Controllable Synthesis of Trifluoromethyl- or gem-Difluorovinyl-containing Analogues of Neonicotinoids by the Reaction of α-(Trifluoromethyl)styrenes with 2-Nitroimino-imidazolidine
Abstract
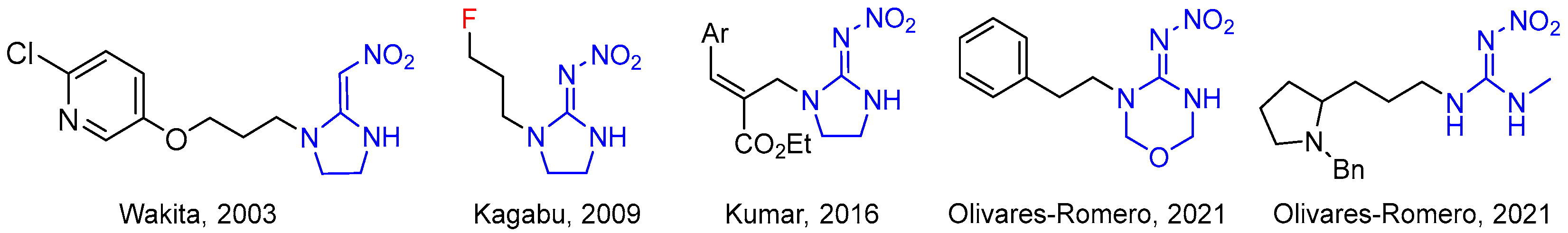
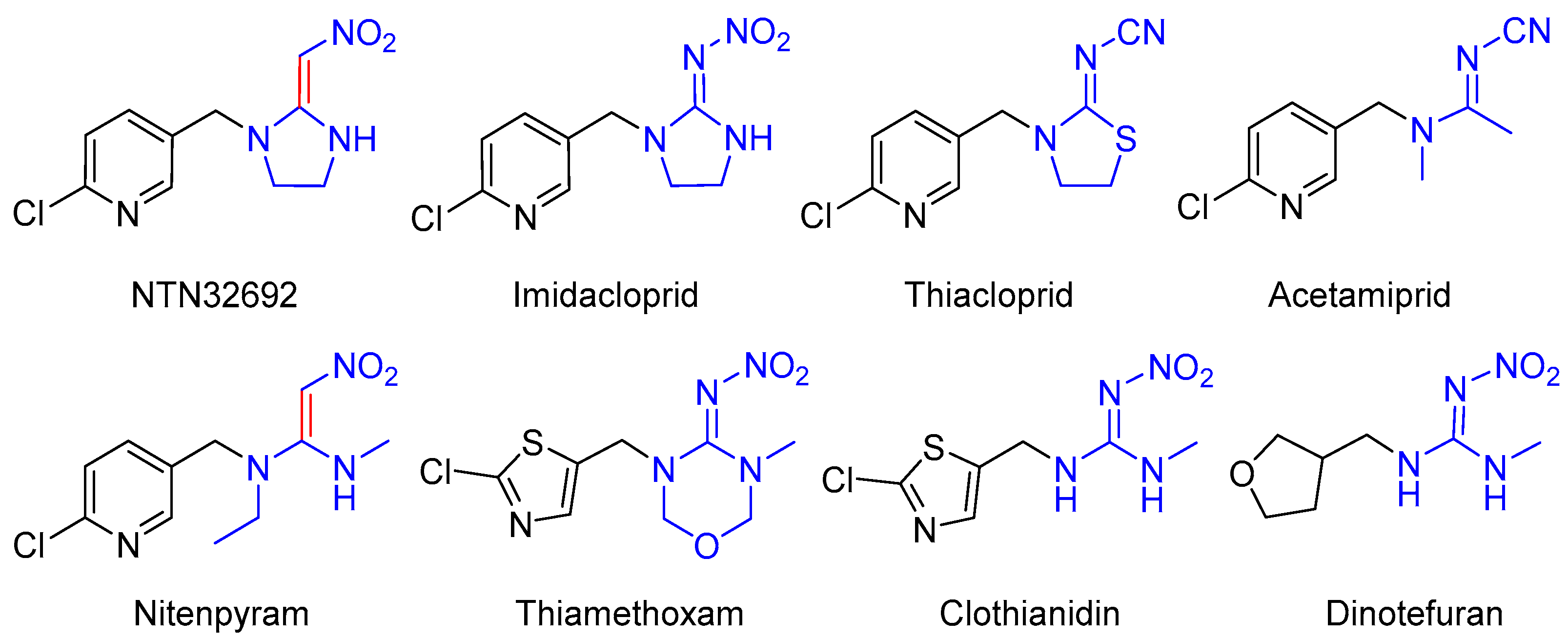
1. Introduction
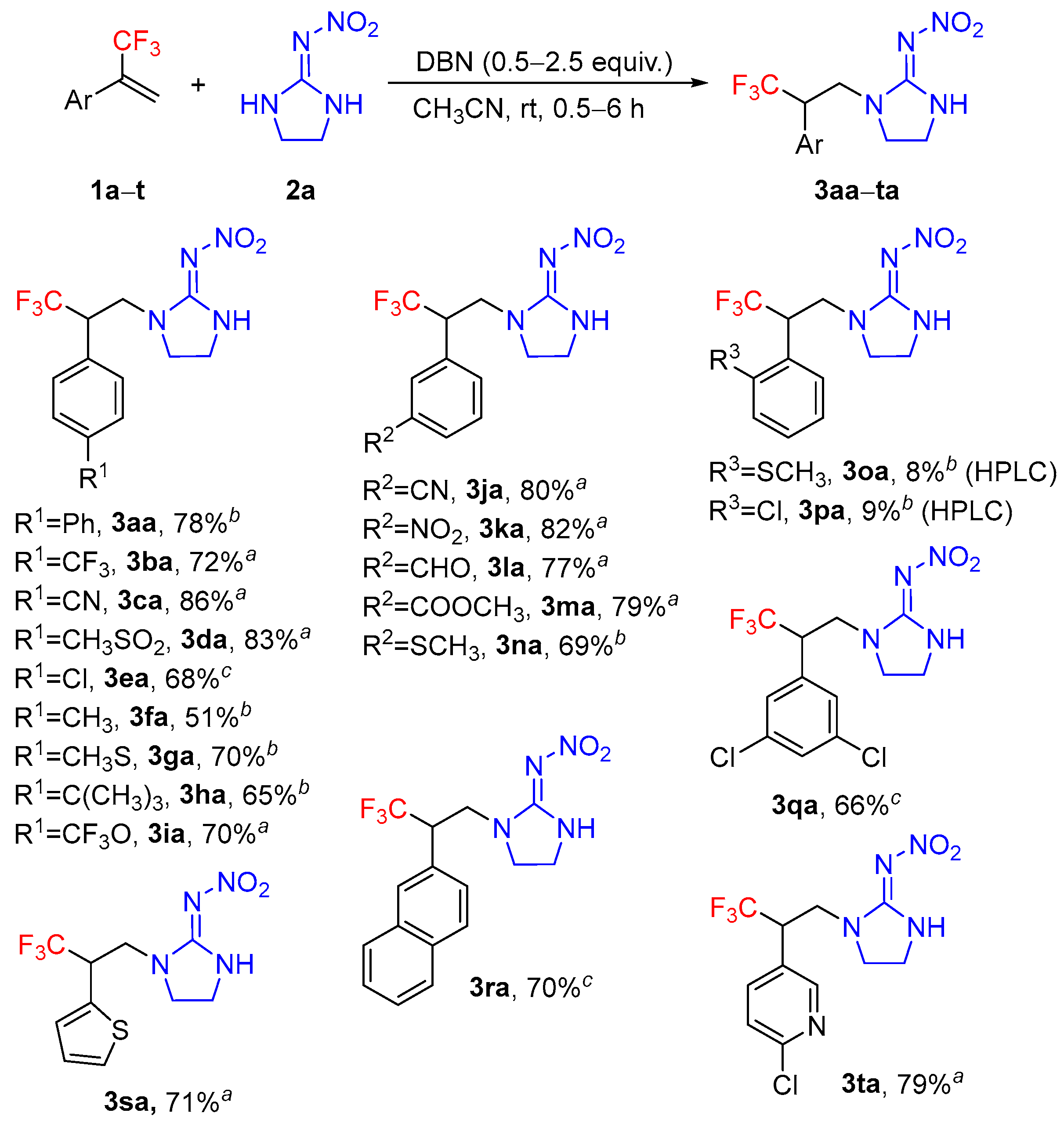
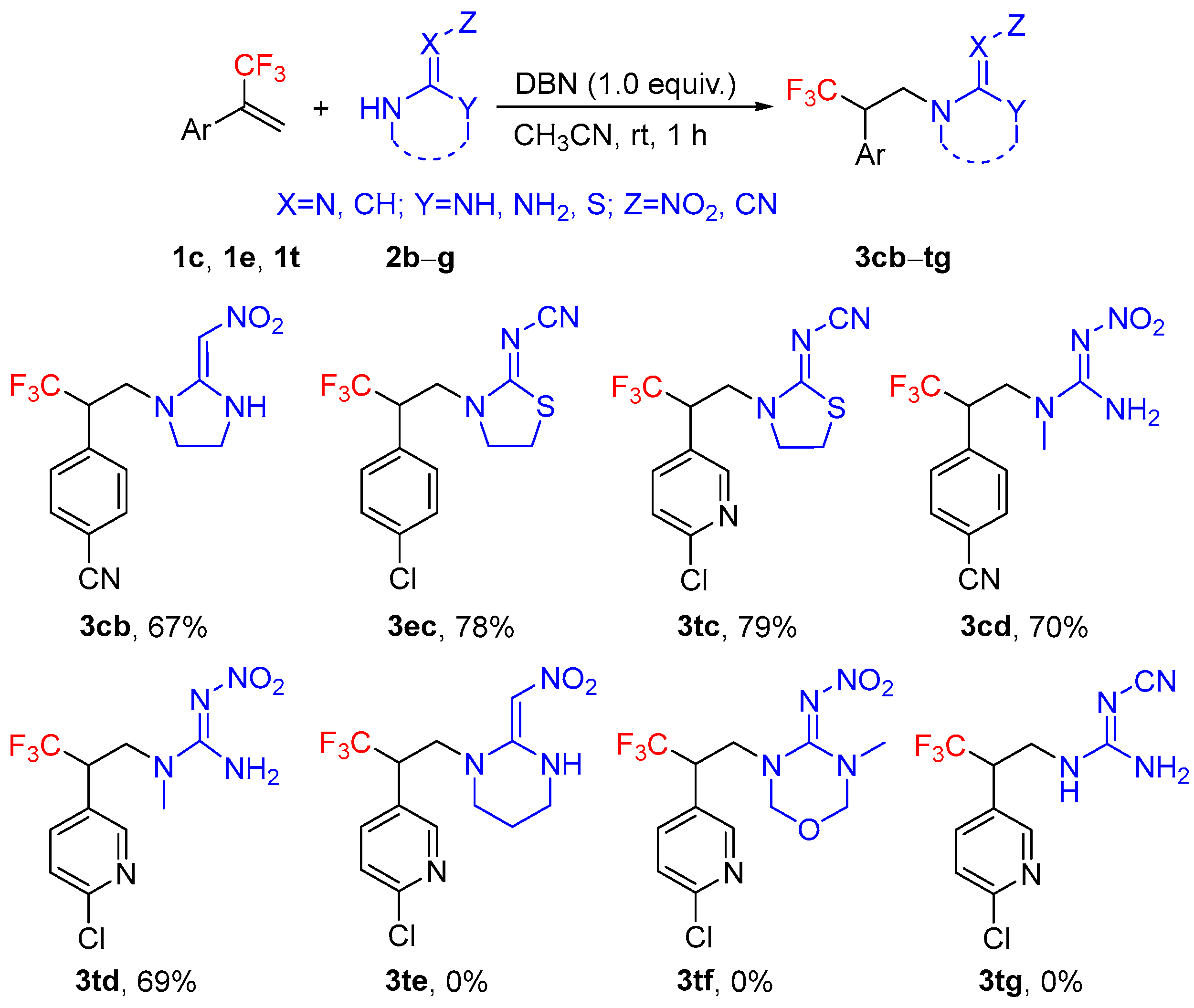
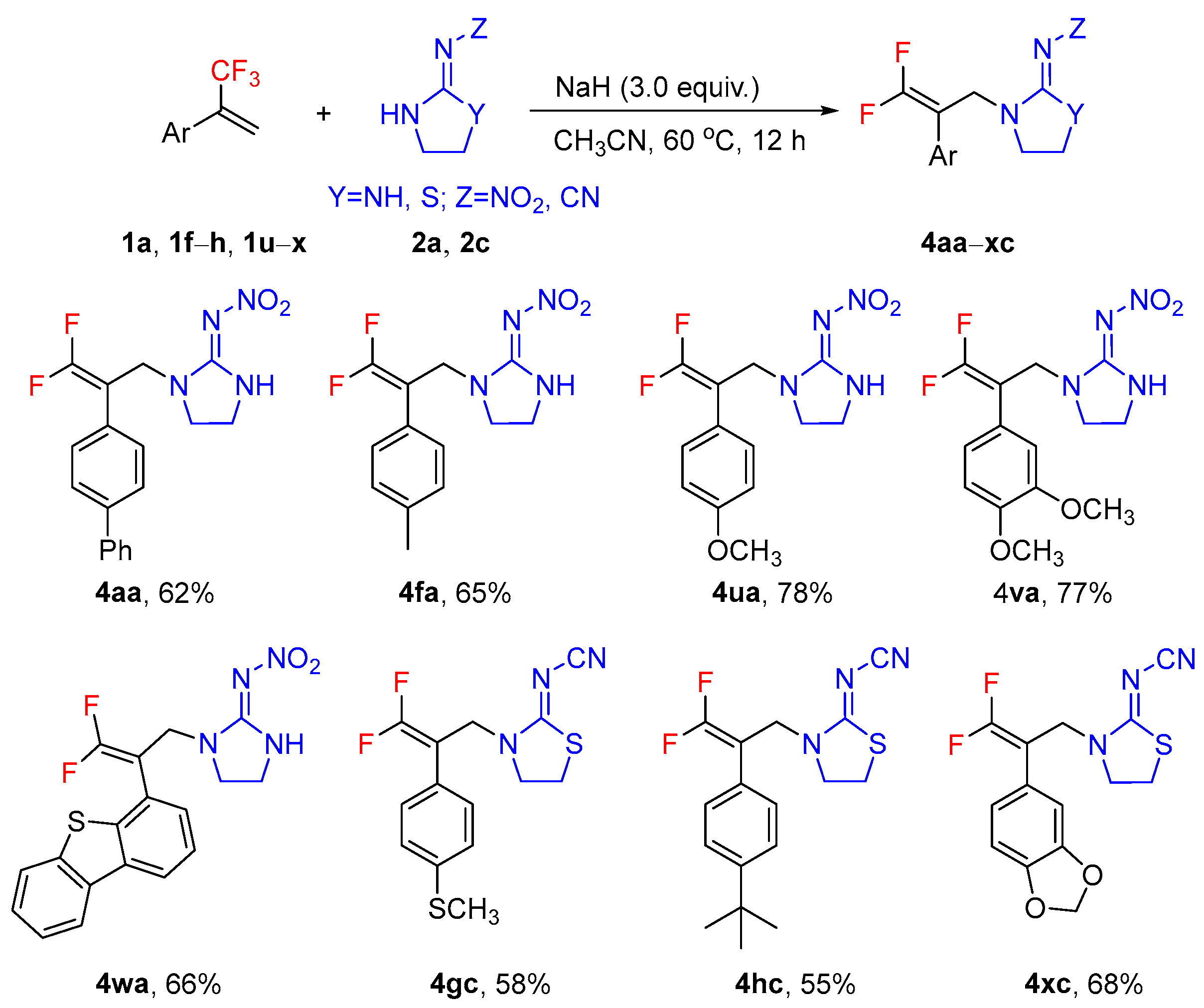
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of the Target Compounds 3aa–td
3.3. General Procedure for the Synthesis of the Target Compounds 4aa–xc
3.4. Procedure for the Synthesis of the Target Compound 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tomizawa, M.; Casida, J.E. Molecular recognition of neonicotinoid insecticides: The determinants of life or death. Acc. Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticides: Highlights of a symposium on strategic molecular designs. J. Agric. Food Chem. 2011, 59, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids—From zero to hero in insecticide chemistry. Pest. Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Ohno, I.; Tomizawa, M.; Durkin, K.A.; Naruse, Y.; Casida, J.E.; Kagabu, S. Molecular features of neonicotinoid pharmacophore variants interacting with the insect nicotinic receptor. Chem. Res. Toxicol. 2009, 22, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kagabu, S. Discovery of imidacloprid and further developments from strategic molecular designs. J. Agric. Food Chem. 2011, 59, 2887–2896. [Google Scholar] [CrossRef]
