater. 2021, 8, rbab033. [Google Scholar] [CrossRef]
- Perche, F.; Uchida, S.; Akiba, H.; Lin, C.Y.; Ikegami, M.; Dirisala, A.; Nakashima, T.; Itaka, K.; Tsumoto, K.; Kataoka, K. Improved brain expression of anti-amyloid β scFv by complexation of mRNA including a secretion sequence with PEG-based block catiomer. Curr. Alzheimer Res. 2016, 14, 295–302. [Google Scholar] [CrossRef]
- Xie, J.; Gonzalez-Carter, D.; Tockary, T.A.; Nakamura, N.; Xue, Y.; Nakakido, M.; Akiba, H.; Dirisala, A.; Liu, X.; Toh, K.; et al. Dual-sensitive nanomicelles enhancing systemic delivery of therapeutically active antibodies specifically into the brain. ACS Nano 2020, 14, 6729–6742. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting nanoparticles to the brain by exploiting the blood-brain barrier impermeability to selectively label the brain endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Lee, W.H. Physicochemical characteristics and in vitro toxicity/anti-SARS-CoV-2 activity of favipiravir solid lipid nanoparticles (SLNs). Pharmaceuticals 2021, 14, 1059. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Li, Y.; Yang, G.Z.; Zhao, C.X. Nanoemulsions for drug delivery. Particuology 2021, 64, 85–97. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Zhang, N.; Li, Q.Y.; Chen, J.; Wang, Q.Z.; Yang, H.B.; Tan, H.P.; Gao, J.F.; Dong, Z.H.; Pang, Z.Q.; et al. Biomimetic liposomes hybrid with platelet membranes for targeted therapy of atherosclerosis. Chem. Eng. J. 2020, 408, 127296. [Google Scholar] [CrossRef]
- Han, Y.; Gao, C.H.; Wang, H.; Sun, J.J.; Liang, M.; Feng, Y.; Liu, Q.Q.; Fu, S.Y.; Cui, L.; Gao, C.S.; et al. Macrophage membrane-coated nanocarriers Co-modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact. Mater. 2021, 6, 529–542. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.J.; Dong, K.; Ren, J.S.; Qu, X.G. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chem. Eur. J. 2014, 21, 829–835. [Google Scholar] [CrossRef]
- Tengjisi; Hui, Y.; Yang, G.Z.; Fu, C.K.; Liu, Y.; Zhao, C.X. Biomimetic core-shell silica nanoparticles using a dual-functional peptide. J. Colloid Interface Sci. 2020, 581, 185–194. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef]
- Liu, D.Z.; Cheng, Y.; Cai, R.Q.; Wang, W.W.; Cui, H.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomedicine 2018, 14, 991–1003. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Chen, F.; Cai, W.B. Biodegradable and renal clearable inorganic nanoparticles. Adv. Sci. 2015, 3, 1500223. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.B.; Phua, S.Z.F.; Lim, W.Q.; Zhang, R.; Feng, L.Z.; Liu, G.F.; Wu, H.W.; Bindra, A.K.; Jana, D.; Liu, Z.; et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv. Mater. 2019, 31, e1901513. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, M.; Pansieri, J.; Heinrich-Balard, L.; Morfin, J.F.; Stransky-Heilkron, N.; Rivory, P.; Mowat, P.; Dumoulin, M.; Cohen, R.; Allémann, É.; et al. Gd-nanoparticles functionalization with specific peptides for ß-amyloid plaques targeting. J. Nanobiotechnology 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Tunki, L.; Kulhari, H.; Reddy, B.B.; Sistla, R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data Brief 2016, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Esposjto, E.; Luca, G.; Nastruzzi, C. Production of lipospheres as carriers for bioactive compounds. Biomaterials 2002, 23, 2283–2294. [Google Scholar] [CrossRef]
