1. Introduction
The emergence and spread of antimicrobial resistance have become a global threat to public health, increasingly reducing the options available to treat life-threatening bacterial infections. Modern medical procedures such as cardiovascular surgery, joint replacement, tooth extraction, and organ transplantation cannot be safely and successfully operated without effective antibacterial agents. However, nosocomial infections caused by vancomycin-resistant enterococci (VRE) are rapidly rising worldwide. Drug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) are routinely found outside the clinic. Therefore, there is an urgent need for the development of new antibacterial treatments with high efficacy and low resistance rates.
Pleuromutilins are semi-synthetic antibiotics derived from natural tricyclic diterpenoid pleuromutilin produced by an edible mushroom,
Pleurotus mutilus [
1,
2]. Pleuromutilins inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit at the peptidyl transferase center [
3,
4,
5]. Pathogens that are resistant to other major antibiotic classes do not have cross-resistance to pleuromutilins [
6]. Early pleuromutilin derivatives, such as tiamulin [
7,
8] and valnemulin [
8], were developed as veterinary medicine to treat respiratory and intestinal infections in farm animals. Retapamulin was the first pleuromutilin for humans, used topically to treat impetigo and skin infections caused by
Staphylococcus aureus and
Streptococcus pyogenes [
9,
10]. Recently, lefamulin was approved by the US Food and Drug Administration (FDA) and the European Commission for the treatment of community-acquired bacterial pneumonia [
10,
11]. Lefamulin marketed in oral and intravenous formulations demonstrated clinical efficacy and an acceptable safety profile, suggesting the general tolerability and low risk of side effects of pleuromutilins in other potential therapeutic areas. The pleuromutilin derivatives often bear modifications at the C14 side chain such as the incorporation of a quaternary amine or amino acid.
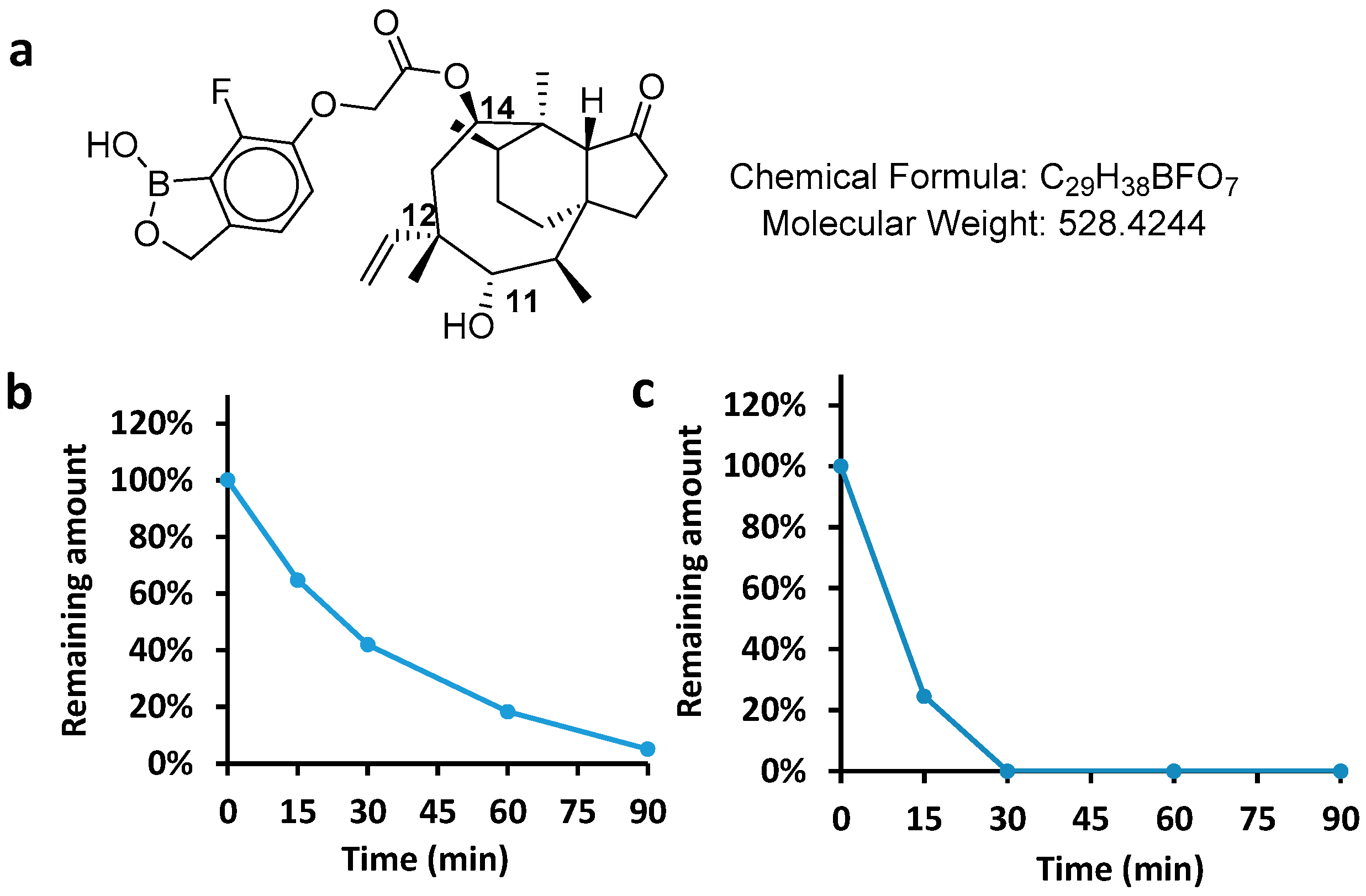
AN11251 is a benzoxaborole-modified pleuromutilin for improving the pharmacologic, physicochemical, and pharmacokinetic properties of this drug [
