Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach
Abstract
1. Introduction
2. Results and Discussion
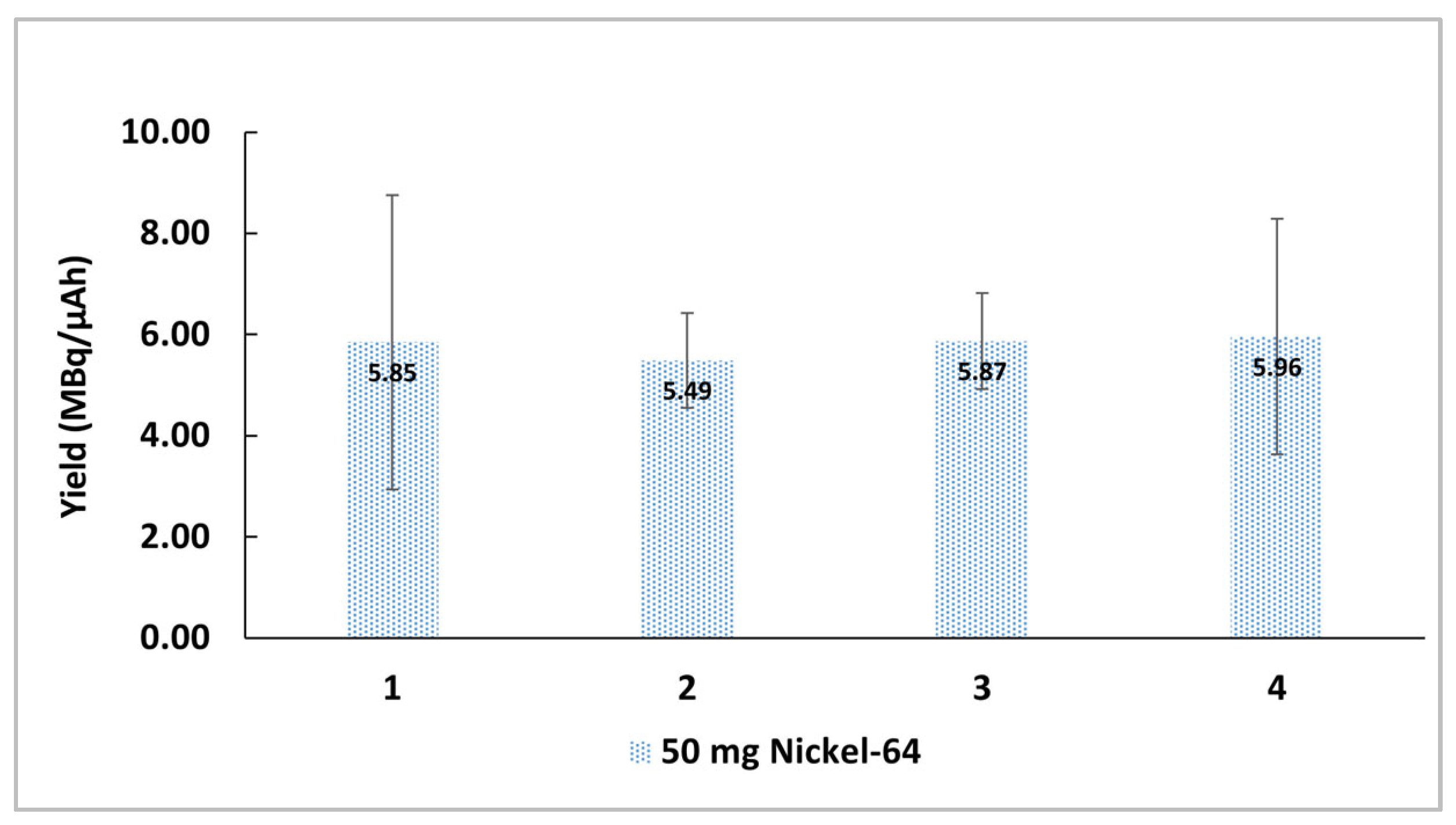
2.1. Copper-64 Production from Solid Targets
2.1.1. Copper-64 Purification
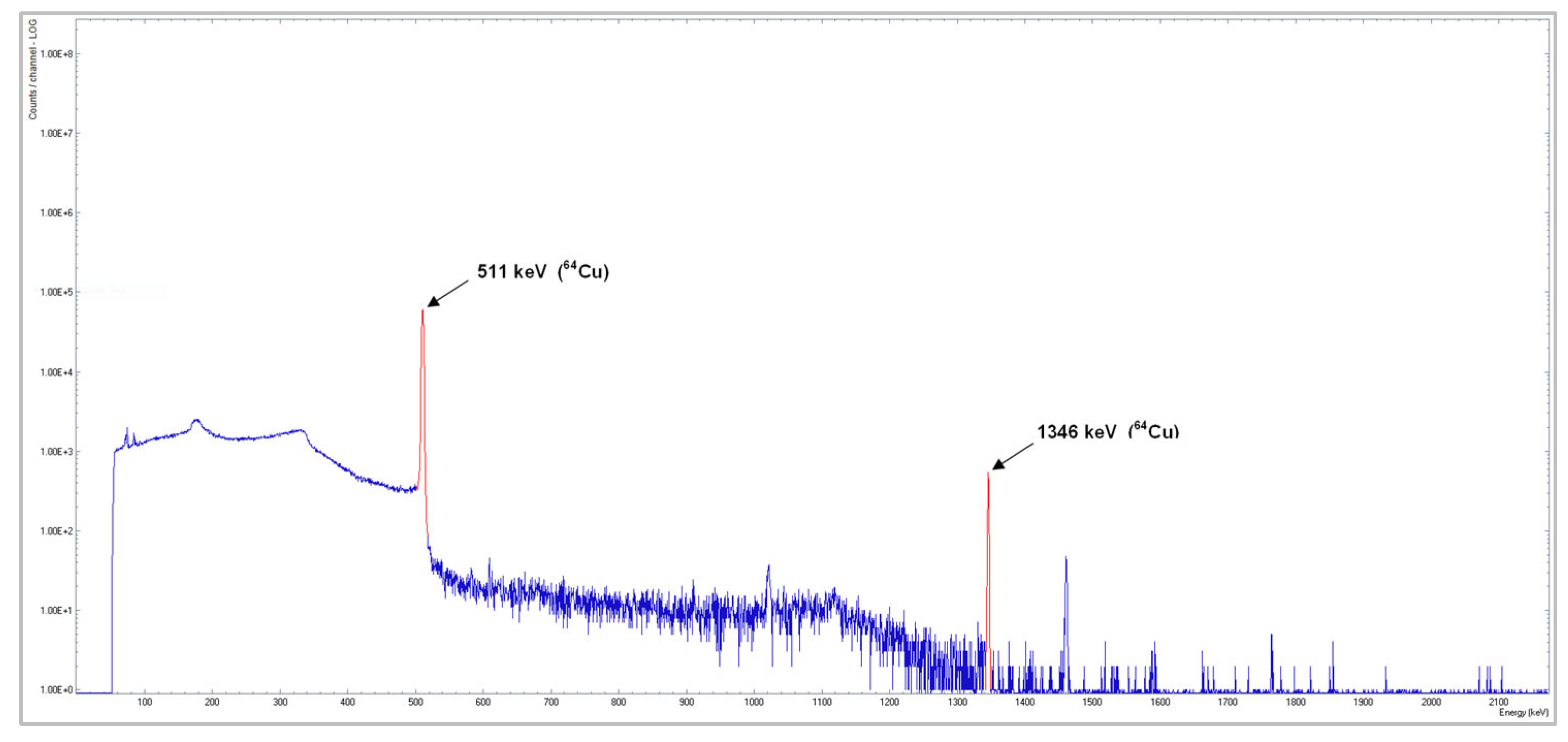
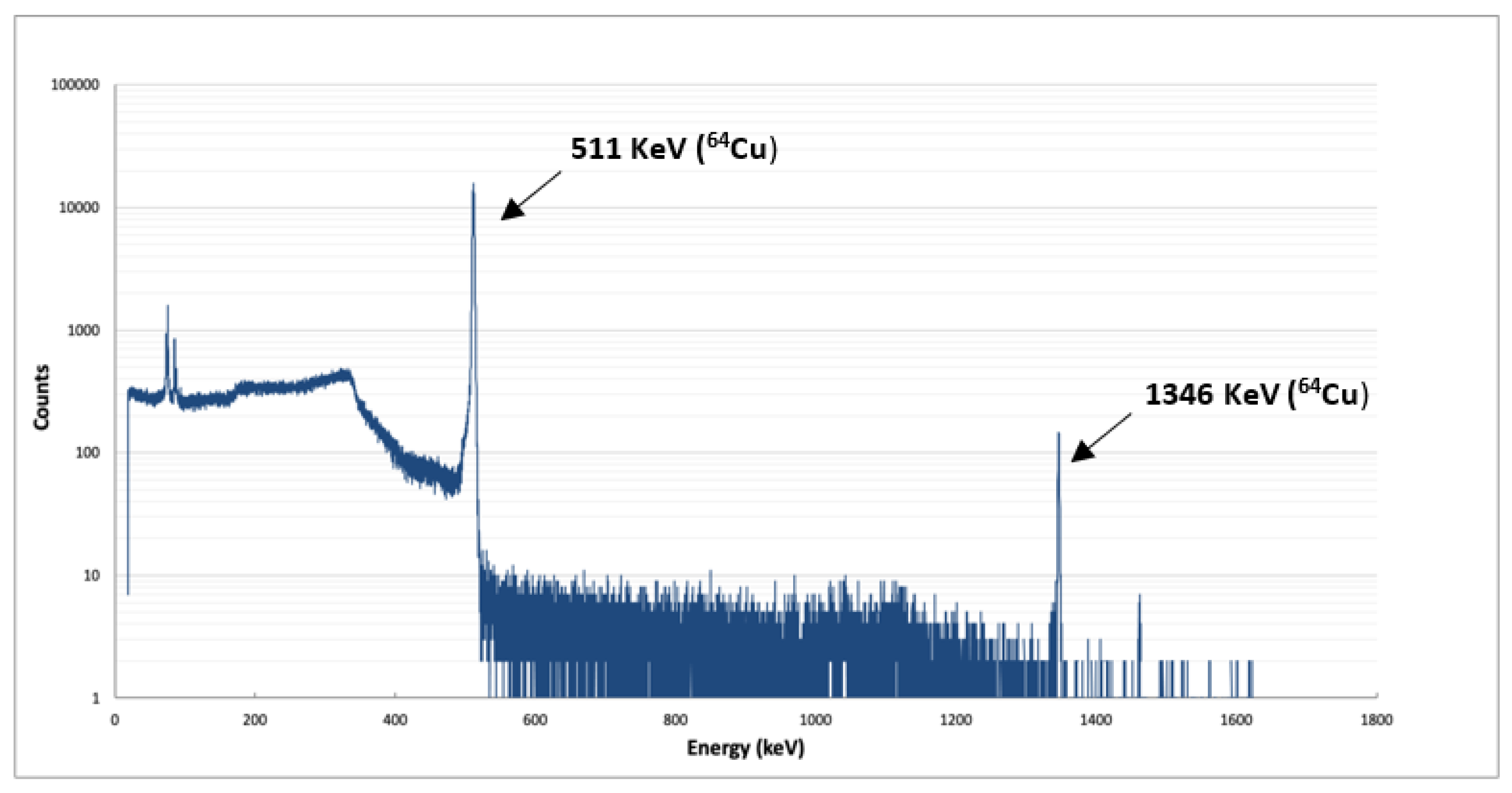
