Synthesis and Evaluation of 68Ga-Labeled (2S,4S)-4-Fluoropyrrolidine-2-Carbonitrile and (4R)-Thiazolidine-4-Carbonitrile Derivatives as Novel Fibroblast Activation Protein-Targeted PET Tracers for Cancer Imaging
Abstract
1. Introduction
2. Results
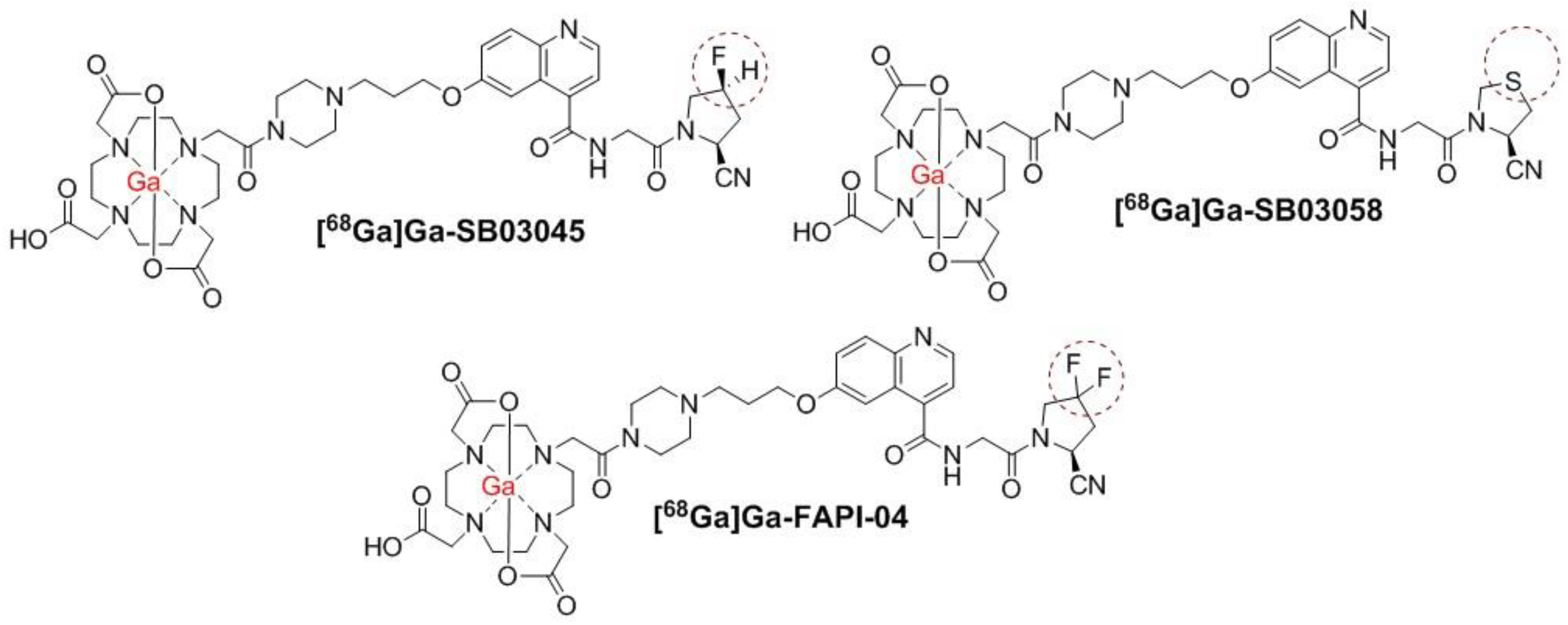
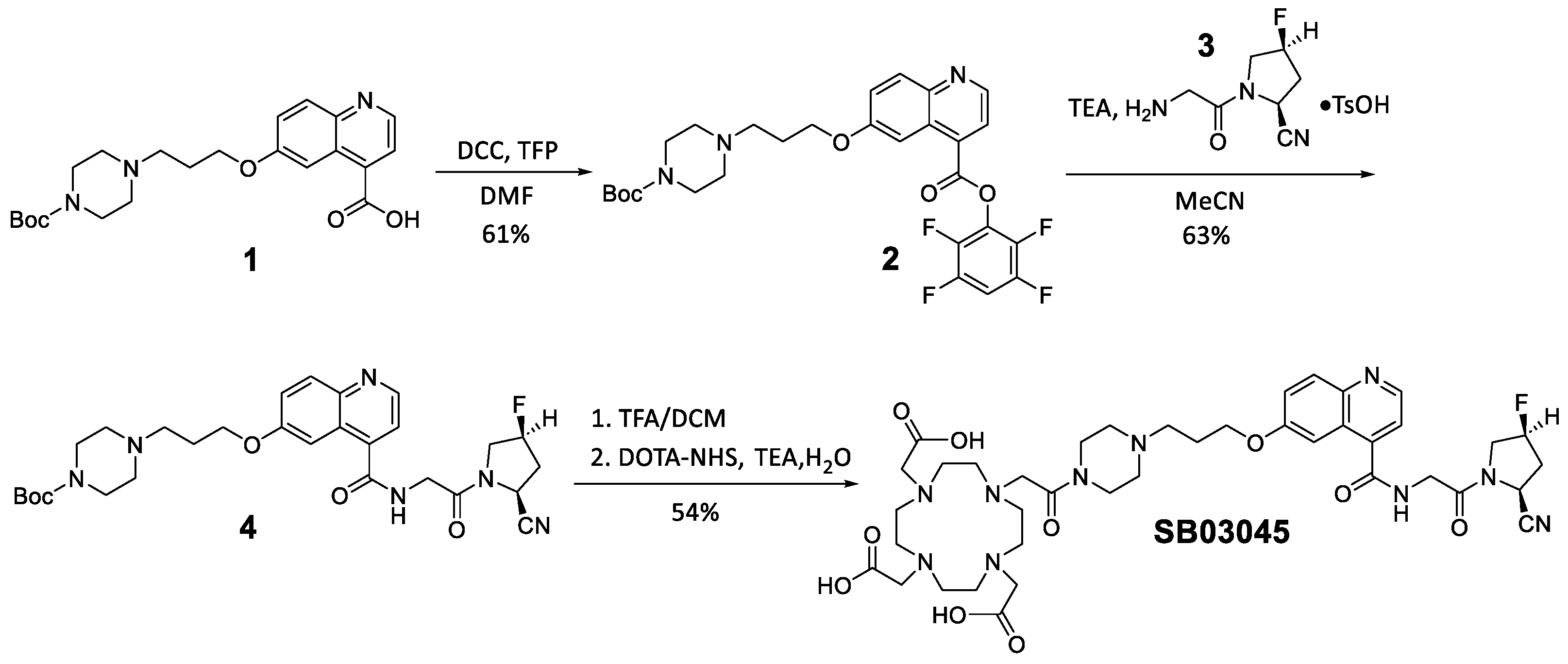
2.1. Synthesis of 68Ga and natGa-Complexed DOTA-Conjugated FAP-Targeted Ligands
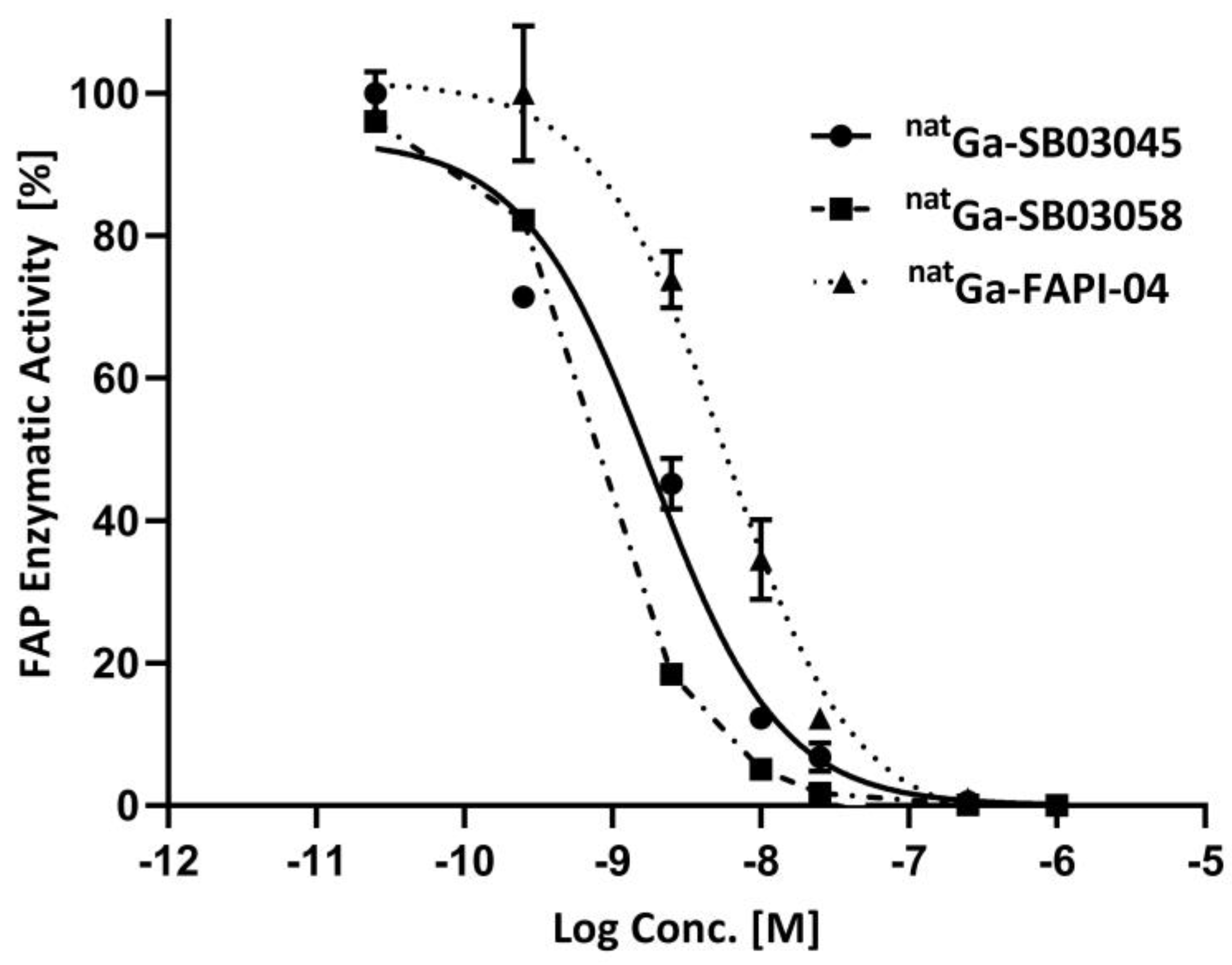
2.2. In Vitro Fluorescence-Based Binding Assay
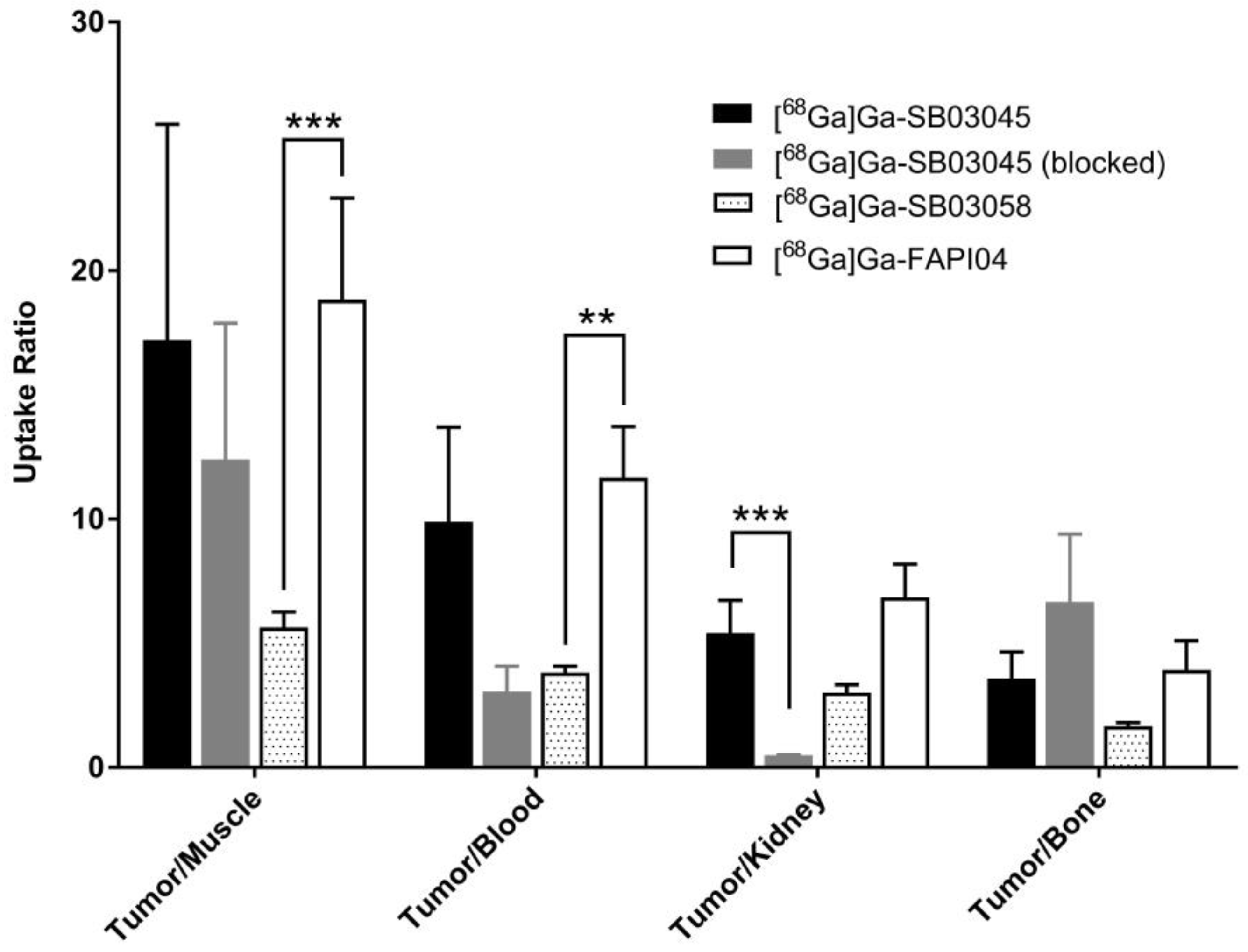
2.3. Ex Vivo Biodistribution and PET/CT Imaging Studies
2.4. Hydrophilicty/LogD7.4 Measurement
3. Discussion
4. Materials and Methods
4.1. Synthesis of natGa-Complexed DOTA-Conjugated FAP-Targeted Ligands
4.2. Cell Culture
4.3. In Vitro Fluorescence Based Binding Assay
4.4. General Procedure for Synthesis of 68Ga-Complexed Radiotracers
4.5. Ex Vivo Biodistribution and PET/CT Imaging Studies
4.6. Hydrophilicty/LogD7.4 Measurement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenblum, J.S.; Kozarich, J.W. Prolyl Peptidases: A Serine Protease Subfamily with High Potential for Drug Discovery. Curr. Opin. Chem. Biol. 2003, 7, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Imlimthan, S.; Moon, E.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Zhang, P.J.; Bi, Y.; Satija, C.; Marjumdar, R.; Stephen, T.L.; Lo, A.; Chen, H.; Mies, C.; June, C.H.; et al. Fibroblast Activation Protein Expression by Stromal Cells and Tumor-Associated Macrophages in Human Breast Cancer. Hum. Pathol. 2013, 44, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Kesch, C.; Yirga, L.; Dendl, K.; Handke, A.; Darr, C.; Krafft, U.; Radtke, J.P.; Tschirdewahn, S.; Szarvas, T.; Fazli, L.; et al. High Fibroblast-Activation-Protein Expression in Castration-Resistant Prostate Cancer Supports the Use of FAPI-Molecular Theranostics. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 385–389. [Google Scholar] [CrossRef]