- Si, W.; Yang, Q.; Zong, Y.; Ren, G.; Zhao, L.; Hong, M.; Xin, Z. Toward understanding the effect of solvent evaporation on the morphology of PLGA microspheres by double emulsion method. Ind. Eng. Chem. Res. 2021, 60, 9196–9205. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int. J. Pharm. 2017, 533, 275–284. [Google Scholar] [CrossRef]
- Sheth, P.; Sandhu, H.; Singhal, D.; Malick, W.; Shah, N.; Kislalioglu, M.S. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr. Drug Deliv. 2012, 9, 269–284. [Google Scholar] [CrossRef]
- Maqbool, F.; Moyle, P.M.; Thurecht, K.J.; Falconer, J.R. Dispersibility of phospholipids and their optimization for the efficient production of liposomes using supercritical fluid technology. Int. J. Pharm. 2019, 563, 174–183. [Google Scholar] [CrossRef]
- Islam, T.; Ragib, A.A.; Ferdosh, S.; Uddin, A.B.M.H.; Akanda, M.J.H.; Mia, M.A.R.; M, R.P.D.; Kamaruzzaman, B.Y.; Sarker, M.Z.I. Development of nanoparticles for pharmaceutical preparations using supercritical techniques. Chem. Eng. Commun. 2022, 209, 1642–1663. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2016, 516, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.-K.; Tan, R.B.H. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Li, R.X. Progress in preparation methods and applications of inorganic nanoparticles. J. Phys. Conf. Ser. 2020, 1676, 012093. [Google Scholar] [CrossRef]
- Li, Y.; Han, Q.L.; Yao, Y.; Li, M.; Dong, P.; Han, L.; Zeng, X.Y.; Liu, J.; Liu, J.M.; Zhang, Y.J.; et al. Comparative study of yttria-stabilized zirconia synthesis by Co-precipitation and solvothermal methods. JOM 2019, 71, 3806–3813. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Müller, T.S.; Karos, R.; Oliveira, P.W.D. Faster synthesis of CIGS nanoparticles using a modified solvothermal method. J. Alloys Compd. 2016, 659, 178–183. [Google Scholar] [CrossRef]
- Chun, Y.G.; Kim, K.H.; Yoon, K.H. Synthesis of CuLnGaSe2 nanoparticles by solvothermal route. Thin Solid Films 2005, 480–481, 46–49. [Google Scholar] [CrossRef]
- Lismont, M.; Páez, C.A.; Dreesen, L. A one-step short-time synthesis of [email protected]2 core–shell nanoparticles. J. Colloid Interface Sci. 2015, 15, 2903–2911. [Google Scholar]
- Sheng, S.; Jin, S.; Cui, K. Thermal decomposition of nanostructured bismuth subcarbonate. Materials 2020, 13, 4287. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.Q.; Ren, W.Y.; Xu, T.; Qian, Z.Y.; He, Z.Y. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef]
- Huang, Y.X.; Tuo, W.W.; Wang, D.; Kang, L.L.; Chen, X.Y.; Luo, M. Restoring the youth of aged red blood cells and extending their lifespan in circulation by remodelling membrane sialic acid. J. Cell. Mol. Med. 2015, 20, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, D.; Wei, X.L.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte–platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Song, S.S.; Ma, J.W.; Yan, Z.Y.; Xie, H.W.; Feng, Y.; Che, S.S. CD47 as a promising therapeutic target in oncology. Front. Immunol. 2022, 13, 757480. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Fang, R.H.; Zhang, L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 2012, 1, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.M.; Fan, J.L.; Long, Y.; Xiao, F.; Daniyal, M.; Tong, C.Y.; Xie, Q.; Jian, Y.Q.; Li, B.; et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 2019, 217, 119301. [Google Scholar] [CrossRef]
- Gao, C.H.; Wang, Y.L.; Sun, J.J.; Han, Y.; Gong, W.; Li, Y.; Feng, Y.; Wang, H.; Yang, M.Y.; Li, Z.P.; et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020, 108, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Meijden, P.E.J.V.D.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Rayes, J.; Watson, S.P.; Nieswandt, B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Investig. 2019, 129, 12–23. [Google Scholar] [CrossRef]
