Intracellular Redox Behavior of Quercetin and Resveratrol Singly and in Mixtures
Abstract
1. Introduction
2. Results
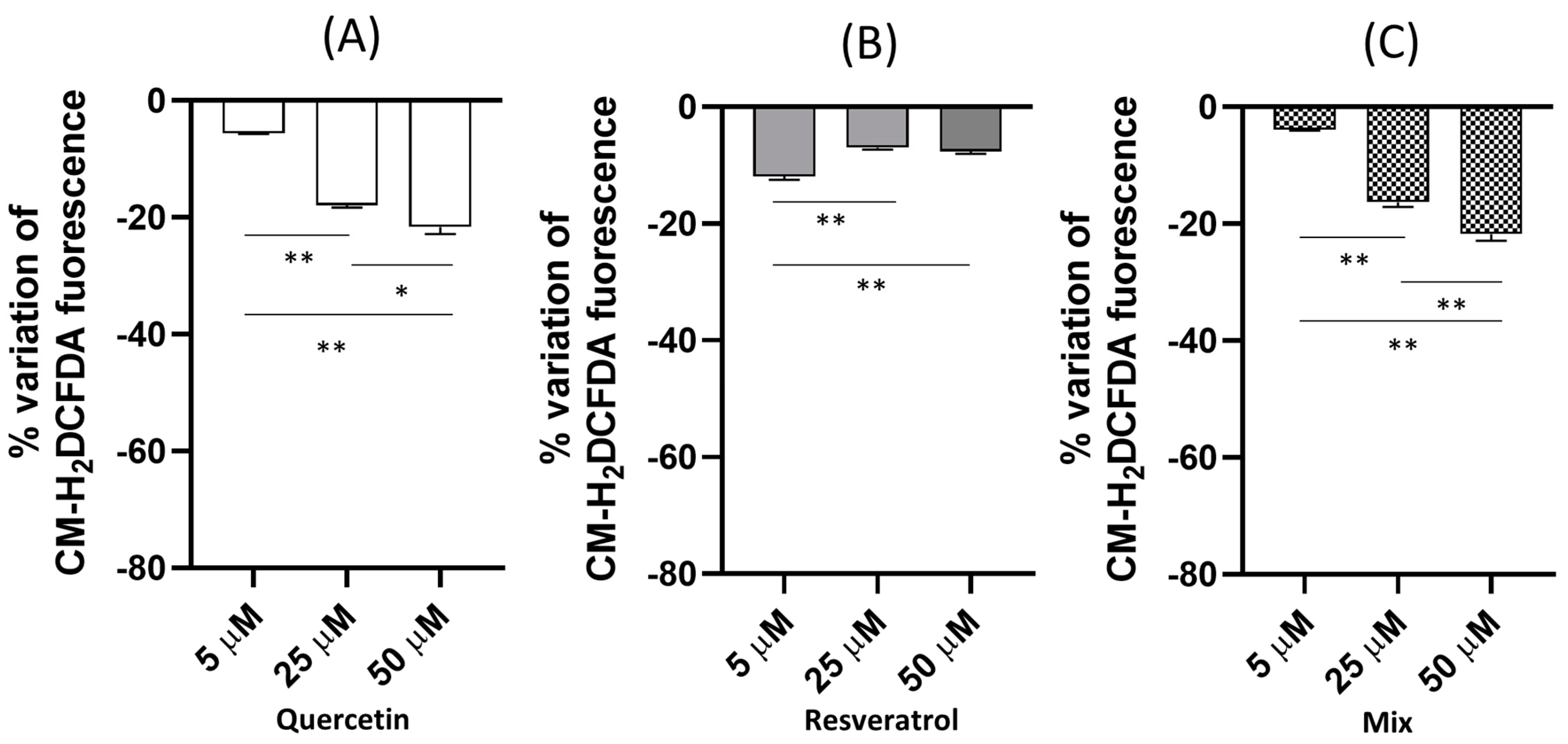
2.1. Dose-Dependent Effect of Quercetin and Resveratrol Singly and in Mixture on the Basal ROS Production of HeLa Cells
2.2. Dose-Dependent Effect of Quercetin and Resveratrol, Singly and in Mixture, on Intracellular ROS under An Exogenous Oxidative Challenge
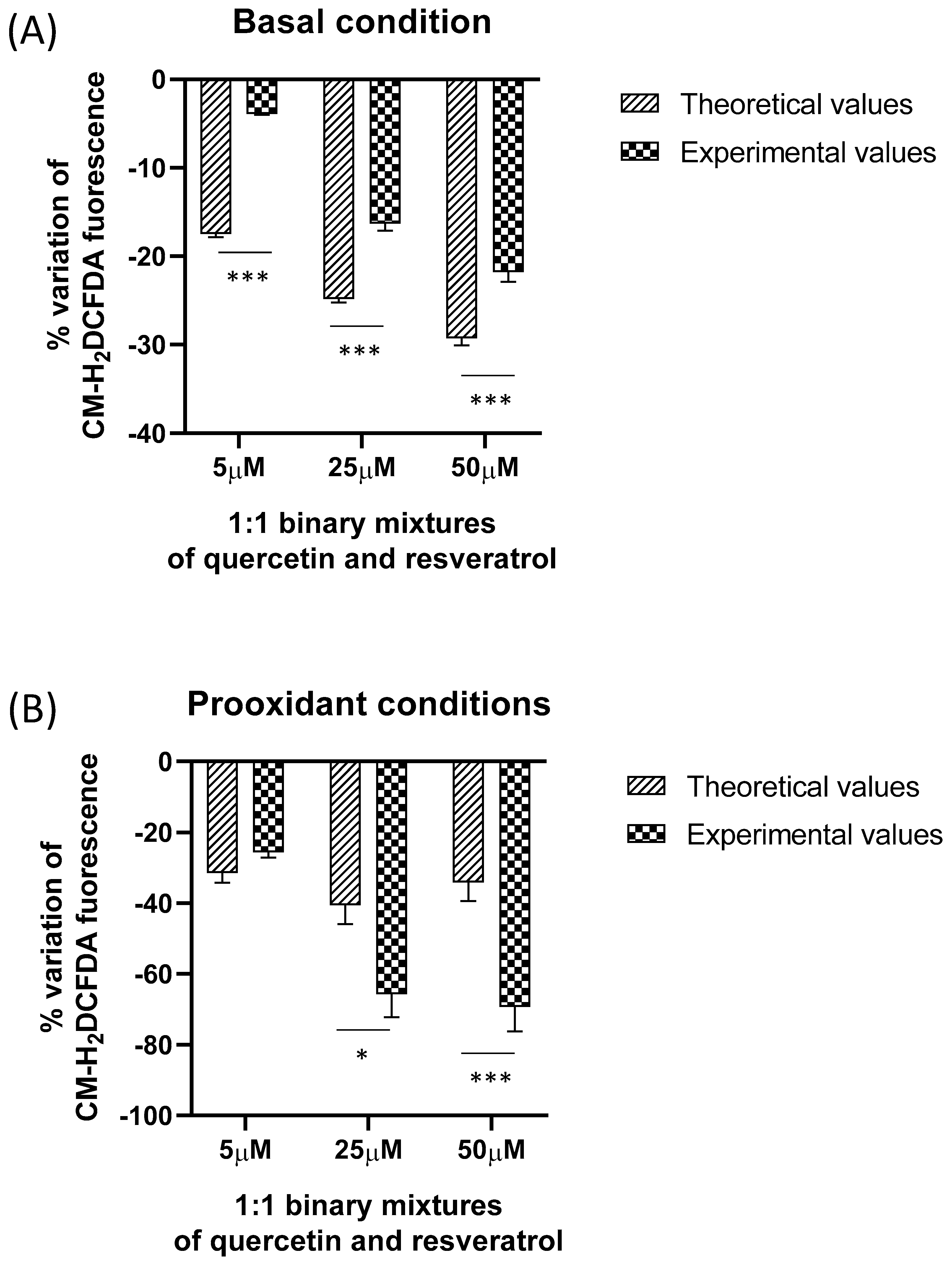
2.3. Comparison between Experimental and Expected Antioxidant Activities
3. Methods
3.1. Materials
3.2. Intracellular Antioxidant/Pro-Oxidant Activity Assay
3.3. Confocal Visualization of HeLa Cells
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Meth. Enzymol. 1990, 186, 343–355. [Google Scholar]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT based quantum chemical descriptors–the importance of group frontier electron density. J. Mol. Model. 2012, 18, 2621–2631. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta 2011, 1807, 1562–1572. [Google Scholar] [CrossRef]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Hodnick, W.F.; MllosavljeviĆ, E.B.; Nelson, J.H.; Pardini, R.S. Electrochemistry of flavonoids: Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vázquez, A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Siche, R.; Ávalos, C.; Arteaga, H.; Saldaña, E.; Vieira, T.M.F.S. Antioxidant capacity of binary and ternary mixtures of orange, grape and starfruit juices. Curr. Nutr. Food Sci. 2016, 12, 65–71. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef]
- DeGraft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–327. [Google Scholar] [CrossRef]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Pires, A.S.; Teixo, R.J.; Tralhão, J.G.; Botelho, M.F. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. Extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef]
- Pashevin, D.A.; Tumanovska, L.V.; Dosenko, V.E.; Nagibin, V.S.; Gurianova, V.L.; Moibenko, A.A. Antiatherogenic effect of quercetin is mediated by proteasome inhibition in the aorta and circulating leukocytes. Pharmacol. Rep. 2011, 63, 1009–1018. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective potentials of flavonoids: Experimental studies and mechanisms of action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef]