2.1.2. Quality Control
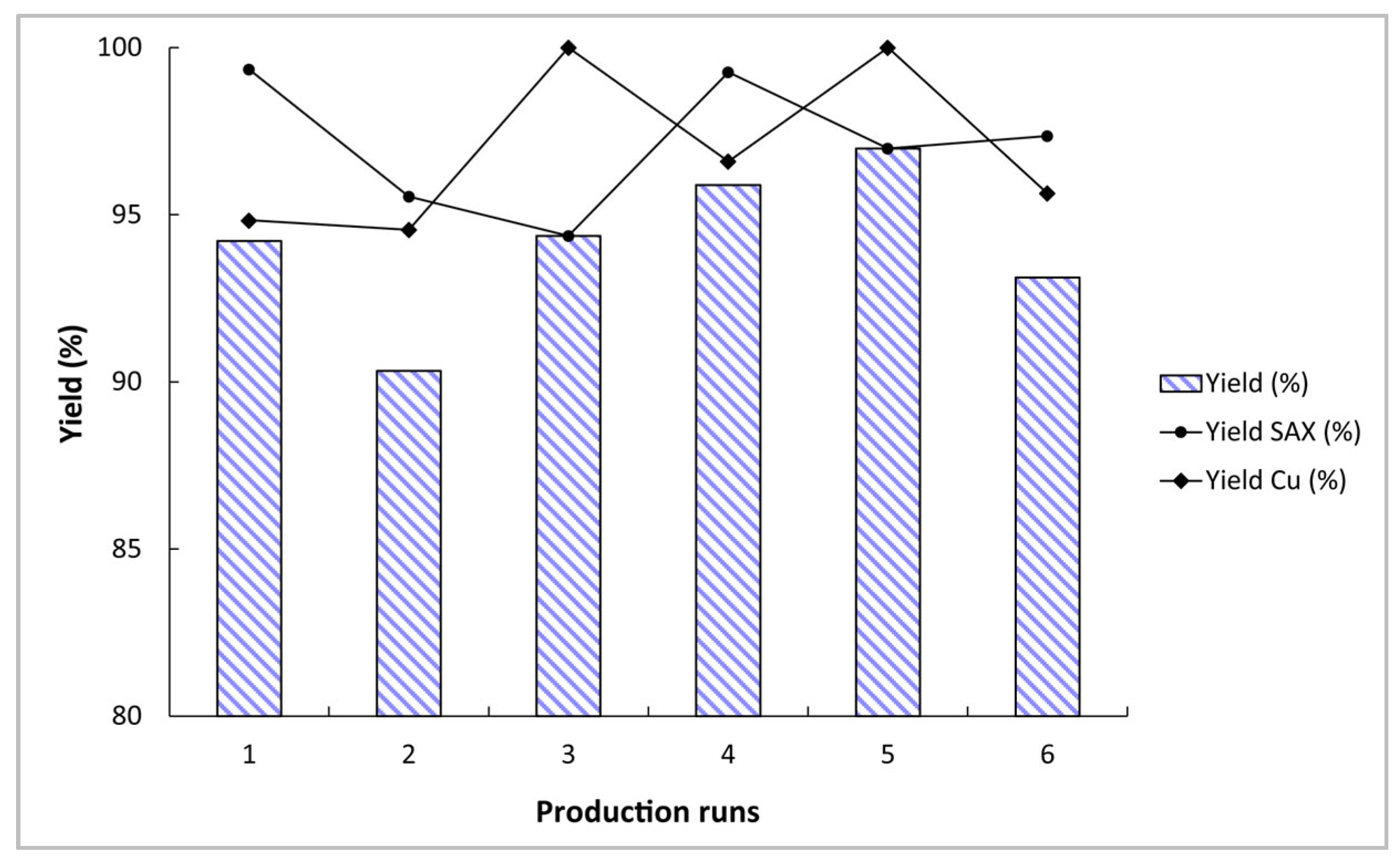
2.2. 64Cu Production from Liquid Targets
2.2.1. 64Cu Purification
2.2.2. Quality Control
2.3. Radiolabeling
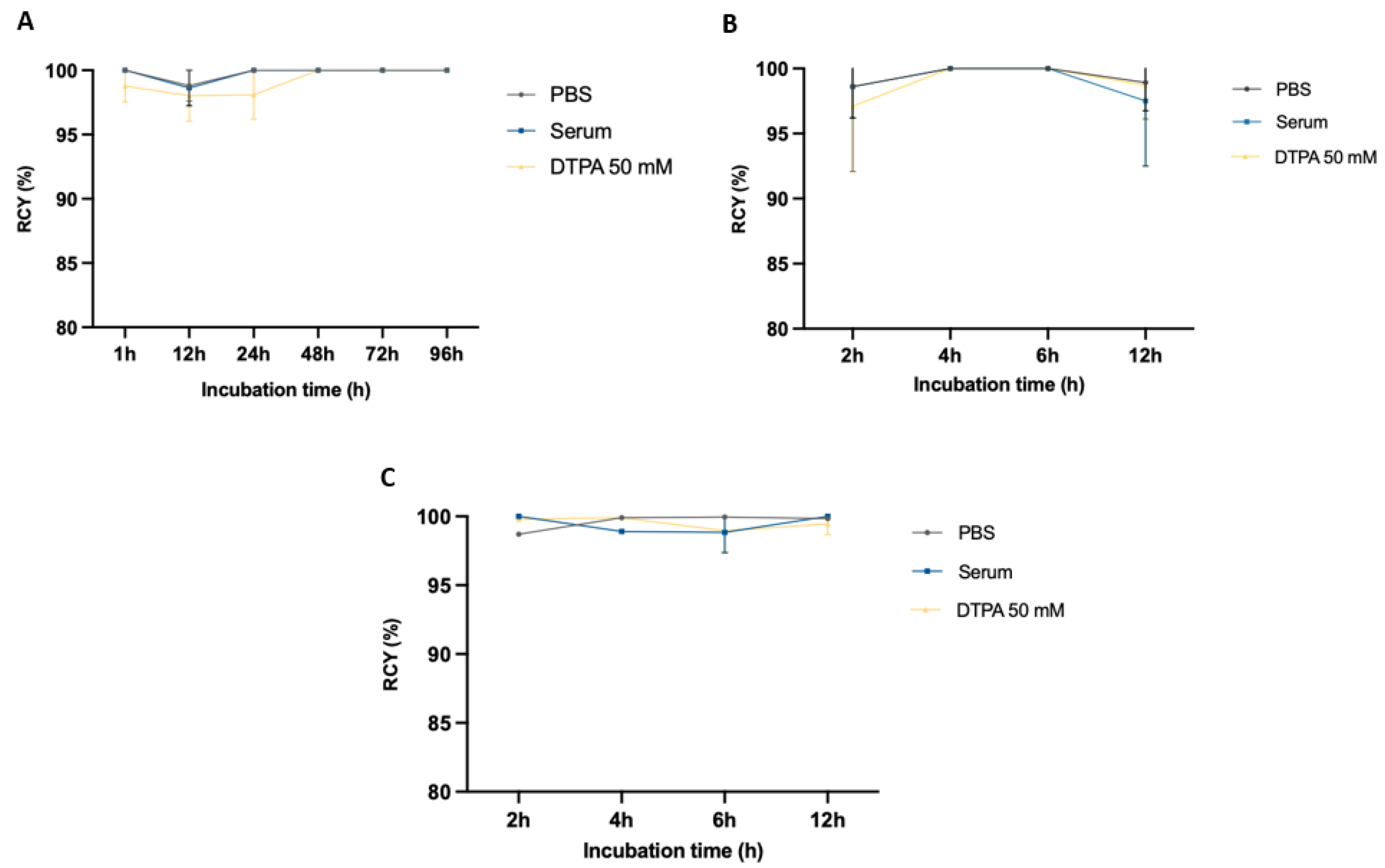
2.4. Stability Studies
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Production and Purification from Solid Target
3.2.1. Target Preparation/Irradiation
3.2.2. Purification
3.3. Production and Purification from Liquid Target
3.3.1. Target Preparation/Irradiation
3.3.2. Purification
3.4. Preparation of Immunoconjugates
Determination of Protein Concentration of the Immunoconjugates
3.5. Radiolabeling of Immunoconjugates
3.6. In Vitro Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jeffery, C.M.; Smith, S.V.; Asad, A.H.; Chan, S.; Price, R.I. Routine production of copper-64 using 11.7MeV protons. AIP Conf. Proc. 2012, 1509, 84–90. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, H.; Wang, F.; Meng, X.; Ren, Q.; Xia, C.; Yang, Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules 2017, 22, 641. [Google Scholar] [CrossRef]
- Bé, M.-M.; Helmer, R.G. Laboratoire National Henri Becquerel—Cu-64_Table. Available online: http://www.lnhb.fr/nuclides/Cu-64_tables.pdf (accessed on 11 April 2023).
