Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions
Abstract
1. Introduction
2. Results and Discussion
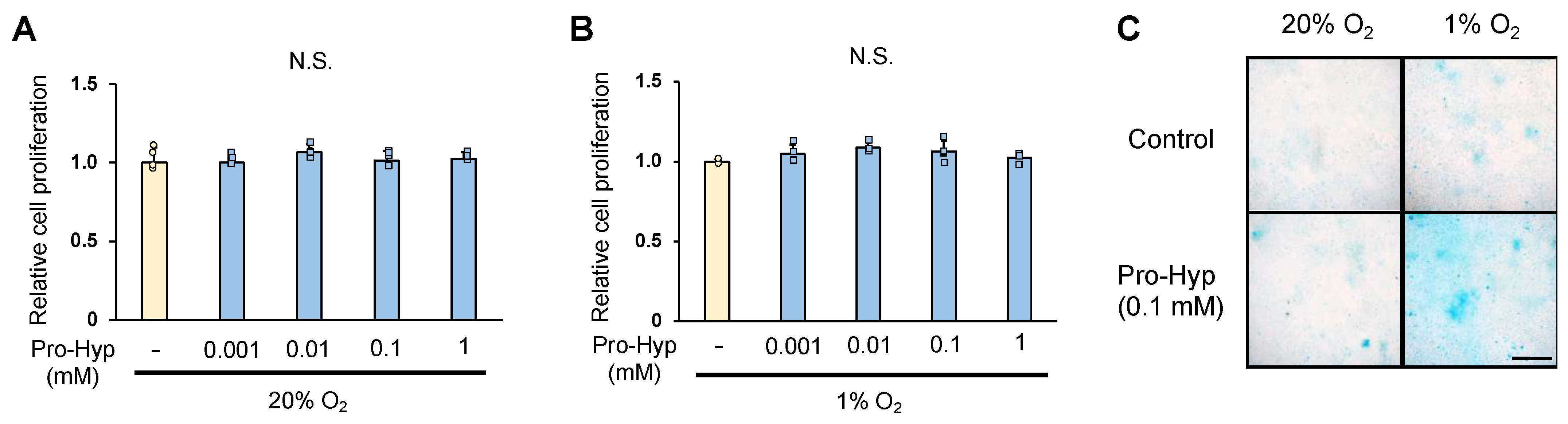
2.1. Pro-Hyp Regulates Chondrogenic ATDC5 Cells Differentiation in Hypoxic Condition
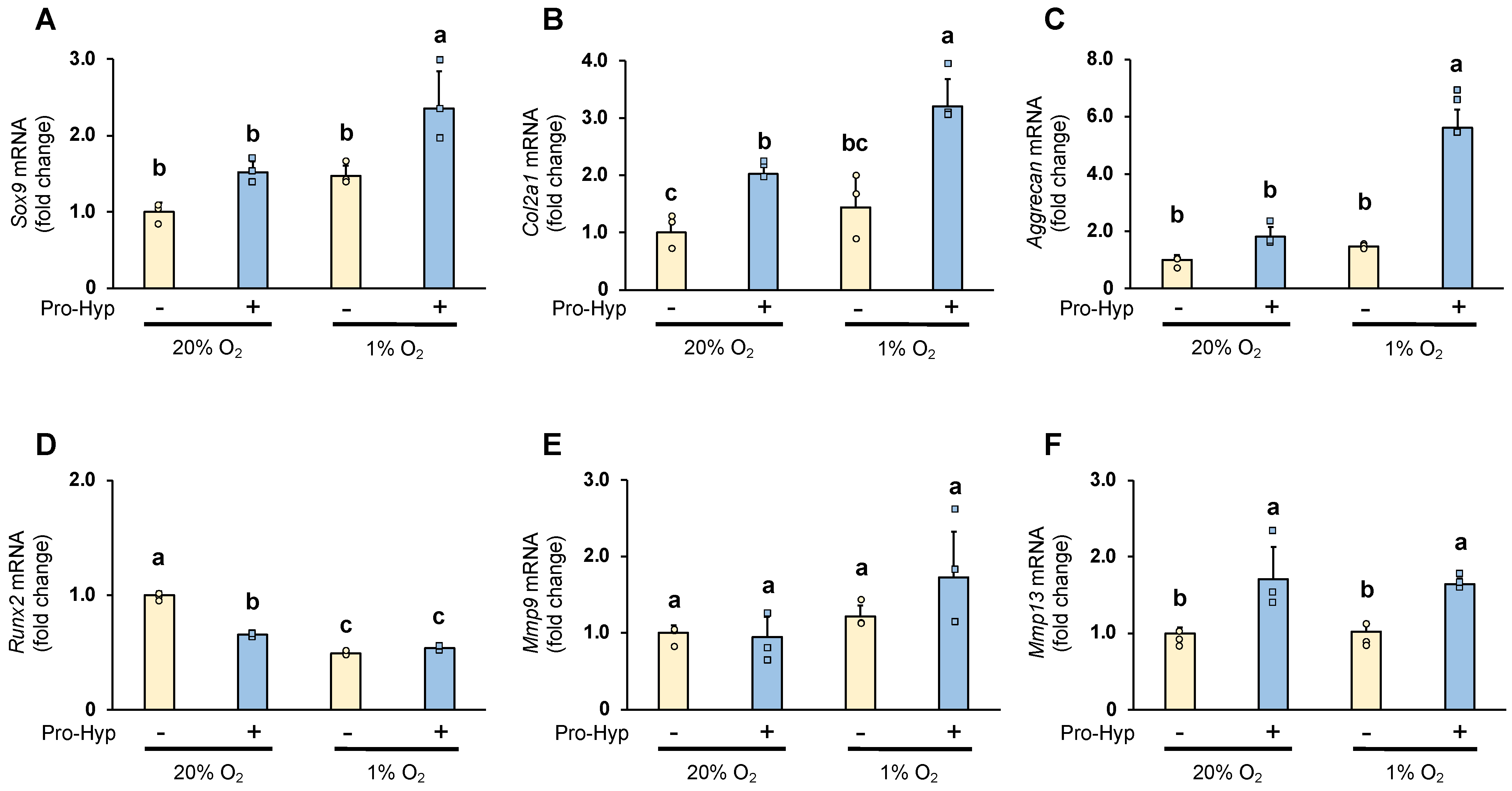
2.2. Pro-Hyp Further Upregulates Chondrogenesis-Specific Genes and ECM Remodeling Regulators under Hypoxic Conditions Compared to Normoxic Conditions
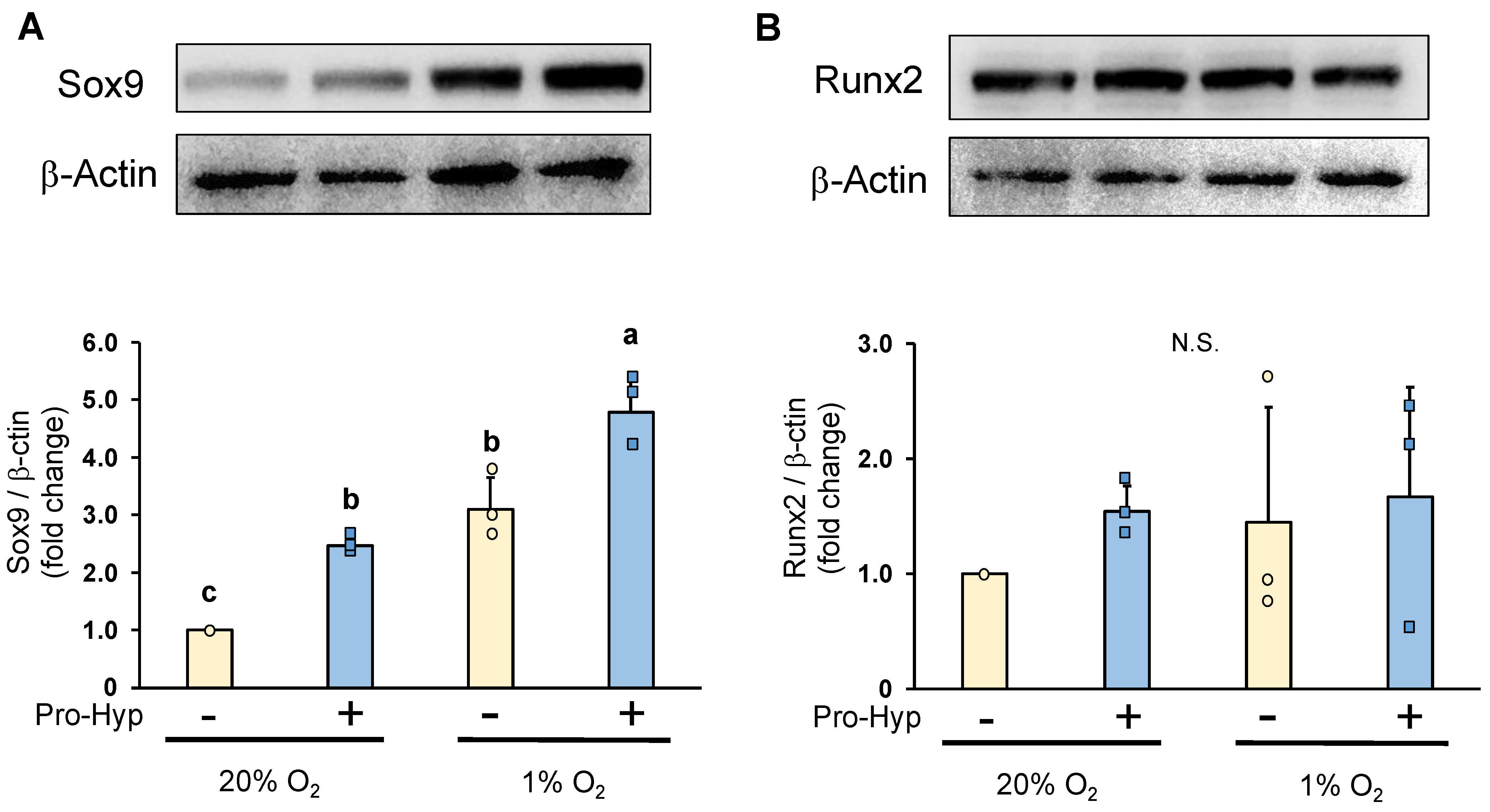
2.3. Pro-Hyp Further Increases Sox9 Protein Level without Affecting Runx2 Protein Level under Hypoxic Conditions Compared to Normoxic Conditions
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture and Treatment
3.3. Cell Proliferation
3.4. Alcian Blue Staining
3.5. RNA Preparation and Quantitative RT-PCR (qPCR)
3.6. Western Blot Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, S.; Everts, V. Molecular pathways of cell-mediated degradation of fibrillar collagen. Matrix Biol. 2019, 75, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Husek, P.; Svagera, Z.; Vsiansky, F.; Franekova, J.; Simek, P. Prolyl-hydroxyproline dipeptide in non-hydrolyzed morning urine and its value in postmenopausal osteoporosis. Clin. Chem. Lab. Med. 2008, 46, 1391–1397. [Google Scholar] [CrossRef]
