Evaluation and Assessment of Trivalent and Hexavalent Chromium on Avena sativa and Soil Enzymes
Abstract
:1. Introduction
2. Results
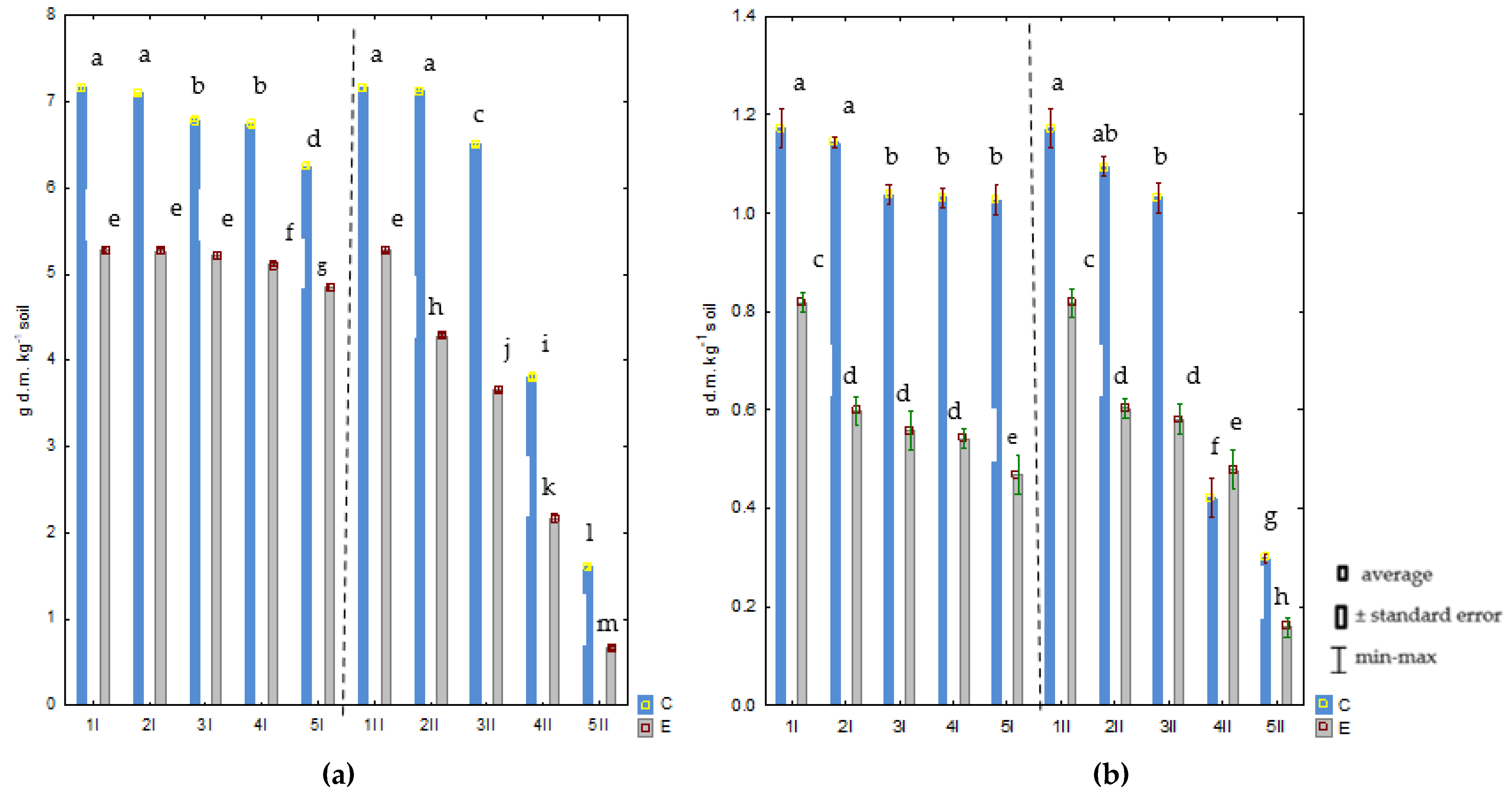
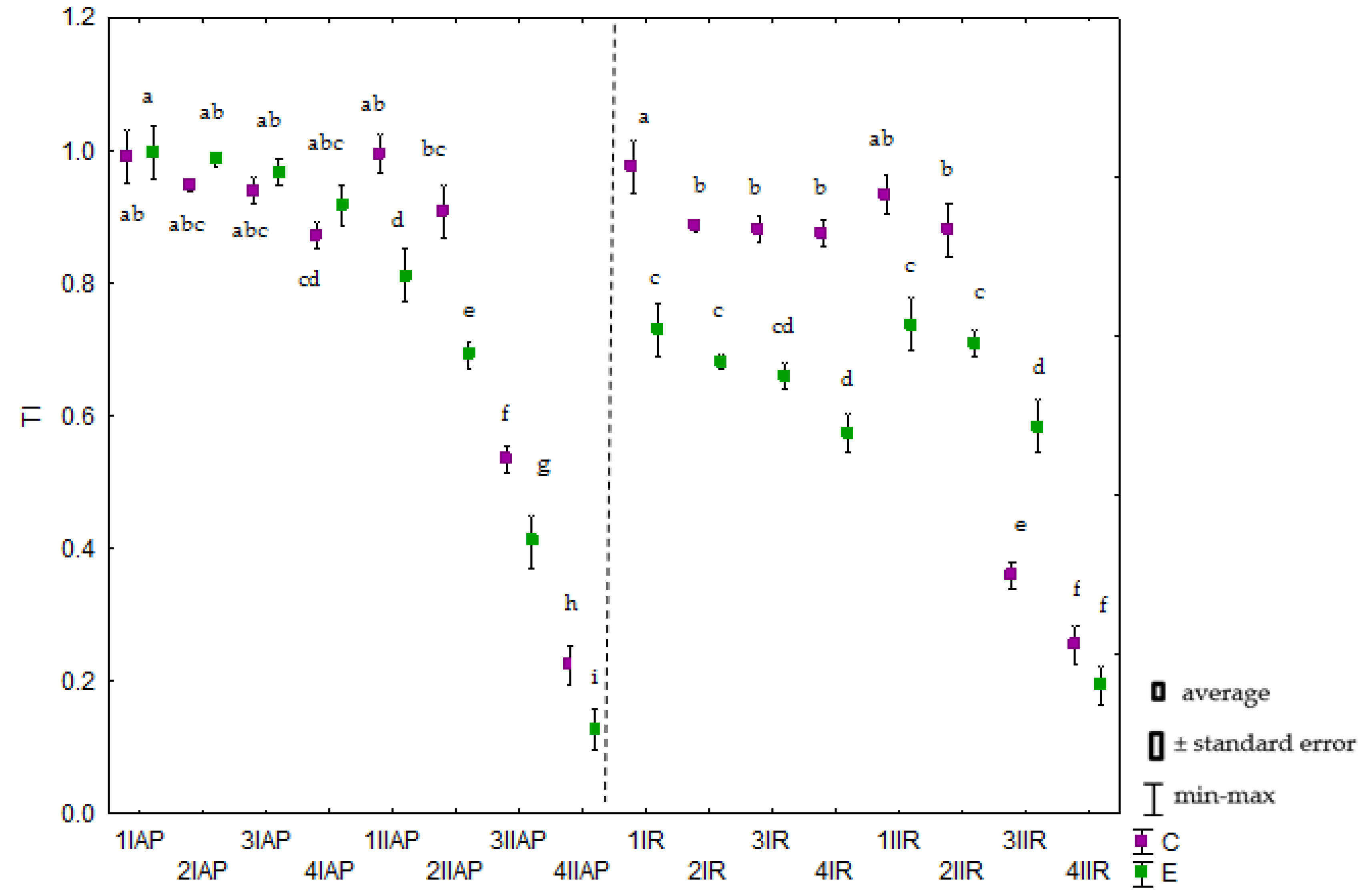
2.1. Effect of Chromium on Avena sativa L. Growth and Development
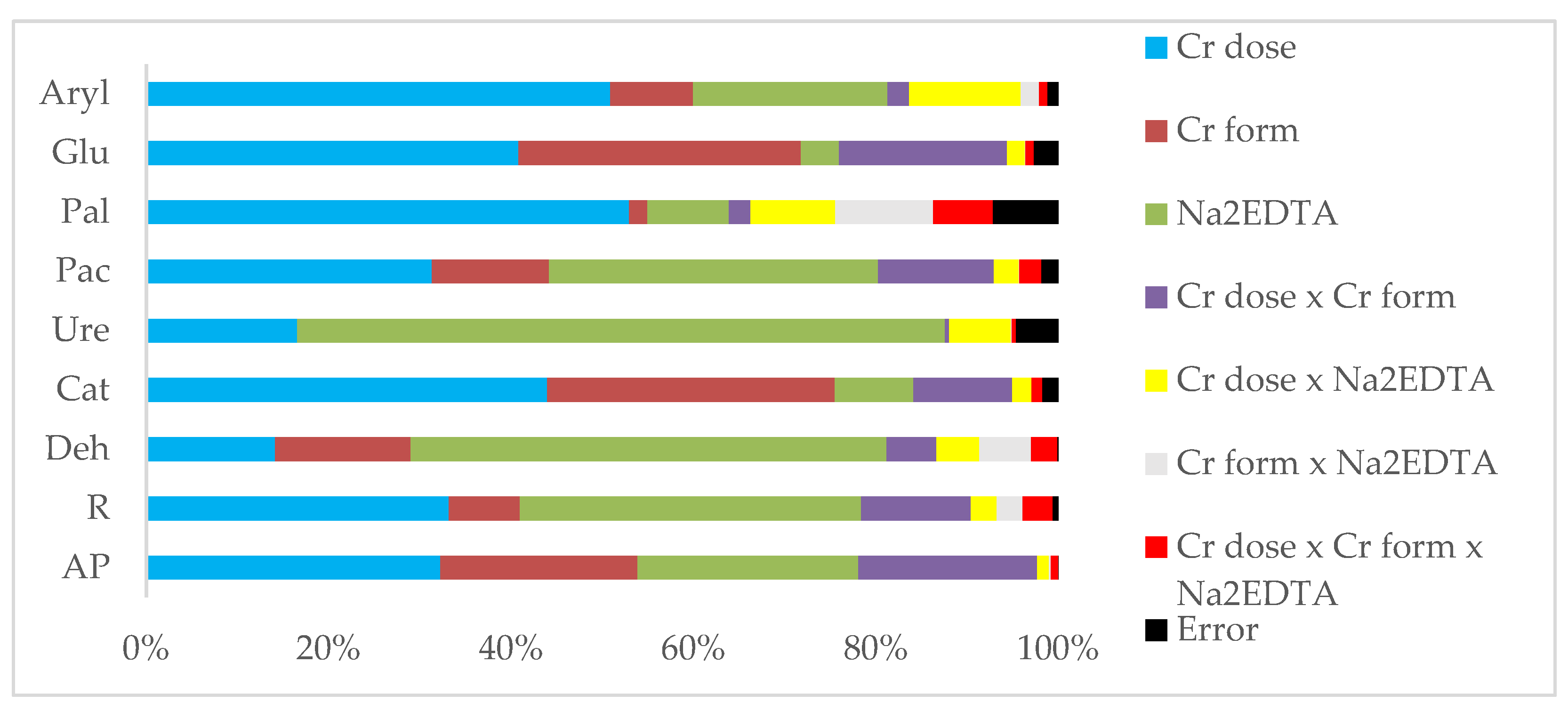
2.2. Effect of Chromium on Biochemical and Physicochemical Parameters of Soil
3. Discussion
3.1. Effect of Chromium on Avena sativa L. Growth and Development
3.2. Effect of Chromium on Biochemical and Physicochemical Parameters of Soil
4. Materials and Methods
4.1. Soil Preparation
4.2. Experimental Procedure
4.3. Assessment of Plant Growth Performance
4.4. Biochemical Determinations
4.5. Physicochemical and Chemical Tests
4.6. Calculations and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fibbi, D.; Doumett, S.; Lepri, L.; Checchini, L.; Gonnelli, C.; Coppini, E.; Bubba, M.D. Distribution and mass balance of hexavalent and trivalent chromium in a subsurface, horizontal flow (SF-h) constructed wetland operating as post-treatment of textile wastewater for water reuse. J. Hazard. Mater. 2012, 199–200, 209–216. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef]
- Li, G.; Yang, X.; Liang, L.; Guo, S. Evaluation of the potential redistribution of chromium fractionation in contaminated soil by citric acid/sodium citrate washing. Arab. J. Chem. 2017, 10, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and soil microorganisms to the toxic effect of chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef]