- Shao, X.S.; Liu, Z.W.; Xu, X.Y.; Li, Z.; Qian, X.H. Overall status of neonicotinoid insecticides in China: Production, application and innovation. J. Pestic. Sci. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Maienfisch, P.; Huerlimann, H.; Rindlisbacher, A.; Gsell, L.; Dettwiler, H.; Haettenschwiler, J.; Sieger, E.; Walti, M. The discovery of thiamethoxam: A second-generation neonicotinoid. Pest. Manag. Sci. 2001, 57, 165–176. [Google Scholar] [CrossRef]
- Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K.A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; et al. Flupyrimin: A novel insecticide acting at the nicotinic acetylcholine receptors. J. Agric. Food Chem. 2017, 65, 7865–7873. [Google Scholar] [CrossRef]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-Baranowska, I. A holistic study of neonicotinoids neuroactive insecticides—Properties, applications, occurrence, and analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef]
- Tian, Z.Z.; Shao, X.S.; Li, Z.; Qian, X.H.; Huang, Q.C. Synthesis, insecticidal activity, and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification. J. Agric. Food Chem. 2007, 55, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Kagabu, S.; Nishimura, K.; Naruse, Y.; Ohno, I. Insecticidal and neuroblocking potencies of variants of the thiazolidine moiety of thiacloprid and quantitative relationship study for the key neonicotinoid pharmacophore. J. Pestic. Sci. 2008, 33, 58–66. [Google Scholar] [CrossRef]
- Krumlinde, P.; Bogár, K.; Bäckvall, J. Synthesis of a neonicotinoide pesticide derivative via chemoenzymatic dynamic kinetic resolution. J. Org. Chem. 2009, 74, 7407–7410. [Google Scholar] [CrossRef] [PubMed]