- Cheng, J.D.; Dunbrack, R.L.; Valianou, M.; Rogatko, A.; Alpaugh, R.K.; Weiner, L.M. Promotion of Tumor Growth by Murine Fibroblast Activation Protein, a Serine Protease, in an Animal Model. Cancer Res. 2002, 62, 4767–4772. [Google Scholar]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D.; Cao, J.; Shen, J.; Geng, J.; Bi, Y.; et al. Fibroblast Activation Protein Overexpression and Clinical Implications in Solid Tumors: A Meta-Analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef]
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Müller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast Activation Protein: A Cell Surface Dipeptidyl Peptidase and Gelatinase Expressed by Stellate Cells at the Tissue Remodelling Interface in Human Cirrhosis: Fibroblast Activation Protein: A Cell Surface Dipeptidyl Peptidase and Gelatinase Expressed by Stellate Cells at the Tissue Remodelling Interface in H. Hepatology 1999, 29, 1768–1778. [Google Scholar]
- Acharya, P.S.; Zukas, A.; Chandan, V.; Katzenstein, A.-L.A.; Puré, E. Fibroblast Activation Protein: A Serine Protease Expressed at the Remodeling Interface in Idiopathic Pulmonary Fibrosis. Hum. Pathol. 2006, 37, 352–360. [Google Scholar] [CrossRef]
- Bauer, S.; Jendro, M.C.; Wadle, A.; Kleber, S.; Stenner, F.; Dinser, R.; Reich, A.; Faccin, E.; Gödde, S.; Dinges, H.; et al. Fibroblast Activation Protein is Expressesed by Rheumatoid Myofibroblast-like Synoviocytes. Arthritis Res. Ther. 2006, 8, R171. [Google Scholar] [CrossRef]