- McDonald, B.; Dunbar, M. Platelets and intravascular immunity: Guardians of the vascular space during bloodstream infections and sepsis. Front. Immunol. 2019, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.P.; Wang, X.Q.; Yin, H.Y.; Cao, X.; Hu, Q.Y.; Lv, W.; Xu, Q.W.; Gu, Z.; Xin, H.L. Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano 2019, 13, 8577–8588. [Google Scholar] [CrossRef]
- Zinger, A.; Soriano, S.; Baudo, G.; Rosa, E.D.; Taraballi, F.; Villapol, S. Biomimetic nanoparticles as a theranostic tool for traumatic brain injury. Adv. Funct. Mater. 2021, 31, 2100722. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.O.; Molinaro, R.; Hartman, K.A.; Boada, C.; Sukhovershin, R.; Rosa, E.D.; Kuri, D.; Zhang, S.R.; Evangelopoulos, M.; Carter, A.M.; et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics 2018, 8, 1131–1145. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2017, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Dou, C.R.; Xia, Y.G.; Li, B.H.; Zhao, M.Y.; Yu, P.; Zheng, Y.Y.; El-Toni, A.M.; Atta, N.F.; Galal, A.; et al. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano 2021, 15, 2263–2280. [Google Scholar] [CrossRef]
- Liu, R.; An, Y.; Jia, W.F.; Wang, Y.S.; Wu, Y.; Zhen, Y.H.; Cao, J.; Gao, H.L. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Control. Release 2020, 321, 589–601. [Google Scholar] [CrossRef]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef]
- Yurdagul, A.; Finney, A.C.; Woolard, M.D.; Orr, A.W. The arterial microenvironment: The where and why of atherosclerosis. Biochem. J. 2016, 473, 1281–1295. [Google Scholar] [CrossRef]
- Nagenborg, J.; Goossens, P.; Biessen, E.A.L.; Donners, M.M.P.C. Heterogeneity of atherosclerotic plaque macrophage origin, phenotype and functions: Implications for treatment. Eur. J. Pharmacol. 2017, 816, 14–24. [Google Scholar] [CrossRef]
- Long, Y.; Xiang, Y.; Liu, S.Y.; Zhang, Y.L.; Wan, J.Y.; Ci, Z.M.; Cui, M.Q.; Shen, L.; Li, N.; Guan, Y.M. Macrophage membrane modified baicalin liposomes improve brain targeting for alleviating cerebral ischemia reperfusion injury. Nanomedicine 2022, 43, 102547. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.D.; Zheng, D.Y.; Lin, X.Y.; Wei, Z.W.; Zhang, D.; Li, Z.F.; Zhang, Y.; Wu, M.; Liu, X.L. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy†. Biomater. Sci. 2018, 6, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Drolez, A.; Vandenhaute, E.; Julien, S.; Gosselet, F.; Burchell, J.; Cecchelli, R.; Delannoy, P.; Dehouck, M.-P.; Mysiorek, C. Selection of a relevant in vitro blood-brain barrier model to investigate pro-metastatic features of human breast cancer cell lines. PLoS ONE 2016, 11, e0151155. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Wu, B.; Wu, Y.T.; Song, X.Y.; Zhang, S.S.; Liu, Z.H. Camouflaging nanoparticles with brain metastatic tumor cell membranes: A new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv. Funct. Mater. 2020, 30, 1909369. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, A.; Jiang, L.D.; Gu, Y.W.; Liu, J.Y. Hybrid membrane-coated biomimetic nanoparticles (HM@BNPs): A multifunctional nanomaterial for biomedical applications. Biomacromolecules 2021, 22, 3149–3167. [Google Scholar] [CrossRef]