- Wakita, T.; Kinoshita, K.; Yamada, E.; Yasui, N.; Kawahara, N.; Naoi, A.; Nakaya, M.; Ebihara, K.; Matsuno, H.; Kodaka, K. The discovery of dinotefuran: A novel neonicotinoid. Pest. Manag. Sci. 2003, 59, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Ohno, I.; Tomizawa, M.; Durkin, K.A.; Casida, J.E.; Kagabu, S. Neonicotinoid substituents forming a water bridge at the nicotinic acetylcholine receptor. J. Agric. Food Chem. 2009, 57, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Kumar, C.N.S.S.P.; Santhoshi, A.; Kumar, K.P.; Murthy, U.S.N.; Rao, V.J. Efficient synthesis of N-allylated 2-nitroiminoimidazolidine analogues from Baylis-Hillman bromides. Synth. Commun. 2017, 47, 131–136. [Google Scholar] [CrossRef]
- Luna-Hernández, S.A.; Bonilla-Landa, I.; Reyes-Luna, A.; Rodríguez-Hernández, A.; Cuapio-Muñoz, U.; Ibarra-Juárez, L.A.; Suarez-Mendez, G.; Barrera-Méndez, F.; Pérez-Landa, I.D.; Enríquez-Medrano, F.J.; et al. Synthesis and insecticidal evaluation of chiral neonicotinoids analogs: The laurel wilt case. Molecules 2021, 26, 4225. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Landa, I.; Cuapio-Muñoz, U.; Luna-Hernández, A.; Reyes-Luna, A.; Rodríguez-Hernández, A.; Ibarra-Juarez, A.; Suarez-Mendez, G.; Barrera-Méndez, F.; Caram-Salas, N.; Enríquez-Medrano, J.F.; et al. L-Proline as a valuable scaffold for the synthesis of novel enantiopure neonicotinoids analogs. J. Agric. Food Chem. 2021, 69, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Kagabu, S.; Aoki, E.; Ohno, I. Is pyridylmethyl group of imidacloprid replaceable with fluoroalkyl moiety as a hydrogen-bond acceptor. J. Pestic. Sci. 2007, 32, 128–130. [Google Scholar] [CrossRef]