- Hsu, L.C.; Liu, Y.T.; Tzou, Y.M. Comparison of the spectroscopic speciation and chemical fractionation of chromium in contaminated paddy soils. J. Hazard. Mater. 2015, 296, 230–238. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical speciation of chromium in water: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Nakkeeran, E.; Patra, C.; Shahnaz, T.; Rangabhashiyam, S.; Selvaraju, N. Continuous biosorption assessment for the removal of hexavalent chromium from aqueous solutions using Strychnos nux vomica fruit shell. Bioresour. Technol. Rep. 2018, 3, 256–260. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Zainab, N.; Amna, K.A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Munis, M.F.H.; Hashem, M.; Alamri, S.; et al. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Agunwamba, J.C. A review on the applicability of activated carbon derived from plant biomass in adsorption of chromium, copper, and zinc from industrial wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melián, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury-and chromium-contaminated soil: A review. J. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, W.; Dang, Z.; Hu, Q.; Wu, F.; Feng, C.; Zhao, X.; Meng, W.; Xing, B.; Giesy, J.P. Refocusing on nonpriority toxic metals in the aquatic environment in China. Environ. Sci. Technol. 2017, 51, 3117–3118. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and tioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Phytotoxicity of chromium on germination, growth and biochemical at-tributes of Hibiscus esculentus L. Am. J. Plant Sci. 2013, 4, 41293. [Google Scholar] [CrossRef] [Green Version]
- Bhalerao, S.A.; Sharma, A.S. Chromium: As an environmental pollutant. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 732–746. [Google Scholar]