- Ai, X.Z.; Wang, S.Y.; Duan, Y.O.; Zhang, Q.Z.; Chen, M.S.; Gao, W.W.; Zhang, L.F. Emerging approaches to functionalizing cell membrane-coated nanoparticles. Biochemistry 2020, 60, 941–955. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, W.; Ma, X.Y.; Tang, L.; Zhang, Y.; Yang, M.; Hu, F.F.; Li, G.L.; Wang, Y.Z. Biomimetic neutrophil and macrophage dual membrane-coated nanoplatform with orchestrated tumor-microenvironment responsive capability promotes therapeutic efficacy against glioma. Chem. Eng. J. 2021, 433, 133848. [Google Scholar] [CrossRef]
- Niu, W.B.; Xiao, Q.; Wang, X.J.; Zhu, J.Q.; Li, J.H.; Liang, X.M.; Peng, Y.M.; Wu, C.T.; Lu, R.J.; Pan, Y.; et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, Q.H.; Wang, X.B.; Wang, P.; Li, T.; Tong, W.Y. Membrane damage effect of therapeutic ultrasound on Ehrlich ascitic tumor cells. Cancer Biother. Radiopharm. 2009, 24, 41–48. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Haworth, K.J.; Fakhri, N.H.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1006–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Chen, W.J.; Zou, M.M.; Lv, R.L.; Wang, D.L.; Hou, F.R.; Feng, H.; Ma, X.B.; Zhong, J.J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Amini, M.; Niemi, E.; Hisdal, J.; Kalvøy, H.; Tronstad, C.; Scholz, H.; Rosales, A.; Martinsen, Ø.G. Monitoring the quality of frozen-thawed venous segments using bioimpedance spectroscopy. Physiol. Meas. 2020, 41, 044008. [Google Scholar] [CrossRef]
- Fu, Q.; Lv, P.P.; Chen, Z.K.; Ni, D.Z.; Zhang, L.J.; Yue, H.; Yue, Z.G.; Wei, W.; Ma, G.H. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane†. Nanoscale 2015, 7, 4020–4030. [Google Scholar] [CrossRef]
- Chai, Z.L.; Hu, X.F.; Wei, X.L.; Zhan, C.Y.; Lu, L.W.; Jiang, K.; Su, B.X.; Ruan, H.T.; Ran, D.N.; Fang, R.H.; et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Control. Release 2017, 264, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Klenk, C.; Heim, D.; Ugele, M.; Hayden, O. Impact of sample preparation on holographic imaging of leukocytes. Opt. Eng. 2019, 59, 102403. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, Q.Q.; Wei, D.; Feng, J.X.; Yao, J.H.; Jiang, T.Z.; Song, Q.X.; Wei, X.B.; Chen, H.Z.; Gao, X.L.; et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.; Ahn, S.B.; Mohamedali, A.; Liu, F. Improved sensitivity in cell surface protein detection by combining chemical labeling with mechanical lysis in a colorectal cancer cell model. Biotechnol. Lett. 2020, 42, 683–695. [Google Scholar] [CrossRef]
- DeCaprio, J.; Kohl, T.O. Using Dounce homogenization to lyse cells for immunoprecipitation. Cold Spring Harb. Protoc. 2019, 2019, 551–554. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, J.; Zhang, C.; Olah, A.; Baer, E. Novel micro-/nano- porous cellular membranes by forced assembly co-extrusion technology. Eur. Polym. J. 2016, 83, 99–113. [Google Scholar] [CrossRef]
- He, W.P.; Frueh, J.; Wu, Z.W.; He, Q. Leucocyte membrane-coated janus microcapsules for enhanced photothermal cancer treatment. Langmuir 2016, 32, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Movahed, S.; Li, D.Q. Microfluidics cell electroporation. Microfluid Nanofluidics 2010, 10, 703–734. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, D.Y.; Li, J.H.; Wu, Y.T.; Zhou, W.B.; Wang, W.; Liang, Z.C.; Li, Z.H. High cell viability microfluidic electroporation in a curved channel. Sens. Actuators B Chem. 2017, 250, 703–711. [Google Scholar] [CrossRef]
- Marqués-Gallego, P.; Kroon, A.I.P.M.d. Ligation strategies for targeting liposomal nanocarriers. BioMed. Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef]
- García-Granados, R.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Metabolic engineering and synthetic biology: Synergies, future, and challenges. Front. Bioeng. Biotechnol. 2019, 7, 36. [Google Scholar] [CrossRef]