- Ramirez-Montagut, T.; Blachere, N.E.; Sviderskaya, E.V.; Bennett, D.C.; Rettig, W.J.; Garin-Chesa, P.; Houghton, A.N. FAPα, a Surface Peptidase Expressed during Wound Healing, Is a Tumor Suppressor. Oncogene 2004, 23, 5435–5446. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Raj, B.K.M.; Calvo, B.; RErrFIG, W.J. Molecular Cloning of Fibroblast Activation Protein a, a Member of the Serine Protease Family Selectively Expressed in Stromal Fibroblasts of Epithelial Cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-Y.; Yeh, T.-K.; Chen, X.; Hsu, T.; Jao, Y.-C.; Huang, C.-H.; Song, J.-S.; Huang, Y.-C.; Chien, C.-H.; Chiu, J.-H.; et al. Substituted 4-Carboxymethylpyroglutamic Acid Diamides as Potent and Selective Inhibitors of Fibroblast Activation Protein. J. Med. Chem. 2010, 53, 6572–6583. [Google Scholar] [CrossRef] [PubMed]
- Ryabtsova, O.; Jansen, K.; Van Goethem, S.; Joossens, J.; Cheng, J.D.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; Van der Veken, P. Acylated Gly-(2-Cyano)Pyrrolidines as Inhibitors of Fibroblast Activation Protein (FAP) and the Issue of FAP/Prolyl Oligopeptidase (PREP)-Selectivity. Bioorg. Med. Chem. Lett. 2012, 22, 3412–3417. [Google Scholar] [CrossRef]
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Extended Structure–Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)Glycyl-2-Cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP). J. Med. Chem. 2014, 57, 3053–3074. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.D.; Valianou, M.; Canutescu, A.A.; Jaffe, E.K.; Lee, H.-O.; Wang, H.; Lai, J.H.; Bachovchin, W.W.; Weiner, L.M. Abrogation of Fibroblast Activation Protein Enzymatic Activity Attenuates Tumor Growth. Mol. Cancer Ther. 2005, 4, 351–360. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, L.; Wu, M.; Wong, M.S.; Li, B.; Corral, S.; Yu, Z.; Nomanbhoy, T.; Alemayehu, S.; Fuller, S.R.; et al. Synthesis and Structure–Activity Relationship of N-Alkyl Gly-Boro-Pro Inhibitors of DPP4, FAP, and DPP7. Bioorg. Med. Chem. Lett. 2005, 15, 4239–4242. [Google Scholar] [CrossRef]