- Ge, D.H.; Chu, X.Q. Multiple-fold C–F bond functionalization for the synthesis of (hetero)cyclic compounds: Fluorine as a detachable chemical handle. Org. Chem. Front. 2022, 9, 2013–2055. [Google Scholar] [CrossRef]
- Chen, S.J.; He, Z.Q.; Chen, G.S.; Zhao, C.; Chen, C.P.; Zhuang, Y.Y.; Chen, L.; Liu, Y.L. Synthesis of CF3-substituted alkylamines, 1,2-bisazoles, and 1,4,5,6-tetrahydro-1,2,4-triazines from newly designed tetrazole-activated trifluoromethyl alkenes. Org. Lett. 2022, 24, 9301–9305. [Google Scholar] [CrossRef]
- Li, W.Y.; Chen, X.F.; Zhou, L. Photocatalytic defluorinative three-component reaction of α-trifluoromethyl alkenes, alkenes, and sodium sulfinates: Synthesis of monofluorocyclopentenes. Org. Lett. 2022, 24, 5946–5950. [Google Scholar] [CrossRef] [PubMed]
- Claraz, A.; Allain, C.; Masson, G. Electroreductive cross-coupling of trifluoromethyl alkenes and redox active esters for the synthesis of gem-difluoroalkene. Chem. Eur. J. 2022, 28, e20210337. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, W.L.; Zuo, Z. Recent advances in the synthesis of difluorinated architectures from trifluoromethyl groups. Adv. Synth. Catal. 2022, 364, 234–267. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Ping, Y.Y.; Kong, W.Q. Synthesis of gem-difluoroalkenes via Ni-catalyzed three-component defluorinative reductive cross-coupling of organohalides, alkenes and trifluoromethyl alkenes. Chin. J. Chem. 2022, 40, 2212–2218. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.C.; Liu, W.F.; Kong, W.Q. Nickel-catalyzed defluorinative asymmetric cyclization of fluoroalkyl-substituted 1,6-enynes for the synthesis of seletracetam. Angew. Chem. Int. Ed. 2022, 61, e202212664. [Google Scholar]