- Zahedi, H.S.; Jazayeri, S.; Ghiasvand, R.; Djalali, M.; Eshraghian, M.R. Effects of polygonum cuspidatum containing resveratrol on inflammation in male professional basketball players. Int. J. Prev. Med. 2013, 4, S1–S4. [Google Scholar]
- Wang, Z.; Zou, J.; Huang, Y.; Cao, K.; Xu, Y.; Wu, J.M. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin. Med. J. 2002, 115, 378–380. [Google Scholar]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Bao, T.; Long, Z.; Jin, B. Exploring the Pharmacological Mechanism of Quercetin-Resveratrol Combination for Polycystic Ovary Syndrome: A Systematic Pharmacological Strategy-Based Research. Sci. Rep. 2019, 9, 18420. [Google Scholar] [CrossRef]
- Singh, V.; Singh, R.; Kujur, P.K.; Singh, R.P. Combination of Resveratrol and Quercetin Causes Cell Growth Inhibition, DNA Damage, Cell Cycle Arrest, and Apoptosis in Oral Cancer Cells. Assay Drug Dev. Technol. 2020, 18, 226–238. [Google Scholar] [CrossRef]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Milton, I.; Portillo, M.P. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur. J. Nutr. 2016, 55, 341–348. [Google Scholar] [CrossRef]
- Zhao, L.; Cen, F.; Tian, F.; Li, M.J.; Zhang, Q.; Shen, H.Y.; Shen, X.C.; Zhou, M.M.; Du, J. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Exp. Ther. Med. 2017, 14, 5942–5948. [Google Scholar] [CrossRef]
- Skroza, D.; Mekinić, I.G.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compost. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Freeman, B.L.; Egget, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, C150–C176. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Gil-Chaves, J.; Sotelo-Mundo, R.R.; Namieśnik, J.; Gorinstein, S.; Gonzales-Aguilar, A. Antioxidant interaction between major polyphenolic compounds found in “Ataulfo” mango pulp: Chlorogenic, gallic, protocatechuic and vanilic acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. Zwitterionic hydrophilic liquid chromatography coupled to mass spectrometry for analysis of beetroot juice and antioxidant interactions between its bioactive compounds. LWT 2018, 93, 641–648. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Investigation of antioxidant activity of selenium compounds and their mixtures with tea polyphenols. Mol. Biol. Rep. 2019, 46, 3019–3024. [Google Scholar] [CrossRef]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration Dependence of the Antioxidant and Prooxidant Activity of Trolox in HeLa Cells: Involvement in the Induction of Apoptotic Volume Decrease. Antioxidants 2020, 9, 1058. [Google Scholar] [CrossRef]
- Giordano, M.E.; Ingrosso, I.; Schettino, T.; Caricato, R.; Giovinazzo, G.; Lionetto, M.G. Intracellular antioxidant activity of grape skin polyphenolic extracts in rat superficial colonocytes: In situ detection by confocal fluorescence microscopy. Front. Physiol. 2016, 7, 177. [Google Scholar] [CrossRef]
- Giordano, M.E.; Caricato, R.; Verri, T.; Lionetto, M.G. The colon epithelium as a target for the intracellular antioxidant activity of hydroxytyrosol: A study on rat colon explants. J. Funct. Foods 2020, 64, 103604. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Changa, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate down stream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., II; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Forstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol as a gene regulator in the vasculature. Curr. Pharm. Biotechnol. 2014, 15, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The antioxidant activity of quercetin in water solution. Biomimetics 2017, 272, 9. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Dudley, J.; Das, S.; Mukherjee, S.; Das, D.K. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J. Nutr. Biochem. 2009, 20, 443–452. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef]
- Erlank, H.; Elmann, A.; Kohen, R.; Kanner, J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011, 51, 2319–2327. [Google Scholar] [CrossRef]
- Li, D.D.; Han, R.M.; Liang, R.; Chen, C.H.; Lai, W.; Zhang, J.P.; Skibsted, L.H. Hydroxyl radical reaction with trans-resveratrol: Initial carbon radical adduct formation followed by rearrangement to phenoxyl radical. J. Phys. Chem. B 2012, 116, 7154–7161. [Google Scholar] [CrossRef] [PubMed]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Brede, O. Elementary reactions of the antioxidant action of trans-stilbene derivatives: Resveratrol, pinosylvin and 4-hydroxystilbene. Phys. Chem. Chem. Phys. 2002, 4, 757–764. [Google Scholar] [CrossRef]