- Xu, Z.-R.; Cai, M.-L.; Chen, S.-H.; Huang, X.-Y.; Zhao, F.-J.; Wang, P. High-Affinity Sulfate Transporter Sultr1;2 Is a Major Transporter for Cr(VI) Uptake in Plants. Environ. Sci. Technol. 2021, 55, 1576–1584. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Srivastava, S.; Nigam, R.; Prakash, S.; Srivastava, M.M. Mobilization of trivalent chromium in presence of organic acids: A hydroponic study of wheat plant (Triticum vulgare). Bull. Environ. Contam. Toxicol. 1999, 63, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kota´s, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 15, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Arslan Ashraf, M.; Ali, Q.; Abbas, Z.; Muhammad Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Zinc-lysine supplementation mitigates oxidative stress in rapeseed (Brassica napus L.) by preventing phytotoxicity of chromium, when irrigated with tannery wastewater. Plants 2020, 9, 1145. [Google Scholar] [CrossRef]
- Hussain, I.; Saleem, M.H.; Mumtaz, S.; Rasheed, R.; Ashraf, M.A.; Maqsood, F.; Rehman, M.; Yasmin, H.; Ahmed, S.; Ishtiaq, C.M.; et al. Choline chloride mediates chromium tolerance in spinach (Spinacia oleracea L.) by restricting its uptake in relation to morpho-physio-biochemical attributes. J. Plant Growth Regul. 2021, 41, 1594–1614. [Google Scholar] [CrossRef]
- Narayani, M.; Shetty, K.V. Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 955–1009. [Google Scholar] [CrossRef]
- Chen, T.; Chang, Q.R.; Liu, J.; Clevers, J.G.P.W.; Kooistra, L. Identification of soil heavy metal sources and improvement in spatial mapping based on soil spectra information: A case study in northwest China. Sci. Total Environ. 2016, 565, 155–164. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com (accessed on 10 December 2022).
- Available online: https://www.eea.europa.eu/publications/lrtap-1990-2019 (accessed on 10 December 2022).