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.-R. Efficient preparation of high-quality (64)Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef]
- IAEA. Cyclotron Produced Radionuclides: Emerging Positron Emitters for Medical Applications: 64Cu and 124I; International Atomic Energy Agency: Vienna, Austria, 2016. [Google Scholar]
- David, T.; Hlinová, V.; Kubíček, V.; Bergmann, R.; Striese, F.; Berndt, N.; Szöllősi, D.; Kovács, T.; Máthé, D.; Bachmann, M.; et al. Improved Conjugation, 64-Cu Radiolabeling, in Vivo Stability, and Imaging Using Nonprotected Bifunctional Macrocyclic Ligands: Bis(Phosphinate) Cyclam (BPC) Chelators. J. Med. Chem. 2018, 61, 8774–8796. [Google Scholar] [CrossRef]
- Niculae, D.; Dusman, R.; Leonte, R.A.; Chilug, L.E.; Dragoi, C.M.; Nicolae, A.; Serban, R.M.; Niculae, D.A.; Dumitrescu, I.B.; Draganescu, D. Biological Pathways as Substantiation of the Use of Copper Radioisotopes in Cancer Theranostics. Front. Phys. 2021, 8, 568296. [Google Scholar] [CrossRef]
- Wadas, T.; Wong, E.; Weisman, G.; Anderson, C. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Yoshii, Y.; Furukawa, T.; Yoshimoto, M.; Matsumoto, H.; Saga, T. Imaging and Therapy Against Hypoxic Tumors with 64Cu-ATSM. In Make Life Visible; Springer: Singapore, 2020; pp. 285–292. [Google Scholar] [CrossRef]
- Wong, P.; Li, L.; Chea, J.; Hu, W.; Poku, E.; Ebner, T.; Bowles, N.; Wong, J.Y.C.; Yazaki, P.J.; Sligar, S.G.; et al. Antibody Targeted PET Imaging of 64Cu-DOTA-Anti-CEA PEGylated Lipid Nanodiscs in CEA Positive Tumors. Bioconjugate Chem. 2020, 31, 743–753. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Q.; Cheng, Y.; Xiang, L.; Shen, G.; Wu, X.; Cai, H.; Li, D.; Zhu, H.; Zhang, R.; et al. 64Cu-labeled melanin nanoparticles for PET/CT and radionuclide therapy of tumor. Nanomedicine 2020, 29, 102248. [Google Scholar] [CrossRef]
- Griessinger, C.M.; Maurer, A.; Kesenheimer, C.; Kehlbach, R.; Reischl, G.; Ehrlichmann, W.; Bukala, D.; Harant, M.; Cay, F.; Brück, J.; et al. 64Cu antibody-targeting of the T-cell receptor and subsequent internalization enables in vivo tracking of lymphocytes by PET. Proc. Natl. Acad. Sci. USA 2015, 112, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kise, S.; Kaizuka, Y.; Watanabe, R.; Sugawa, T.; Furukawa, T.; Fujii, H.; Uehara, T. Copper-64-Labeled Antibody Fragments for Immuno-PET/Radioimmunotherapy with Low Renal Radioactivity Levels and Amplified Tumor-Kidney Ratios. ACS Omega 2021, 6, 21556–21562. [Google Scholar] [CrossRef] [PubMed]
- Baruta, S.; Leonte, R.; Cocioaba, D.; Craciun, L.; Ur, C.A.; Niculae, D. Cyclotron production of 64Cu by proton irradiation of enriched 64Ni target: Validation of Geant4 simulation parameters through experimental data. Front. Phys. 2022, 10, 1038014. [Google Scholar] [CrossRef]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Alves, V.H.; Carmo, S.J.C.D.; Alves, F.; Abrunhosa, A.J. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef]
- Svedjehed, J.; Pärnaste, M.; Gagnon, K. Demystifying solid targets: Simple and rapid distribution-scale production of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11. Nucl. Med. Biol. 2022, 104–105, 1–10. [Google Scholar] [CrossRef]
- Hrynchak, I.; Santos, L.; Falcão, A.; Gomes, C.M.; Abrunhosa, A.J. Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies. Int. J. Mol. Sci. 2021, 22, 10745. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M. Targeted therapy of cancer with radiolabeled antibodies. J. Nucl. Med. 2002, 43, 693–713. [Google Scholar] [PubMed]
- Leonte, R.A.; Cocioabă, D.; Chilug, L.E.; Ilie, S.I.B.; Eșanu, T.R.; Burghelea, B.; Chiriacescu, A.; Crăciun, L.S.; Niculae, D. Process validation for production of copper radioisotopes in a TR-19 variable energy cyclotron. AIP Conf. Ser. 2020, 2295, 020022. [Google Scholar] [CrossRef]