- Yang, N.C.; Lee, C.H.; Song, T.Y. Evaluation of resveratrol oxidation in vitro and the crucial role of bicarbonate ions. Biosci. Biotechnol. Biochem. 2010, 74, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Skora, N.C.; Whelan, J.; Villa, B.P.; Straub, K.D. ‘Antioxidant’ berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021, 165, 67–78. [Google Scholar] [CrossRef] [PubMed]



| Initial Velocity (ΔF/F0 x min−1) | Area under Curve (AUC) | |
|---|---|---|
| 300 µM H2O2 | 0.356 ± 0.017 a | 200.3 ± 7.4 a |
| 300 µM H2O2 + 5 µM quercetin | 0.154 ± 0.008 b | 128.9 ± 4.9 b |
| 300 µM H2O2 + 25 µM quercetin | 0.069 ± 0.004 c | 74.40 ± 2.9 c |
| 300 µM H2O2 + 50 µM quercetin | 0.077 ± 0.004 c | 84.39 ± 3.2 c |
| 300 µM H2O2 | 0.326 ± 0.020 a | 213.3 ± 7.9 a |
| 300 µM H2O2 + 5 µM resveratrol | 0.382 ± 0.019 a,b | 245.7 ± 8.4 a,b |
| 300 µM H2O2 + 25 µM resveratrol | 0.429 ± 0.022 b | 279.9 ± 9.2 b |
| 300 µM H2O2 + 50 µM resveratrol | 0.428 ± 0.018 b | 285.0 ± 10.4 b |
| 300 µM H2O2 | 0.386 ± 0.022 a | 203.2 ± 6.8 a |
| 300 µM H2O2 + 5 µM mix | 0.263 ± 0.015 b | 168.3 ± 6.3 b |
| 300 µM H2O2 + 25 µM mix | 0.086 ± 0.006 c | 94.2 ± 3.7 c |
| 300 µM H2O2 + 50 µM mix | 0.096 ± 0.005 c | 105.8 ± 4.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, M.E.; Lionetto, M.G. Intracellular Redox Behavior of Quercetin and Resveratrol Singly and in Mixtures. Molecules 2023, 28, 4682. https://doi.org/10.3390/molecules28124682
Giordano ME, Lionetto MG. Intracellular Redox Behavior of Quercetin and Resveratrol Singly and in Mixtures. Molecules. 2023; 28(12):4682. https://doi.org/10.3390/molecules28124682
Chicago/Turabian StyleGiordano, Maria Elena, and Maria Giulia Lionetto. 2023. "Intracellular Redox Behavior of Quercetin and Resveratrol Singly and in Mixtures" Molecules 28, no. 12: 4682. https://doi.org/10.3390/molecules28124682
APA StyleGiordano, M. E., & Lionetto, M. G. (2023). Intracellular Redox Behavior of Quercetin and Resveratrol Singly and in Mixtures. Molecules, 28(12), 4682. https://doi.org/10.3390/molecules28124682