- Du, D.H.; Peng, H.; He, L.; Bai, S.P.; Li, Z.H.; Teng, H.L. Synthesis of remote fluoroalkenyl ketones by photo-induced ring-opening addition of cyclic alkoxy radicals to fluorinated alkenes. Org. Biomol. Chem. 2022, 20, 9313–9318. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.B.; Qiu, K.Y.; Guo, M. Recent advance in the C–F bond functionalization of trifluoromethyl-containing compounds. Org. Chem. Front. 2021, 8, 3915–3942. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.L.; Cho, S.H. Defluorinative C−C bond-forming reaction of trifluoromethyl alkenes with gem-(diborylalkyl)lithiums. Org. Lett. 2022, 24, 2705–2710. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, C.C.; Zhou, L.; Lou, Y.X.; Yang, K.; Song, Q.L. Ni-Catalyzed radical-promoted defluoroalkylborylation of trifluorom. Org. Lett. 2022, 24, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, M.; Biremond, T.; Jubault, P.; Poisson, T. Electrochemical synthesis of gem-difluoro- and γ-fluoro allyl boronates and silans. Chem. Eur. J. 2022, 28, e202202194. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, C.; Lan, Y.; Lin, Z.Y.; Zhang, L.C.; Wang, C. Photocatalytic hydroacylation of trifluoromethyl alkenes. Chem. Commun. 2019, 55, 12691–12694. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, H.; Liu, Q.; Chen, K.; Liu, Z.Y.; Feng, C. Nickel-catalyzed anti-markovnikov hydroalkylation of trifluoromethylalkenes. ACS Catal. 2022, 12, 9410–9417. [Google Scholar] [CrossRef]
- Hu, M.; Tan, B.B.; Ge, S.Z. Enantioselective cobalt-catalyzed hydroboration of fluoroalkyl substituted alkenes to access chiral fluoroalkylboronates. J. Am. Chem. Soc. 2022, 144, 15333–15338. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, Y.Z.; Guo, Y.Y.; Liu, S.S.; Shen, X. Reductive quenching-initiated catalyst-controlled divergent alkylation of α-CF3-olefins. Chem. Catal. 2022, 2, 1380–1393. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Zheng, Y.; Yuan, C.P.; Guan, J.P.; Ye, Z.P.; Xiao, J.A.; Xiang, H.Y.; Chen, K.; Chen, X.Q.; Yang, H. Photoredox-catalyzed deoxygenation of hexafluoroacetone hydrate enables hydroxypolyfluoroalkylation of alkenes. Angew. Chem. Int. Ed. 2022, e202211035. [Google Scholar]
- Deng, Y.P.; He, J.J.; Cao, S.; Qian, X.H. Advances in cycloaddition and hydroaddition reaction of α-(trifluoromethyl)styrenes without defluorination: An alternative approach to CF3-containing compounds. Chin. Chem. Lett. 2022, 33, 2363–2371. [Google Scholar] [CrossRef]
- Bégué, J.; Bonnet-Delpon, D.; Rock, M.H. Addition of organolithium reagents to α-(trifluoromethyl)styrene: Concise synthesis of functionalised gem-difluoroalkenes. J. Chem. Soc. Perkin Trans. 1996, 1, 1409–1413. [Google Scholar] [CrossRef]
- Zeng, H.; Cai, Y.Y.; Jiang, H.F.; Zhu, C.L. Two C(sp3)−F bond activation in a CF3 group: Ipso-defluorinative amination triggered 1,3-diamination of (trifluoromethyl)alkenes with indoles, carbazoles, pyrroles, and sulfonamides. Org. Lett. 2021, 23, 66–70. [Google Scholar] [CrossRef]
- Zeng, H.; Li, H.Y.; Li, C.X.; Jiang, H.F.; Zhu, C.L. Bond energy enabled amine distinguishing strategy: Chemo-, regioselective 1,3-diamination of (trifluoromethyl)alkenes with different amines by two C(sp3)–F bond cleavages. Org. Chem. Front. 2022, 9, 1383–1388. [Google Scholar] [CrossRef]