- Scott, A.M.; Wiseman, G.; Adjei, A.; Lee, F.-T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; Larson, S.M.; et al. A Phase I Dose-Escalation Study of Sibrotuzumab in Patients with Advanced or Metastatic Fibroblast Activation Protein-Positive Cancer. Clin. Cancer Res. 2003, 9, 1639–1647. [Google Scholar]
- Welt, S.; Divgi, C.R.; Scott, A.M.; Garin-Chesa, P.; Finn, R.D.; Graham, M.; Carswell, E.A.; Cohen, A.; Larson, S.M.; Old, L.J. Antibody Targeting in Metastatic Colon Cancer: A Phase I Study of Monoclonal Antibody F19 against a Cell-Surface Protein of Reactive Tumor Stromal Fibroblasts. J. Clin. Oncol. 1994, 12, 1193–1203. [Google Scholar] [CrossRef]
- Milo, L.J.; Lai, J.H.; Wu, W.; Liu, Y.; Maw, H.; Li, Y.; Jin, Z.; Shu, Y.; Poplawski, S.E.; Wu, Y.; et al. Chemical and Biological Evaluation of Dipeptidyl Boronic Acid Proteasome Inhibitors for Use in Prodrugs and Pro-Soft Drugs Targeting Solid Tumors. J. Med. Chem. 2011, 54, 4365–4377. [Google Scholar] [CrossRef]
- Chai, X.; Sun, G.; Fang, Y.; Hu, L.; Liu, X.; Zhang, X. Tumor-Targeting Efficacy of a BF211 Prodrug through Hydrolysis by Fibroblast Activation Protein-α. Acta Pharmacol. Sin. 2018, 39, 415–424. [Google Scholar] [CrossRef]
- Riet, T.; Abken, H. Chimeric Antigen Receptor T Cells: Power Tools to Wipe out Leukemia and Lymphoma. Expert Rev. Hematol. 2015, 8, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-Cell for Acute Lymphoblastic Leukemia (ALL) Treatment: A Review Study. Cancer Gene Ther 2022, 29, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, C.-T.; Ma, T.-T.; Li, Z.-Y.; Zhou, L.-N.; Mu, B.; Leng, F.; Shi, H.-S.; Li, Y.-O.; Wei, Y.