12,
13]. AN11251 is a pre-clinical candidate for treatments of Onchocerciasis or river blindness and lymphatic filariasis. The diseases are caused by parasitic nematodes which carry obligate endosymbiotic
Wolbachia bacteria. AN11251 can kill
Wolbachia-infected cells and adult worms in vitro, and the in vivo efficacy was established with the infection mouse model to be superior or comparable to existing antibacterial treatments. Its great efficacy and lessened risk of drug resistance warrant further consideration for therapeutics against bacterial pathogens of global and public health concerns.
We set out to investigate the PK properties and antibacterial potency of AN11251 for the development of an antibacterial agent with new indication. Here in this paper, the ADME (absorption, distribution, metabolism, and excretion) and PK (pharmacokinetics) profile were first measured, such as the PPB (plasma protein binding), metabolic stability, half-life, volume of distribution [
14], and so on. We also demonstrated the good to excellent activities of AN11251 against Gram-positive bacteria and
Mycobacterium. Finally, the human dose of AN11251 for antibacterial use was predicted using a PK/PD (pharmacokinetics and pharmacodynamics) model [
15].
3. Materials and Methods
3.1. Materials
AN11251 was provided by Calibr at Scripps Research (San Diego, CA, USA). All the other chemicals were purchased from Sigma (St Louis, MO, USA) without further purification. Rats were purchased from Charles River (Wilmington, NC, USA) and fasted overnight before dosing. Yeast Extract (OXOID, from Thermo Fisher (Waltham, MA, USA)), Sheep Blood (Yuanye Bio-Technology in Shanghai, China), Haemophilus Test Medium Base (HTM, HalingBio in Shanghai, China), Tryptic Soy Broth (TSA, BD (Franklin Lakes, NJ, USA)), Cation Adjusted Muller-Hinton broth (CAMHB, BD), 7H9 broth (BD), OADC (BD), glycerol (Sigma), Tyloxapol (Sigma), and Chocolate Agar (BD) were from commercial sources as indicated. Rifampicin (RIF, Sigma), Ciprofloxacin (CIP, MedChemExpress (South Brunswick, NJ, USA)), Alamar BlueTM Cell Viability Reagent from Thermo Fisher, and other chemical reagents were purchased.