- Kusubata, M.; Koyama, Y.; Tometsuka, C.; Shigemura, Y.; Sato, K. Detection of endogenous and food-derived collagen dipeptide prolyl hydroxyproline (Pro-Hyp) in allergic contact dermatitis-affected mouse ear. Biosci. Biotechnol. Biochem. 2015, 79, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A. The collagen triple-helix structure. Matrix Biol. 1995, 15, 545–554. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on the growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Ogura, K.; Taniuchi, Y.; Kataoka, A.; Inoue, N.; Sugihara, F.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen- derived dipeptide prolyl-hydroxyproline promotes differentiation of MC3T3-E1 osteoblastic cells. Biochem. Biophys. Res. Commun. 2014, 453, 498–501. [Google Scholar] [CrossRef]
- Ide, K.; Takahashi, S.; Sakai, K.; Taga, Y.; Ueno, T.; Dickens, D.; Jenkins, R.; Falciani, F.; Sasaki, T.; Ooi, K.; et al. The dipeptide prolyl-hydroxyproline promotes cellular homeostasis and lamellipodia-driven motility via active β1-integrin in adult tendon cells. J. Biol. Chem. 2021, 297, 10089. [Google Scholar] [CrossRef]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef]
- Yasuda, H.; Oh, C.D.; Chen, D.; de Crombrugghe, B.; Kim, J.H. A novel regulatory mechanism of type II collagen expression via a SOX9-dependent enhancer in Intron 6. J. Biol. Chem. 2017, 292, 528–538. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Bi, W.M.; Deng, J.M.; Zhang, Z.P.; Behringer, R.R.; de Crombrugge, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T.; Heppenstall, R.B. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis an in vitro and in viro studyin rats and rabbits. J. Bone Jt. Surg. 1971, 53, 719–728. [Google Scholar] [CrossRef]