- Clean-Up of Polluted Environment? Front Plant Sci. 2018, 9, 1476. [CrossRef] [Green Version]
- Yan, X.; Wang, J.; Song, H.; Peng, Y.; Zuo, S.; Gao, T.; Duan, X.; Qin, D.; Dong, J. Evaluation of the phytoremediation potential of dominant plant species growing in a chromium salt–producing factory wasteland, China. Environ. Sci. Pollut. Res. 2020, 27, 7657–7671. [Google Scholar] [CrossRef]
- Huda, A.K.M.N.; Hossain, M.; Mukta, R.H.; Khatun, M.R.; Haque, M.A. EDTA–enhanced Cr detoxification and its potential toxicity in rice (Oryza sativa L.). Plant Stress 2021, 2, 100014. [Google Scholar] [CrossRef]
- Hong, P.K.A.; Banerji, S.K.; Regmi, T. Extraction, recovery, and biostability of EDTA for remediation of lead, copper, zinc and nickel. Soil Sci. Soc. Am. J. 1999, 47, 47–51. [Google Scholar] [CrossRef]
- Grčman, H.; Velikonja-Bolta, Š.; Vodnik, D.; Kos, B.; Leštan, D. EDTA enhanced heavy metal phytoextraction: Metal accumulation, leaching and toxicity. Plant Soil 2001, 235, 105–114. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil Effect mechanism toxicity and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Vodnik, D.; Macek, I.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part II: Can remediated soil be used as a plant substrate. Sci. Total. Environ. 2014, 475, 142–152. [Google Scholar] [CrossRef]
- Jez, E.; Lestan, D. EDTA retention and emissions from remediated soil. Chemosphere 2016, 151, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dipu, S.; Kumar, A.A.; Thanga, S.G. Effect of chelating agents in phytoremediation of heavy metals. Remediat. J. 2012, 22, 133–146. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Finžgar, N.; Leštan, D. Multi-step leaching of Pb and Zn contaminated soils with EDTA. Chemosphere 2007, 66, 824–832. [Google Scholar] [CrossRef]
- Zou, Z.; Qiu, R.; Zhang, W.; Dong, H.; Zhao, Z.; Zhang, T.; Wei, X.; Cai, X. The study of operating variables in soil washing with EDTA. Environ. Pollut. 2009, 157, 229–236. [Google Scholar] [CrossRef]
- Udovic, M.; Domen, L. Fractionation and bioavailability of Cu in soil remediated by EDTA leaching and processed by earthworms. Environ. Sci. Pollut. Res. 2010, 17, 561–570. [Google Scholar] [CrossRef]
- Neugschtner, R.W.; Tlustos, P.; Komarek, M.; Szakova, J.; Jakoubkova, L. Chemically enhanced phytoextraction of risk elements from a contaminated agricultural soil using Zea mays and Triticum aestivum: Performance and metal mobilization over a three year period. Int. J. Phytorem. 2012, 14, 754–771. [Google Scholar] [CrossRef]
- Wu, G.; Kanga, H.; Zhang, X.; Shao, H.; Chu, L.; Ruand, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Abideen, S.N.U.; Abideen, A.A. Protein level and heavy metals (Pb, Cr, and Cd) concentrations in wheat (Triticum aestivum) and in oat (Avena sativa) plants. IJIAS 2013, 3, 284–289. [Google Scholar]
- Tobiasz-Salach, R.; Pyrek-Bajcar, E.; Bobrecka-Jamro, D. Assessing the possible use of hulled and naked oat grains as energy source. Econtechmod. Inter. Quart. J. 2016, 15, 35–40. [Google Scholar]
- Proszak-Miąsik, D.; Jarecki, W.; Nowak, K. Selected parameters of oat straw as an alternative energy raw material. Energies 2022, 15, 331. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-Garcia, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Akinci, I.E.; Akinci, S. Effect of chromium toxicity on germination and early seedling growth in melon (Cucumis melo L.). Afr. J. Biotechnol. 2010, 9, 4589–4594. [Google Scholar]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. Biomed. Res. Int. 2014, 2014, 934182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Dey, U.; Mondal, N.K. Ultrastructural deformation of plant cell under heavy metal stress in Gram seedlings. Cogent Environ. Sci. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Zheng, B.; Yuan, H.; Yan, D. Jasrotia, S. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, L.M.; Gress, J.; De, J.; Rathinasabapathi, B.; Marchi, G.; Chen, Y.; Ma, L.Q. Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulat or Pterisvittata. Chemosphere 2016, 147, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; Lytle, C.M.; Qian, J.-H.; Terry, N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 1998, 206, 293–299. [Google Scholar] [CrossRef]
- Shanker, A.K.; Djanaguiraman, M.; Venkateswarlu, B. Chromium interactions in plants: Current status and future strategies. Metallomics 2009, 1, 375–383. [Google Scholar] [CrossRef]
- Bareen, F.; Khadija, R.; Muhammad, S.; Aisha, N. Uptake and leaching of Cu, Cd, and Cr after EDTA application in sand columns using sorghum and pearl millet. Pol. J. Environ. Stud. 2019, 28, 2065–2077. [Google Scholar] [CrossRef]
- Ali, S.Y.; Chaudhury, S. EDTA-enhanced phytoextraction by tagetes sp. and effect on bioconcentration and translocation of heavy metals. Environ. Proc. 2016, 3, 735. [Google Scholar] [CrossRef]
- Naseem, S.; Yasin, M.; Ahmed, A.; Faisal, M. Chromium accumulation and toxicity in corn (Zea mays L.) seedlings. Pol. J. Environ. Stud. 2015, 24, 899–904. [Google Scholar]
- Nagarajan, M.; Ganesh, K.S. Effect of chromium on growth, biochemicals and nutrient accumulation of paddy (Oryza sativa L.). Int. Lett. Nat. Sci. 2014, 23, 63–71. [Google Scholar] [CrossRef]
- Diwan, H.; Ahmad, A.; Iqbal, M. Chromium-induced alterations in photosynthesis and associated attributes in Indian mustard. J. Environ. Biol. 2012, 33, 239–244. [Google Scholar] [PubMed]