-Q. Immunotherapy Targeting Fibroblast Activation Protein Inhibits Tumor Growth and Increases Survival in a Murine Colon Cancer Model. Cancer Sci. 2010, 101, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein–Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68 Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Ashworth, D.M.; Atrash, B.; Baker, G.R.; Baxter, A.J.; Jenkins, P.D.; Jones, D.M.; Szelke, M. 4-Cyanothiazolidides as Very Potent, Stable Inhibitors of Dipeptidyl Peptidase IV. Bioorg. Med. Chem. Lett. 1996, 6, 2745–2748. [Google Scholar] [CrossRef]
- Verena, A.; Zhang, Z.; Kuo, H.-T.; Merkens, H.; Zeisler, J.; Wilson, R.; Bendre, S.; Wong, A.A.W.L.; Bénard, F.; Lin, K.-S. Synthesis and Preclinical Evaluation of Three Novel 68Ga-Labeled Bispecific PSMA/FAP-Targeting Tracers for Prostate Cancer Imaging. Molecules 2023, 28, 1088. [Google Scholar] [CrossRef]
- Olberg, D.E.; Arukwe, J.M.; Grace, D.; Hjelstuen, O.K.; Solbakken, M.; Kindberg, G.M.; Cuthbertson, A. One Step Radiosynthesis of 6-[ 18 F]Fluoronicotinic Acid 2,3,5,6-Tetrafluorophenyl Ester ([ 18 F]F-Py-TFP): A New Prosthetic Group for Efficient Labeling of Biomolecules with Fluorine-18. J. Med. Chem. 2010, 53, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Gopalan, B.; Lingam, V.S.P.R.; Shah, D.M. Preparation of Glycinamide Derivatives as Dipeptidyl Peptidase IV Inhibitors. Patent WO2005075426 A1, 18 August 2005. [Google Scholar]
- Lin, K.-S.; Pan, J.; Amouroux, G.; Turashvili, G.; Mesak, F.; Hundal-Jabal, N.; Pourghiasian, M.; Lau, J.; Jenni, S.; Aparicio, S.; et al. In Vivo Radioimaging of Bradykinin Receptor B1, a Widely Overexpressed Molecule in Human Cancer. Cancer Res. 2015, 75, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Edosada, C.Y.; Quan, C.; Tran, T.; Pham, V.; Wiesmann, C.; Fairbrother, W.; Wolf, B.B. Peptide Substrate Profiling Defines Fibroblast Activation Protein as an Endopeptidase of Strict Gly 2 -Pro 1 -Cleaving Specificity. FEBS Lett. 2006, 580, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Kamer, K.J.; Raines, R.T. Origin of the Stability Conferred upon Collagen by