- Stephan, M.T.; Irvine, D.J. Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.Y.; Lemons, P.K.; Cheng, H. A facile approach to functionalize cell membrane-coated nanoparticles. Theranostics 2016, 6, 1012–1022. [Google Scholar] [CrossRef]
- Wang, R.R.; Wang, C.W.; Dai, Z.Z.; Chen, Y.Z.; Shen, Z.W.; Xiao, G.; Chen, Y.F.; Zhou, J.N.; Zhuang, Z.R.; Wu, R.H. An amyloid-β targeting chemical exchange saturation transfer probe for in vivo detection of Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 3859–3867. [Google Scholar] [CrossRef]
- Spencer, B.; Trinh, L.; Rockenstein, E.; Mante, M.; Florio, J.; Adame, A.; El-Agnaf, O.M.A.; Kim, C.; Masliah, E.; Rissman, R.A. Systemic peptide mediated delivery of an siRNA targeting α-syn in the CNS ameliorates the neurodegenerative process in a transgenic model of Lewy body disease. Neurobiol. Dis. 2019, 127, 163–177. [Google Scholar] [CrossRef]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-targeting increases the transfer of solid lipid nanoparticles with donepezil cargo across a culture model of the blood–brain barrier. Pharmaceutics 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Cox, A.; Cassina, V.; Nardo, L.; Salerno, D.; Marrano, C.A.; Missana, N.; Andreozzi, P.; Silva, P.J.; Stellacci, F.; et al. The clustering of mApoE anti-amyloidogenic peptide on nanoparticle surface does not alter its performance in controlling beta-amyloid aggregation. Int. J. Mol. Sci. 2020, 21, 1066. [Google Scholar] [CrossRef] [PubMed]
- Séguy, L.; Guyon, L.; Maurel, M.; Verdié, P.; Davis, A.; Corvaisier, S.; Lisowski, V.; Dallemagne, P.; Groo, A.C.; Malzert-Fréon, A. Active targeted nanoemulsions for repurposing of tegaserod in Alzheimer’s disease treatment. Pharmaceutics 2021, 13, 1626. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Galstyan, A.; Cox, A.; Shatalova, E.S.; Sun, T.; Rashid, M.-H.; Grodzinski, Z.; Chiechi, A.; Fuchs, D.-T.; Patil, R.; et al. Signature effects of vector-guided systemic nano bioconjugate delivery across blood-brain barrier of normal, Alzheimer’s, and tumor mouse models. ACS Nano 2022, 16, 11815–11832. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.H.; Zhu, L.N.; Wang, L.; Liu, X.Y.; Xiao, F.; Xie, Y.Z.X.; Zheng, W.F.; Jiang, X.Y. Screening on-chip fabricated nanoparticles for penetrating the blood–brain barrier. Nanoscale 2022, 14, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; He, T.; Chai, Z.; Samulski, R.J.; Li, C.W. Blood-brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 2018, 176, 71–83. [Google Scholar] [CrossRef]
- Díaz-Perlas, C.; Oller-Salvia, B.; Sánchez-Navarro, M.; Teixidó, M.; Giralt, E. Branched BBB-shuttle peptides: Chemoselective modification of proteins to enhance blood–brain barrier transport†. Chem. Sci. 2018, 9, 8409–8415. [Google Scholar] [CrossRef]
- Falanga, A.; Melone, P.; Cagliani, R.; Borbone, N.; D’Errico, S.; Piccialli, G.; Netti, P.; Guarnieri, D. Design, synthesis and characterization of novel co-polymers decorated with peptides for the selective nanoparticle transport across the cerebral endothelium. Molecules 2018, 23, 1655. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.F.; Shao, K.; Huang, R.Q.; Ye, L.Y.; Lou, J.N.; Jiang, C. A leptin derived 30-amino-acid peptide modified pegylated poly-L-lysine dendrigraft for brain targeted gene delivery. Biomaterials 2010, 31, 5246–5257. [Google Scholar] [CrossRef]