- Saravanan, A.; Jayasree, R.; Hemavathy, R.V.; Jeevanantham, S.; Hamsini, S.; Senthil, K.P.; Yuvaraj, D. Phytoremediation of Cr (VI) ion contaminated soil using Black gram (Vigna mungo): Assessment of removal capacity. J. Environ. Chem. Eng. 2019, 7, 103052. [Google Scholar]
- Ramana, S.; Biswas, A.K.; Singh, A.B.; Ahirwar, N.K.; Subba Rao, A. Tolerance of ornamental succulent plant crown of thorns (Euphorbia milli) to chromium and its remediation. Int. J. Phytoremediation 2014, 17, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Abbasi, M.S.; Amin, F.; Jahangir, T.M.; Soomro, N.U. Evaluation of chromium phyto-toxicity, phyto-tolerance, and phyto-accumulation using biofuel plants for effective phytoremediation. Int. J. Phytoremediation 2019, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bareen, E.F.; Tahira, S.A. Efficiency of seven different cultivated plant species for phytoextraction of toxic metals from tannery effluent contaminated soil using EDTA. Soil Sediment Contam. 2010, 19, 160–173. [Google Scholar] [CrossRef]
- Han, F.X.; Sridhar, B.B.M.; Monts, D.L.; Su, Y. Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytol. 2004, 162, 489–499. [Google Scholar] [CrossRef]
- Ebrahimi, M. Effect of EDTA treatment method on leaching of Pb and Cr by Phragmites australis (Cav.) Trin. Ex Steudel (common reed). Caspian J. Environ. Sci. 2015, 13, 153–166. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Huang, S.; Peng, B.; Yang, Z.; Chai, L.; Zhou, L. Chromium accumulation, microorganism population and enzyme activities in soils around chromium-containing slag heap of steel alloy factory. Trans. Nonferrous Met. Soc. China 2009, 19, 241–248. [Google Scholar] [CrossRef]
- Belyaeva, O.N.; Haynes, R.J.; Birukova, O.A. Barley yield and soil microbial and enzyme activities as affected by contamination of two soils with lead, zinc or copper. Biol. Fertil. Soils 2005, 41, 85–94. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiology Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Min, X.; Yang, Z.; Chai, L.; Zhang, S.; Wang, Y. Physicochemical and biological quality of soil in hexavalent chromium-contaminated soils as affected by chemical and microbial remediation. Environ. Sci. Pollut. Res. 2014, 21, 379–388. [Google Scholar] [CrossRef]
- Tokunaga, T.K.; Wan, J.; Firestone, M.K.; Hazen, T.C.; Olson, K.R.; Herman, D.J.; Sutton, S.R.; Lanzirotti, A. In situ reduction of chromium(VI) in heavily contaminated soils through organic carbon amendment. J. Environ. Qual. 2003, 32, 1641–1649. [Google Scholar] [CrossRef] [Green Version]
- Dotaniya, M.L.; Rajendiran, S.; Meena, V.D.; Saha, J.K.; Vassanda Coumar, M.; Kundu, S.; Patra, A.K. Influence of chromium contamination on carbon mineralization and enzymatic activities in Vertisol. Agric. Res. 2017, 6, 91–96. [Google Scholar] [CrossRef]
- Quilchano, C.; Maranon, T. Dehydrogenase activity in Mediterranean forest soils. Biol. Fertil. Soils 2002, 35, 102–107. [Google Scholar] [CrossRef]
- Wyszkowska, J. Soil contamination with chromium and its enzymatic activity and yielding. Polish J. Environ. Stud. 2002, 11, 79–84. [Google Scholar]
- Baathe, E. Effects of heavy metals in soil microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Peng, B.; Huang, S.H.; Yang, Z.H.; Chai, L.Y.; Xu, Y.Z.; Su, C.Q. Inhibitory effect of Cr(VI) on activities of soil enzymes. J. Cent. South. Univ. Technol. 2009, 16, 594–598. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Zheng, D.; Feng, S. Effects of cadmium, zinc and lead on soil enzyme activities. J. Environ. Sci. 2006, 18, 1135–1141. [Google Scholar] [CrossRef]
- Stępniewska, Z.; Wolińska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Samborska, A.; Stępniewska, Z.; Stępniewski, W. Influence of different oxidation states of chromium (VI, III) on soil urease activity. Geoderma 2004, 122, 317–322. [Google Scholar] [CrossRef]
- Al-Khashman, O.A.; Shawabkeh, R.A. Metals distribution in soils around the cement factory in southern Jordan. Environ. Pollut. 2006, 140, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Schulin, R. Heavy metal contamination along a soil transect in the vicinity of the iron smelter of Kremikovtzi (Bulgaria). Geoderma 2007, 140, 52–61. [Google Scholar] [CrossRef]
- Tome, V.F.; Blanco, R.P.; Lozano, J.C. The ability of Helianthus annuus L. and Brassica juncea to uptake and translocate natural uranium and 226Ra under different milieu conditions. Chemosphere 2009, 74, 293–300. [Google Scholar] [CrossRef]
- Mahmood-ul-Hassan, M.; Suthar, V.; Ahmad, R.; Yousra, M. Heavy metal phytoextraction—Natural and EDTA-assisted remediation of contaminated calcareous soils by sorghum and oat. Environ. Monit. Assess. 2017, 189, 591. [Google Scholar] [CrossRef]
- Komárek, M.; Tlustoš, P.; Száková, J.; Chrastn, V.; Balík, J. The role of Fe- and Mn-oxides during EDTA enhanced phytoextraction of heavy metals. Plant Soil Environ. 2007, 53, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Römkens, P.; Bouwman, L.; Japenga, J.; Draaisma, C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ. Pollut. 2002, 116, 109–121. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Phytoremediation of heavy metal contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J. Environ. Qual. 2001, 30, 1919–1926. [Google Scholar] [CrossRef]
- Available online: https://zpe.gov.pl/a/soils-in-poland (accessed on 10 December 2022).
- World’s Worst Pollution Problems 2015. The New Top Six Toxic Threats: A Priority List for Remediation. Available online: http://www.worstpolluted.org/docs/WWPP_2015_Final.pdf (accessed on 21 November 2020).