- Etherington, P.J.; Winlove, P.; Taylor, P.; Paleolog, E.; Miotla, J.M. VEGF release is associated with reduced oxygen tensions in experimental inflammatory arthritis. Clin. Exp. Rheumatol. 2002, 20, 799–805. [Google Scholar]
- Grimshaw, M.J.; Mason, R.M. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthr. Cartil. 2000, 8, 386–392. [Google Scholar] [CrossRef]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Stickens, D.; Behonick, D.J.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.J.; Schorpp-Kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef] [PubMed]
- Borzí, R.M.; Olivotto, E.; Pagani, S.; Vitellozzi, R.; Neri, S.; Battistelli, M.; Falcieri, E.; Facchini, A.; Flamigni, F.; Penzo, M.; et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010, 62, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Fichter, M.; Körner, U.; Schömburg, J.; Jennings, L.; Cole, A.A.; Mollenhauer, J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J. Orthop. Res. 2006, 24, 63–70. [Google Scholar] [CrossRef]
- Abdelgawad, M.E.; Søe, K.; Andersen, T.L.; Merrild, D.M.; Christiansen, P.; Kjærsgaard-Andersen, P.; Delaisse, J.M. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone 2014, 67, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.; Wu, L.; King, K.B.; Hämmerle, H.; Cs-Szabo, G.; Mollenhauer, J. The effects of collagen fragments on the extracellular matrix metabolism of bovine and human chondrocytes. Connect. Tissue Res. 2001, 42, 71–86. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Sox9 | CCAGCAAGAACAAGCCACAC | GCTCAGTTCACCGATGTCCA |
| Col2α1 | AGGTGCTCAAGGTTCTCGTG | GCTCCAGGAAGACCAGGTTC |
| Aggrecan | CCAAACCAGCCTGACAACTT | TCTAGCATGCTCCACCACTG |
| Runx2 | TAAGAAGAGCCAGGAGGTGC | AGGTACGTGTGGTAGTGAGTG |
| MMP9 | TGAATCAGCTGGCTTTTGTG | GTGGATAGCTCGGTGGTGTT |
| MMP13 | AGGCCTTCAGAAAAGCCTTC | TCCTTGGAGTGATCCAGACC |
| β-actin | AAGGCCAACCGTGAAAAGAT | GTGGTACGACCAGAGGCATAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimira, Y.; Sato, T.; Sakamoto, M.; Osawa, Y.; Mano, H. Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions. Molecules 2023, 28, 4664. https://doi.org/10.3390/molecules28124664
Kimira Y, Sato T, Sakamoto M, Osawa Y, Mano H. Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions. Molecules. 2023; 28(12):4664. https://doi.org/10.3390/molecules28124664
Chicago/Turabian StyleKimira, Yoshifumi, Takahiro Sato, Mayu Sakamoto, Yoshihiro Osawa, and Hiroshi Mano. 2023. "Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions" Molecules 28, no. 12: 4664. https://doi.org/10.3390/molecules28124664
APA StyleKimira, Y., Sato, T., Sakamoto, M., Osawa, Y., & Mano, H. (2023). Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions. Molecules, 28(12), 4664. https://doi.org/10.3390/molecules28124664