- He, J.J.; Liu, C.; Deng, Y.P.; Zeng, Q.D.; Zhang, Y.; Liu, Y.; Zheng, P.; Cao, S. DBN-Mediated addition reaction of α-(trifluoromethyl)styrenes with diazoles, triazoles, tetrazoles, and primary, secondary, and secondary cyclic amines. Org. Lett. 2022, 24, 2299–2304. [Google Scholar] [CrossRef]
- Chen, F.L.; Xu, X.F.; He, Y.L.; Huang, G.P.; Zhu, S.L. NiH-Catalyzed migratory defluorinative olefin cross-coupling: Trifluoromethyl-substituted alkenes as acceptor olefins to form gem-difluoroalkenes. Angew. Chem. Int. Ed. 2020, 59, 5398–5402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yang, J.D.; Chen, J.P. Chemoselective catalytic hydrodefluorination of trifluoromethylalkenes towards mono-/gem-difluoroalkenes under metal-free condition. Nat. Commun. 2021, 12, 2835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Cao, X.F.; Chen, X.; Li, Z.; Xu, X.Y. The structure modification of seven-membered aza-brigded neonicotinoids in order to investigate their impact on honey bees. J. Chem. Res. 2021, 835–844. [Google Scholar] [CrossRef]

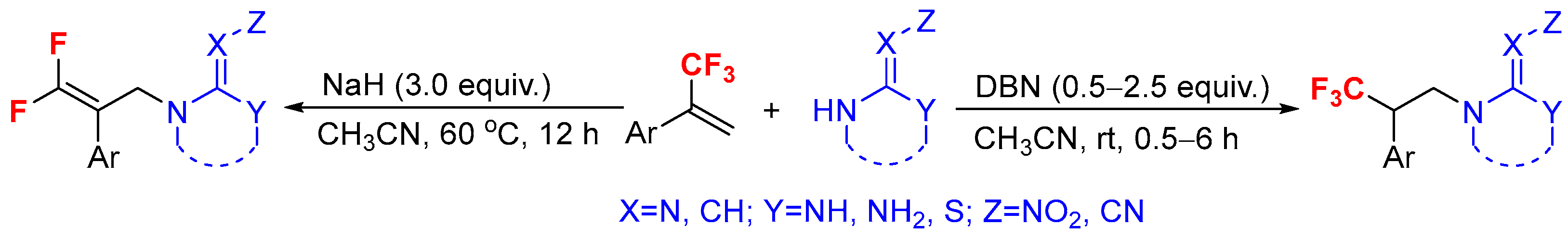


 | ||||
|---|---|---|---|---|
| Entry | Base (equiv.) | Solvent | Temp (°C) | 3aa/4aa (%) b |
| 1 | LiHMDS (3.0) | CH3CN | 25 | 0/0 |
| 2 | KOH (3.0) | CH3CN | 25 | 2/9 |
| 3 | Cs2CO3 (3.0) | CH3CN | 25 | 15/21 |
| 4 | KOtBu (3.0) | CH3CN | 25 | 27/23 |
| 5 | Et3N (3.0) | CH3CN | 25 | 0/0 |
| 6 | TMEDA (3.0) c | CH3CN | 25 | 0/0 |
| 7 | DIPEA (3.0) c | CH3CN | 25 | 0/0 |
| 8 | DMAP (3.0) c | CH3CN | 25 | 0/0 |
| 9 | DABCO (3.0) c | CH3CN | 25 | 0/0 |
| 10 | TMG (3.0) c | CH3CN | 25 | 60/0 |
| 11 | TBD (3.0) c | CH3CN | 25 | 76/0 |
| 12 | DBU (3.0) c | CH3CN | 25 | 78/0 |
| 13 | DBN (3.0) c | CH3CN | 25 | 97/0 |
| 14 | DBN (3.0) | MeOH | 25 | 2/0 |
| 15 | DBN (3.0) | toluene | 25 | 17/0 |