- PN-ISO-11466:2002; Polish Committee for Standardization. Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic: London, UK, 1998; pp. 316–365. [Google Scholar]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A dangerous pollutant distorting the biological properties of soill. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. The impact of permethrin and cypermethrin on plants, soil enzyme activity, and microbial communities. J. Mol. Sci. 2023, 24, 2892. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of zeolite to neutralise nickel in a soil environment. Environ. Monit. Assess. 2018, 190, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PN ISO 11047:2001; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc in Aqua Regia Extracts of Soil—Flame and Electrothermal Atomic Absorption Spectrometric Methods. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of soil contaminated with nickel, cadmium and cobalt. Int. J. Phytoremediation 2021, 23, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Dell Inc. Dell Statistica (Data Analysis Software System); Version 13.1; Dell Inc.: Tulsa, OK, USA, 2022.

| Cr Dose mg kg−1 d.m. Soil | Aboveground Parts | Roots | Soil | |||
|---|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | |
| Control | ||||||
| 0 | 1.22 f | 1.34 e | 12.90 h | 25.30 f | 19.30 g | 20.10 e |
| 40 | 1.66 d | 6.21 b | 41.30 d | 45.40 c | 43.30 d | 61.50 b |
| Na2EDTA | ||||||
| 0 | 0.88 g | 0.50 g | 28.40 e | 24.20 g | 17.60 h | 19.20 f |
| 40 | 2.19 c | 16.30 a | 47.90 b | 86.80 a | 44.10 c | 64.90 a |
| Cr dose mg kg−1 d.m. Soil | D µg kg−1 | TF | AF | BFAG | BFR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(VI) | Cr(III) | Cr(VI) | Cr(VI) | |
| Control | ||||||||||
| 0 | 23.86 f | 39.25 b | 0.10 c | 0.05 d | 0.73 f | 1.33 b | 0.06 b | 0.07 b | 0.67 f | 1.26 c |
| 40 | 52.73 a | 23.48 g | 0.04 e | 0.14 b | 0.99 d | 0.84 e | 0.04 b | 0.10 b | 0.95 e | 0.74 f |
| Na2EDTA | ||||||||||
| 0 | 27.86 d | 22.42 h | 0.03 f | 0.02 g | 1.66 a | 1.29 b | 0.05 b | 0.03 b | 1.61 a | 1.26 bc |
| 40 | 33.06 c | 24.54 e | 0.05 de | 0.19 a | 1.14 c | 1.59 a | 0.05 b | 0.25 a | 1.09 d | 1.34 b |
| (a) | ||||||||||||
| Cr Dose mg kg−1 d.m. Soil | Dehydrogenases | Catalase | Urease | |||||||||
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | |||||||
| Control | ||||||||||||
| 5 | −0.01 a | −0.37 i | −0.02 a | −0.01 a | −0.04 a | −0.04 a | ||||||
| 10 | −0.15 c | −0.49 j | −0.03 a | −0.01 a | −0.03 a | −0.03 a | ||||||
| 20 | −0.16 d | −0.88 o | −0.04 a | −0.02 a | −0.03 a | −0.03 a | ||||||
| 40 | −0.17 e | −0.95 p | −0.04 a | −0.02 a | −0.04 a | −0.06 ab | ||||||
| −0.12 A | −0.67 C | −0.03 D | −0.01 B | −0.04 A | −0.04 A | |||||||
| Na2EDTA | ||||||||||||
| 5 | −0.10 b | −0.45 k | −0.01 a | −0.01 a | −0.08 abc | −0.07 ab | ||||||
| 10 | −0.23 f | −0.75 l | −0.02 a | −0.01 a | −0.08 abc | −0.04 a | ||||||
| 20 | −0.25 g | −0.78 m | −0.03 a | −0.01 a | −0.123 bcd | −0.16 be | ||||||
| 40 | −0.35 h | −0.83 n | −0.03 a | −0.01 a | −0.21 e | −0.20 e | ||||||
| −0.23 B | −0.70 C | −0.02 C | −0.01 A | −0.12 B | −0.12 B | |||||||
| (b) | ||||||||||||
| Cr dose mg kg−1 d.m. Soil | Acid Phosphatase | Alkaline Phosphatase | β-glucosidase | Arylsulfatase | ||||||||
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | |||||
| Control | ||||||||||||
| 5 | −0.08 abc | −0.02 ab | −0.01 bc | −0.06 cd | −0.01 a | −0.06 abc | −0.01 a | −0.36 c | ||||
| 10 | −0.11 cd | −0.18 d | −0.08 cd | −0.10 cd | −0.02 a | −0.06 abc | −0.09 ab | −0.37 c | ||||
| 20 | −0.12 cd | −0.35 e | −0.09 cd | −0.11 d | −0.03 a | −0.16 cd | −0.13 b | −0.46 cde | ||||
| 40 | −0.13 cd | −0.52 f | −0.11 d | −0.12 d | −0.03 a | −0.23 d | −0.14 b | −0.54 e | ||||