Fluorination. Bioorg. Med. Chem. Lett. 2009, 19, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, Y.; Ji, X.; Deng, G.; Leng, Y.; Liu, H. Synthesis and Biological Evaluation of Pyrrolidine-2-Carbonitrile and 4-Fluoropyrrolidine-2-Carbonitrile Derivatives as Dipeptidyl Peptidase-4 Inhibitors for the Treatment of Type 2 Diabetes. Bioorg. Med. Chem. 2013, 21, 7418–7429. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Hiratate, A.; Takahashi, M.; Saito, M.; Munetomo, E.; Kitano, K.; Saito, H.; Takaoka, Y.; Yamamoto, K. Synthesis and Structure–Activity Relationships of Potent 3- or 4-Substituted-2-Cyanopyrrolidine Dipeptidyl Peptidase IV Inhibitors. Bioorg. Med. Chem. 2004, 12, 6053–6061. [Google Scholar] [CrossRef]
- Meng, L.; Fang, J.; Zhao, L.; Wang, T.; Yuan, P.; Zhao, Z.; Zhuang, R.; Lin, Q.; Chen, H.; Chen, X.; et al. Rational Design and Pharmacomodulation of Protein-Binding Theranostic Radioligands for Targeting the Fibroblast Activation Protein. J. Med. Chem. 2022, 65, 8245–8257. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder–Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2022, 63, 952–958. [Google Scholar] [CrossRef]
- Toms, J.; Kogler, J.; Maschauer, S.; Daniel, C.; Schmidkonz, C.; Kuwert, T.; Prante, O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18 F-Labeled FAP Inhibitor. J. Nucl. Med. 2020, 61, 1806–1813. [Google Scholar] [CrossRef]
- Kelly, J.M.; Jeitner, T.M.; Ponnala, S.; Williams, C.; Nikolopoulou, A.; DiMagno, S.G.; Babich, J.W. A Trifunctional Theranostic Ligand Targeting Fibroblast Activation Protein-α (FAPα). Mol. Imaging Biol. 2021, 23, 686–696. [Google Scholar] [CrossRef]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.