| 16 | DBN (3.0) | CH2Cl2 | 25 | 57/0 |
| 17 | DBN (3.0) | DMSO | 25 | 82/0 |
| 18 | DBN (3.0) | NMP | 25 | 93/0 |
| 19 | DBN (3.0) | THF | 25 | 95/0 |
| 20 | DBN (2.5) | CH3CN | 25 | 97/0 |
| 21 | DBN (2.0) | CH3CN | 25 | 82/0 |
| 22 d | NaH (3.0) | CH3CN | 25 | 0/30 |
| 23 d | NaH (3.0) | CH3CN | 40 | 0/43 |
| 24 d | NaH (3.0) | CH3CN | 60 | 0/71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Sun, Z.; Deng, Y.; Liu, Y.; Zheng, P.; Cao, S. Controllable Synthesis of Trifluoromethyl- or gem-Difluorovinyl-containing Analogues of Neonicotinoids by the Reaction of α-(Trifluoromethyl)styrenes with 2-Nitroimino-imidazolidine. Molecules 2023, 28, 3530. https://doi.org/10.3390/molecules28083530
He J, Sun Z, Deng Y, Liu Y, Zheng P, Cao S. Controllable Synthesis of Trifluoromethyl- or gem-Difluorovinyl-containing Analogues of Neonicotinoids by the Reaction of α-(Trifluoromethyl)styrenes with 2-Nitroimino-imidazolidine. Molecules. 2023; 28(8):3530. https://doi.org/10.3390/molecules28083530
Chicago/Turabian StyleHe, Jingjing, Zhudi Sun, Yupian Deng, Ying Liu, Pai Zheng, and Song Cao. 2023. "Controllable Synthesis of Trifluoromethyl- or gem-Difluorovinyl-containing Analogues of Neonicotinoids by the Reaction of α-(Trifluoromethyl)styrenes with 2-Nitroimino-imidazolidine" Molecules 28, no. 8: 3530. https://doi.org/10.3390/molecules28083530
APA StyleHe, J., Sun, Z., Deng, Y., Liu, Y., Zheng, P., & Cao, S. (2023). Controllable Synthesis of Trifluoromethyl- or gem-Difluorovinyl-containing Analogues of Neonicotinoids by the Reaction of α-(Trifluoromethyl)styrenes with 2-Nitroimino-imidazolidine. Molecules, 28(8), 3530. https://doi.org/10.3390/molecules28083530