| −0.11 B | −0.27 C | −0.07 C | −0.10 D | −0.02 A | −0.13 C | −0.09 A | −0.43 B | |||||
| Na2EDTA | ||||||||||||
| 5 | −0.01 a | −0.10 bcd | −0.04 bce | 0.07 a | −0.02 a | −0.06 abc | −0.37 c | −0.48 de | ||||
| 10 | −0.02 a | −0.13 cd | −0.05 cd | 0.04 ab | −0.05 ab | −0.15 bce | −0.41 cd | −0.50 de | ||||
| 20 | −0.08 abc | −0.37 e | −0.06 cd | −0.02 bc | −0.05 ab | −0.16 bce | −0.45 cde | −0.51 de | ||||
| 40 | −0.10 bcd | −0.37 e | −0.09 cd | −0.07 cd | −0.06 abc | −0.24 d | −0.50 de | −0.52 e | ||||
| −0.05 A | −0.24 C | −0.05 B | 0.01 A | −0.05 B | −0.15 D | −0.43 B | −0.50 C | |||||
| Cr Dose mg kg−1 d.m. Soil | Corg | NTotal | pHKCl | HAC | EBC | CEC | BS% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | (mmol(+) kg−1 Soil) | |||||||||||||
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | |
| Control | ||||||||||||||
| 0 | 0.72 a | 0.72 a | 0.13 ab | 0.13 ab | 6.10 c | 6.10 c | 9.19 g | 9.19 g | 33.00 e | 33.00 e | 42.19 e | 42.19 e | 78.21 ef | 78.21 ef |
| 5 | 0.70 a | 0.65 cd | 0.13 ab | 0.12 bc | 5.95 cd | 5.95 cd | 9.19 g | 9.75 def | 37.00 d | 42.00 a | 46.19 d | 51.75 a | 80.10 bcd | 81.16 bc |
| 10 | 0.66 bc | 0.65 cd | 0.13 ab | 0.10 ef | 5.95 cd | 5.90 d | 9.94 de | 10.50 c | 33.00 e | 41.00 ab | 42.94 e | 51.50 a | 76.85 fg | 79.610 cde |
| 20 | 0.60 e | 0.64 cd | 0.12 bcd | 0.10 ef | 5.85 de | 5.90 d | 10.13 cd | 11.25 b | 31.00 e | 37.00 d | 41.13 e | 48.25 bcd | 75.37 g | 76.68 fg |
| 40 | 0.60 e | 0.64 d | 0.12 bcd | 0.09 f | 5.70 e | 5.90 d | 10.50 c | 11.44 b | 40.00 abc | 32.00 c | 50.50 ab | 43.44 e | 79.21 de | 73.67 h |
| 0.66 A | 0.66 A | 0.12 A | 0.11 B | 5.91 B | 5.95 B | 9.79 C | 10.43 B | 37.00 B | 39.20 A | 46.79 B | 49.63 A | 78.85 B | 78.77 B | |
| r | −0.89 | −0.63 | −0.96 | −0.90 | −0.97 | −0.64 | 0.91 | 0.90 | −0.12 | −0.99 | −0.02 | −0.99 | −0.38 | −0.98 |
| Na2EDTA | ||||||||||||||
| 0 | 0.71 a | 0.71 a | 0.14 a | 0.14 a | 6.70 a | 6.70 a | 9.56 efg | 9.56 efg | 40.00 abc | 40.00 abc | 49.56 abc | 49.56 abc | 80.71 bcd | 80.71 bcd |
| 5 | 0.687 b | 0.68 b | 0.14 a | 0.11 cde | 6.65 a | 6.65 a | 9.94 de | 8.06 i | 33.00 e | 40.00 abc | 42.94 e | 48.06 cd | 76.84 fg | 83.23 a |
| 10 | 0.68 b | 0.68 b | 0.13 ab | 0.11 cde | 6.60 a | 6.65 a | 11.63 ab | 8.63 h | 31.00 e | 38.00 cd | 42.63 e | 46.63 d | 72.72 h | 81.50 b |
| 20 | 0.67 b | 0.67 b | 0.13 ab | 0.11 de | 6.35 b | 6.55 a | 12.00 a | 9.19 g | 31.00 e | 39.00 bcd | 43.00 e | 48.19 bcd | 72.08 h | 80.93 bc |
| 40 | 0.67 b | 0.66 bc | 0.12 bc | 0.11 de | 5.95 cd | 6.55 a | 12.00 a | 9.38 fg | 31.00 e | 32.00 e | 43.00 e | 41.38 e | 72.08 h | 77.34 f |
| 0.68 A | 0.68 A | 0.13 A | 0.12 B | 6.45 A | 6.62 A | 11.03 A | 8.55 D | 33.20 C | 37.80 B | 44.23 C | 46.35 B | 74.89 C | 81.44 A | |
| r | −0.68 | −0.84 | −0.88 | −0.60 | −0.99 | −0.88 | 0.79 | 0.89 | −0.63 | −0.92 | −0.51 | −0.84 | −0.75 | −0.99 |
| Variable Factors | AP | R | Deh | Cat | Ure | Pac | Pal | Glu | Aryl | Corg | NTotal | pH | HAC | EBC | CEC | BS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Cr | −0.26 | −0.31 | −0.15 | −0.85 * | −0.35 | −0.32 | −0.51 * | −0.63 * | −0.65 * | −0.76 * | −0.72 * | −0.55 * | 0.71 * | −0.24 | −0.03 | −0.56 * |
| AP | 1.00 | 0.96 * | 0.98 * | −0.04 | 0.88 * | 0.96 * | 0.58 * | −0.48 * | −0.27 | −0.07 | −0.21 | −0.60 * | −0.72 * | 0.18 | −0.04 | 0.54 * |
| R | 1.00 | 0.96 * | 0.05 | 0.93 * | 0.94 * | 0.58 * | −0.38 * | −0.06 | −0.07 | −0.15 | −0.55 * | −0.80 * | 0.42 * | 0.21 | 0.72 * | |
| Deh | 1.00 | −0.11 | 0.88 * | 0.96 * | 0.55 * | −0.55 * | −0.32 | −0.16 | −0.31 | −0.69 * | −0.69 * | 0.27 | 0.07 | 0.57 * | ||
| Cat | 1.00 | 0.05 | 0.07 | 0.17 | 0.77 * | 0.69 * | 0.72 * | 0.67 * | 0.65 * | −0.48 * | 0.20 | 0.07 | 0.40 * | |||