- Farshbaf, M.; Mojarad-Jabali, S.; Hemmati, S.; Khosroushahi, A.Y.; Motasadizadeh, H.; Zarebkohan, A.; Valizadeh, H. Enhanced BBB and BBTB penetration and improved anti-glioma behavior of bortezomib through dual-targeting nanostructured lipid carriers. J. Control. Release 2022, 345, 371–384. [Google Scholar] [CrossRef]
- Fattahi, H.; Esmaeil, N.; Aliomrani, M. Apamin as a BBB shuttle and its effects on T cell population during the experimental autoimmune encephalomyelitis-induced model of multiple sclerosis. Neurotox. Res. 2021, 39, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Wang, S.G.; Huang, Y.; Seefeldt, T.; Alqahtani, Y.; Guan, X.M. 2-(2-Cholesteroxyethoxyl)ethyl 3′-S-glutathionylpropionate and its self-assembled micelles for brain delivery: Design, synthesis and evaluation. Int. J. Pharm. 2021, 600, 120520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Os, W.L.v.; Tian, X.B.; Zu, G.Y.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 35, 456–465. [Google Scholar] [CrossRef]
- Maderna, E.; Colombo, L.; Cagnotto, A.; Fede, G.D.; Indaco, A.; Tagliavini, F.; Salmona, M.; Giaccone, G. In situ tissue labeling of cerebral amyloid using HIV-related Tat peptide. Mol. Neurobiol. 2018, 55, 6834–6840. [Google Scholar] [CrossRef]
- Rousselle, C.; Clair, P.; Temsamani, J.; Scherrmann, J.-M. Improved brain delivery of benzylpenicillin with a peptide-vector-mediated strategy. J. Drug Target. 2002, 10, 309–315. [Google Scholar] [CrossRef]
- Yang, L.C.; Sun, J.; Xie, W.J.; Liu, Y.N.; Liu, J. Dual-functional selenium nanoparticles bind to and inhibit amyloid β fiber formation in Alzheimer’s disease. J. Mater. Chem. B 2017, 5, 5954–5967. [Google Scholar] [CrossRef] [PubMed]
- Rusiecka, I.; Ruczyński, J.; Kozłowska, A.; Backtrog, E.; Mucha, P.; Kocić, I.; Rekowski, P. TP10-dopamine conjugate as a potential therapeutic agent in the treatment of Parkinson’s disease. Bioconjug. Chem. 2019, 30, 760–774. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.N.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B 2016, 145, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, X.; Zheng, X.Y.; Shao, X.Y.; Liu, Q.F.; Zhang, Q.Z.; Qian, Y. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 2014, 35, 456–465. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, S.T.; Yang, P.; Wang, P.Z.; Lu, S.; Sheng, D.Y.; Qian, K.; Cao, J.X.; Lu, W.; Zhang, Q.Z. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J. Control. Release 2020, 320, 347–362. [Google Scholar] [CrossRef]








| Cell | Separation Methods | Properties | Limitations |
|---|---|---|---|
| Erythrocyte | Extrusion, ultrasound, freeze–thaw, and hypotonicity | Easy availability. | Poor targeting ability. |
| Long circulatory lifespan (~120 days in humans and ~50 days in mice) and wide circulation range. | |||
| Uniform in size and shape, with a good surface area to volume ratio, without organelles and any DNA. | |||
| Good biocompatibility, biodegradability, and non-immunogenicity. | |||
| Platelet | Extrusion, freeze–thaw, and ultrasound | High targeting efficiency. | Small proportion of blood and undesirable activated. |
| Controlled drug release. | |||
| Lower immunogenicity. | |||
| Long systemic circulation (around 7–10 days). | |||
| Targeting to plaque. | |||
| Leukocyte | Extrusion and hypotonicity | Adhesion capacity. | Organization residency restrictions. |
| Migratory and chemotactic capacity in disease states. | |||
| High loading capacity. | |||
| Macrophage | Extrusion and hypotonicity | Good targeting ability to AD lesions. | Organization residency restrictions. |
| Innate immune evasion ability. | |||