-C.R.; Restifo, N.P.; Rosenberg, S.A. Immune Targeting of Fibroblast Activation Protein Triggers Recognition of Multipotent Bone Marrow Stromal Cells and Cachexia. J. Exp. Med. 2013, 210, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Zarrad, F.; Nader, M.; Kersting, D.; Weber, M.; Kazek, S.; Sraieb, M.; Hamacher, R.; et al. Pitfalls and Common Findings in 68 Ga-FAPI PET: A Pictorial Analysis. J. Nucl. Med. 2022, 63, 890–896. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendre, S.; Zhang, Z.; Colpo, N.; Zeisler, J.; Wong, A.A.W.L.; Bénard, F.; Lin, K.-S. Synthesis and Evaluation of 68Ga-Labeled (2S,4S)-4-Fluoropyrrolidine-2-Carbonitrile and (4R)-Thiazolidine-4-Carbonitrile Derivatives as Novel Fibroblast Activation Protein-Targeted PET Tracers for Cancer Imaging. Molecules 2023, 28, 3481. https://doi.org/10.3390/molecules28083481
Bendre S, Zhang Z, Colpo N, Zeisler J, Wong AAWL, Bénard F, Lin K-S. Synthesis and Evaluation of 68Ga-Labeled (2S,4S)-4-Fluoropyrrolidine-2-Carbonitrile and (4R)-Thiazolidine-4-Carbonitrile Derivatives as Novel Fibroblast Activation Protein-Targeted PET Tracers for Cancer Imaging. Molecules. 2023; 28(8):3481. https://doi.org/10.3390/molecules28083481
Chicago/Turabian StyleBendre, Shreya, Zhengxing Zhang, Nadine Colpo, Jutta Zeisler, Antonio A. W. L. Wong, François Bénard, and Kuo-Shyan Lin. 2023. "Synthesis and Evaluation of 68Ga-Labeled (2S,4S)-4-Fluoropyrrolidine-2-Carbonitrile and (4R)-Thiazolidine-4-Carbonitrile Derivatives as Novel Fibroblast Activation Protein-Targeted PET Tracers for Cancer Imaging" Molecules 28, no. 8: 3481. https://doi.org/10.3390/molecules28083481
APA StyleBendre, S., Zhang, Z., Colpo, N., Zeisler, J., Wong, A. A. W. L., Bénard, F., & Lin, K.-S. (2023). Synthesis and Evaluation of 68Ga-Labeled (2S,4S)-4-Fluoropyrrolidine-2-Carbonitrile and (4R)-Thiazolidine-4-Carbonitrile Derivatives as Novel Fibroblast Activation Protein-Targeted PET Tracers for Cancer Imaging. Molecules, 28(8), 3481. https://doi.org/10.3390/molecules28083481