| Ure | 1.00 | 0.89 * | 0.68 * | −0.33 | −0.00 | −0.12 | −0.06 | −0.42 * | −0.77 * | 0.41 * | 0.20 | 0.69 * | ||||
| Pac | 1.00 | 0.63 * | −0.41 * | −0.236 | −0.03 | −0.16 | −0.54 * | −0.74 * | 0.27 | 0.06 | 0.60 * | |||||
| Pal | 1.00 | −0.04 | 0.08 | 0.34 | 0.19 | −0.150 | −0.59 * | 0.25 | 0.09 | 0.49 * | ||||||
| Glu | 1.00 | 0.82 * | 0.60 * | 0.70 * | 0.86 * | −0.15 | 0.22 | 0.19 | 0.20 | |||||||
| Aryl | 1.00 | 0.47 * | 0.63 * | 0.68 * | −0.34 | 0.49 * | 0.44 * | 0.46 * | ||||||||
| Corg | 1.00 | 0.63 * | 0.53 * | −0.26 | 0.01 | −0.08 | 0.16 | |||||||||
| NTotal | 1.00 | 0.69 * | −0.31 | 0.14 | 0.06 | 0.27 | ||||||||||
| pH | 1.00 | 0.07 | −0.05 | −0.03 | −0.08 | |||||||||||
| HAC | 1.00 | −0.50 * | −0.23 | −0.88 * | ||||||||||||
| EBC | 1.00 | 0.96 * | 0.85 * | |||||||||||||
| CEC | 1.00 | 0.67 * |
| Variable Factors | AP | R | Deh | Cat | Ure | Pac | Pal | Glu | Aryl | Corg | NTotal | pH | HAC | EBC | CEC | BS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Cr | −0.84 * | −0.82 * | −0.68 * | −0.55 * | −0.43 * | −0.79 * | −0.64 * | −0.94 * | −0.64 * | −0.71 * | −0.84 * | −0.18 | 0.46 * | −0.47 * | −0.35 | −0.59 * |
| AP | 1.00 | 0.97 * | 0.86 * | 0.21 | 0.73 * | 0.96 * | 0.51 * | 0.74 * | 0.21 | 0.33 | 0.58 * | −0.33 | −0.10 | 0.52 * | 0.51 * | 0.39 * |
| R | 1.00 | 0.89 * | 0.27 | 0.65 * | 0.94 * | 0.51 * | 0.75 * | 0.24 | 0.40 * | 0.61 * | −0.28 | −0.15 | 0.48 * | 0.45 * | 0.39 * | |
| Deh | 1.00 | 0.22 | 0.64 * | 0.91 * | 0.45 * | 0.55 * | 0.12 | 0.43 * | 0.54 * | −0.42 * | −0.06 | 0.09 | 0.08 | 0.09 | ||
| Cat | 1.00 | −0.25 | 0.26 | 0.54 * | 0.59 * | 0.62 * | 0.68 * | 0.61 * | 0.65 * | −0.69 * | 0.14 | −0.06 | 0.54 * | |||
| Ure | 1.00 | 0.71 * | 0.28 | 0.26 | −0.15 | −0.02 | 0.13 | −0.73 * | 0.49 * | 0.12 | 0.27 | −0.24 | ||||
| Pac | 1.00 | 0.48 * | 0.65 * | 0.16 | 0.33 | 0.60 * | −0.36 * | −0.09 | 0.37 * | 0.35 | 0.28 | |||||
| Pal | 1.00 | 0.62 * | 0.22 | 0.54 * | 0.41 * | 0.17 | −0.48 * | 0.20 | 0.06 | 0.44 * | ||||||
| Glu | 1.00 | 0.71 * | 0.71 * | 0.82 * | 0.32 | −0.51 * | 0.54 | 0.41 * | 0.67 * | |||||||
| Aryl | 1.00 | 0.78 * | 0.82 * | 0.66 * | −0.46 * | 0.19 | 0.06 | 0.42 * | ||||||||
| Corg | 1.00 | 0.75 * | 0.52 * | −0.60 * | −0.04 | −0.22 | 0.37 * | |||||||||
| NTotal | 1.00 | 0.37 * | −0.47 * | 0.29 | 0.16 | 0.48 * | ||||||||||
| pH | 1.00 | −0.78 * | 0.11 | −0.12 | 0.57 * | |||||||||||
| HAC | 1.00 | −0.25 | 0.04 | −0.80 | ||||||||||||
| EBC | 1.00 | 0.96 * | 0.77 * | |||||||||||||
| CEC | 1.00 | 0.56 * |
| Type of Soil | Granulometric Composition (%) | pHKCl | Corg | Ntotal | HAC | EBC | CEC | BS% | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | g kg−1 | mmol(+) kg−1 Soil | ||||||
| ls | 69.41 | 27.71 | 2.88 | 6.09 | 6.18 | 1.27 | 8.81 | 24.00 | 32.81 | 73.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Evaluation and Assessment of Trivalent and Hexavalent Chromium on Avena sativa and Soil Enzymes. Molecules 2023, 28, 4693. https://doi.org/10.3390/molecules28124693
Boros-Lajszner E, Wyszkowska J, Kucharski J. Evaluation and Assessment of Trivalent and Hexavalent Chromium on Avena sativa and Soil Enzymes. Molecules. 2023; 28(12):4693. https://doi.org/10.3390/molecules28124693
Chicago/Turabian StyleBoros-Lajszner, Edyta, Jadwiga Wyszkowska, and Jan Kucharski. 2023. "Evaluation and Assessment of Trivalent and Hexavalent Chromium on Avena sativa and Soil Enzymes" Molecules 28, no. 12: 4693. https://doi.org/10.3390/molecules28124693
APA StyleBoros-Lajszner, E., Wyszkowska, J., & Kucharski, J. (2023). Evaluation and Assessment of Trivalent and Hexavalent Chromium on Avena sativa and Soil Enzymes. Molecules, 28(12), 4693. https://doi.org/10.3390/molecules28124693