| Long circulation ability in vivo. | |||
| Cancer cell | Extrusion and Dounce homogenizer | Strong homologous targeting ability. | Homologous tumor targeting. |
| Method | Procedures | Advantages | Disadvantages |
|---|---|---|---|
| Co-extrusion | The mixed solution formed by mixing the cell membrane suspension and the NPs suspension is co-extruded through a porous filter membrane of specified size with an extruder for many times | The steps are simple and easy to use. | Time-consuming and labor-intensive. Low synthesis rate |
| The multi-layer target product can be prepared | |||
| Ultrasound | The mixture formed by mixing the cell membrane suspension and the NPs suspension is sonicated at a certain frequency for a specified time | Less loss of raw materials; mass production is possible. | Uneven coating, easy to form polydisperse particles. NPs are easily broken |
| The biomimetic NPs formed are highly stable. | |||
| Membrane hybrids can be formed | |||
| Microfluidic electroporation | The cell membrane suspension and NPs suspension are mixed separately in the instrument, flow through the electroporation area, and finally the product is collected in the chip | High synthesis rate and good parallelism | Complex operation process |
| Target Receptor or Transport Pathway | Name | Peptide Sequence | Ref. |
|---|---|---|---|
| Low-density lipoprotein receptor | Angiopep-2 | TFFYGGSRGKRNFKTEEY | [130] |
| ApoB | SSVIDALQYKLEGTTRLTRKRGLKLATALSLSNKFVEGS | [131] | |
| ApoE | LRKLRKRLL | [132] | |
| mApoE | CWGLRKLRKRLLR | [133] | |
| Peptide-22 | Ac-CMPRLRGC-NH2 | [134] | |
| Transferrin receptor | B6 | CGHKAKGPRK | [135] |
| D-T7 | d-HRPYIAH | [136] | |
| T7 | HAIYPRH | [136] | |
| THR | THRPPMWSPVWP-NH2 | [137] | |
| THRre | pwvpswmpprht-NH2 | [138] | |
| CRT | CRTIGPSVC | [139] | |
| Leptin receptor | Leptin30 | YQQILTSMPSRNVIQISNDLENLRDLLHVL | [140] |
| Nicotinic acetylcholine receptor | RVG29 | YTIWMPENPRPGTPCDIFTNSRGKRASNG-OH | [57] |
| DCDX | GreirtGraerwsekf-OH | [116] | |
| D8 | DRTGDRDADREDW | [141] | |
| Potassium or calcium channel | Apamin | CNCKAPETALCARRCQQH-NH2 | [142] |
| MiniAp-4 | H-[Dap]KAPETAL D-NH2 | [135] | |
| Glutathione transporter | GSH | γ-l-glutamyl-CG-OH | [143] |
| G23 | HLNILSTLWKYRC | [144] | |
| Adsorption-mediated endocytosis | TAT(47-57) | YGRKKRRQRRR-NH2 | [145] |
| SynB1 | RGGRLSYSRRRFSTSTGR | [146] | |
| Unknown receptor | CGN | d-GNHPLAKYNGT | [136] |
| TGN | TGNYKALHPHNG | [147] | |
| TP10 | AGYLLGKINLKALAALAKKIL-NH2 | [148] | |
| Aβ aggregates | LVFFA | LVFFA | [149] |
| KLVFF | KLVFF | [64] | |
| LPFFD | LPFFD | [147] | |
| QSH | QSHYRHISPAQV | [150] | |
| Sphingomyelin and ganglioside GT1B on neurons | Tet1 | HLNILSTLWKYR | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Na, Y.; Yin, S.; Yan, C.; Gu, J.; Zhang, N.; Geng, F. Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease. Molecules 2023, 28, 2336. https://doi.org/10.3390/molecules28052336
Zhong X, Na Y, Yin S, Yan C, Gu J, Zhang N, Geng F. Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease. Molecules. 2023; 28(5):2336. https://doi.org/10.3390/molecules28052336
Chicago/Turabian StyleZhong, Xinyu, Yue Na, Shun Yin, Chang Yan, Jinlian Gu, Ning Zhang, and Fang Geng. 2023. "Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease" Molecules 28, no. 5: 2336. https://doi.org/10.3390/molecules28052336
APA StyleZhong, X., Na, Y., Yin, S., Yan, C., Gu, J., Zhang, N., & Geng, F. (2023). Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease. Molecules, 28(5), 2336. https://doi.org/10.3390/molecules28052336