- Obata, A.; Kasamatsu, S.; McCarthy, D.W.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Production of therapeutic quantities of (64)Cu using a 12 MeV cyclotron. Nucl. Med. Biol. 2003, 30, 535–539. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- IAEA. Copper-64 Radiopharmaceuticals: Production, Quality Control and Clinical Applications; International Atomic Energy Agency: Vienna, Austria, 2022. [Google Scholar]
- Bryan, J.N.; Jia, F.; Mohsin, H.; Sivaguru, G.; Anderson, C.J.; Miller, W.H.; Henry, C.J.; Lewis, M.R. Monoclonal antibodies for copper-64 PET dosimetry and radioimmunotherapy. Cancer Biol. Ther. 2011, 11, 1001–1007. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.C.; Pinkston, K.L.; Robinson, H.; Harvey, B.R.; Wilganowski, N.; Gore, K.; Sevick-Muraca, E.M.; Azhdarinia, A. Comparison of DOTA and NODAGA as chelators for (64)Cu-labeled immunoconjugates. Nucl. Med. Biol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Yapp, D.T.T.; Crisp, S.; Sutherland, B.W.; Ng, S.S.W.; Gleave, M.; Bensimon, C.; Jurek, P.; Kiefer, G.E. Comparison of bifunctional chelates for (64)Cu antibody imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2117–2126. [Google Scholar] [CrossRef]
- Cooper, M.S.; Sabbah, E.; Mather, S.J. Conjugation of chelating agents to proteins and radiolabeling with trivalent metallic isotopes. Nat. Protoc. 2006, 1, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.I.; Alves, V.H.; Carmo, S.J.C.D.; Silva, M.; Hrynchak, I.; Alves, F.; Falcão, A.; Abrunhosa, A.J. Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets. Pharmaceuticals 2022, 15, 723. [Google Scholar] [CrossRef] [PubMed]

| Cyctroton | Solid Target | Liquid Target |
|---|---|---|
| ACSI TR-19 | IBA Cyclone® Kiube | |
| Nickel-64 (mg) | 48 ± 1 | 100.6 ± 3.6 |
| Integrated current [μA·h] | 150 ± 0.2 | 230.7 ± 94.1 |
| Number of runs | 2 | 10 |
| Proton energy [MeV] extracted/on target | 14.2/11.7 | 18/16.9 |
| Beam current [μA] | 25 ± 1.2 | 54.5 ± 7.8 |
| Beam time [h] | 6 | 4.1 ± 1.3 |
| Activity at EOB [GBq] | 13.5 ± 0.5 | 2.8 ± 1.3 |
| Solid Target | Liquid Target | |
|---|---|---|
| [64Cu]CuCl2 (μL) | 38 | 90 |
| [64Cu]CuCl2 (MBq) | 94 | 108 |
| % Radiochemical purity | ||
| DOTA-Trastuzumab (μg) | 285 | 281 |
| NODAGA-Nanobody (μg) | 882 | 881 |
| NOTA-Nanobody (μg) | 497 | 630 |
| % Radiochemical yield (n.d.c.) * | ||
| [64Cu]Cu-DOTA-Trastuzumab | 100 | 88.5 |
| [64Cu]Cu-NODAGA-Nanobody | 100 | 93.57 |
| [64Cu]Cu-NOTA-Nanobody | 100 | 82.76 |
| % Radiochemical purity | ||
| [64Cu]CuCl2 | 100 | 100 |
| SA (MBq/μg) | ||
| [64Cu]Cu-DOTA-Trastuzumab | 0.33 | 0.30 |
| [64Cu]Cu-NODAGA-Nanobody | 0.11 | 0.15 |
| [64Cu]Cu-NOTA-Nanobody | 0.19 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrynchak, I.; Cocioabă, D.; Fonseca, A.I.; Leonte, R.; do Carmo, S.J.C.; Cornoiu, R.; Falcão, A.; Niculae, D.; Abrunhosa, A.J. Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach. Molecules 2023, 28, 4670. https://doi.org/10.3390/molecules28124670
Hrynchak I, Cocioabă D, Fonseca AI, Leonte R, do Carmo SJC, Cornoiu R, Falcão A, Niculae D, Abrunhosa AJ. Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach. Molecules. 2023; 28(12):4670. https://doi.org/10.3390/molecules28124670
Chicago/Turabian StyleHrynchak, Ivanna, Diana Cocioabă, Alexandra I. Fonseca, Radu Leonte, Sérgio J. C. do Carmo, Roxana Cornoiu, Amílcar Falcão, Dana Niculae, and Antero J. Abrunhosa. 2023. "Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach" Molecules 28, no. 12: 4670. https://doi.org/10.3390/molecules28124670
APA StyleHrynchak, I., Cocioabă, D., Fonseca, A. I., Leonte, R., do Carmo, S. J. C., Cornoiu, R., Falcão, A., Niculae, D., & Abrunhosa, A. J. (2023). Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach. Molecules, 28(12), 4670. https://doi.org/10.3390/molecules28124670