3.2. Detection of AN11251 in Biological Samples
An aliquot of 40 µL AN11251 in DMSO solution was mixed with acetonitrile 200 µL (containing Labetalol, tolbutamide, Verapamil, dexamethasone, glyburide, and Celecoxib 100 ng/mL as internal standard for each) in a 96-well plate. Then the mixture was vortexed for 10 min at 800 rpm and centrifuged for 15 min at 3220×
g at 4 °C. An aliquot of 50 µL supernatant was transferred to another clean 96-well plate and centrifuged for 5 min at 3220×
g at 4 °C. The supernatant was directly injected for LC-MS/MS analysis. The instrument was LC-MS/MS-CS-Triple Quad 6500 plus. Chromatographic separations were carried out using ACQUITY UPLC HSS T3 LC column (1.8 μm × 2.1 × 50 mm). The mobile phases were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The analytes were eluted using mobile phase B of 20% with a linear increase to 95% over 1 min and maintained for 0.4 min, followed by a return to the starting solution mixture in 0.1 min. The flow rate was 0.6 mL/min and the injection volume was 10 µL. The retention time of AN11251 was 0.99 min, and the retention time of celecoxib was 1.02 min. Negative ion electrospray tandem mass spectrometric analysis was carried out at unit resolution with collision-induced dissociation and selective reaction monitoring [
12]. The MS analysis used SRM detection. The calculated
m/
z value of AN11251 as [M − H] was 527.2, and the found
m/
z value was 573.3 for [M − H + HCOOH]. The calculated
m/
z value of the internal standard celecoxide was 316.0, and the found
m/
z value for [M − H + HCOOH + H
2O] was 380.0.
3.3. Plasma Protein Binding
Plasma protein binding of AN11251 was determined by equilibrium dialysis using an HT-Dialysis plate (Model HTD 96b) and the dialysis membrane (molecular weight cut off 12–14 KDa) in triplicate. Human plasma (BIOMEX GmbH) was mixed from more than 6 individuals and rat plasma was mixed from more than 10 rats. AN11251 was detected at the concentration of 2 µM, and Warfarin was the control compound. The samples were matched with opposite blank buffer to obtain a final volume of 100 µL with a volume ratio of the matrix: Dialysis Buffer (100 mM sodium phosphate and 150 mM NaCl, pH 7.4) (1:1) in each well. The stop solution (acetonitrile containing tolbutamide at 200 ng/mL, labetalol at 200 ng/mL) was added to the T0 sample of AN11251 and to the control sample. The plate was sealed and shaken at 800 rpm for 10 min. Then these T0 samples were stored at 4 °C pending further processing along with other post-dialysis samples. An aliquot of 100 µL loading matrix containing AN11251 or Warfarin was transferred to the donor side and 100 µL dialysis buffer was loaded to the receiving side of the well. The plate was rotated at 100 rpm in a humidified incubator with 5% CO
2 at 37 °C for 4 h. At the end of the dialysis, all samples were further processed by protein precipitation for LC-MS/MS analysis. The %bound was calculated using the following equations:
F = Free compound concentration as determined by the calculated concentration on the buffer side of the membrane.
T = Total compound concentration as determined by the calculated concentration on the matrix side of the membrane.
3.4. Metabolic Stability in Microsomes
Microsomes from humans and rats were purchased from Corning (Shanghai, China) and Xenotech (Kansas City, KS, USA), respectively. The working solution of liver microsomes was prepared in 100 mM phosphate buffer at 0.56 mg/mL. The quench solution was cold (4 °C) acetonitrile (ACN) containing 200 ng/mL Verapamil and 200 ng/mL Imipramine as internal standards (IS). An amount of 445 µL of the microsomal working solution was transferred into the pre-warmed plates T120 (incubate time: 120 min) and NCF 120 (no co-factor NADPH regenerating system), then the plates were incubated at 37 °C for 10 min with constant shaking. An amount of 54 µL of the liver microsomes was transferred into a blank plate, and 6 µL NADPH and 180 µL quenching solution were added to the blank plate. The microsomal working solution (0.56 mg/mL) and the compound working solution (100 µM) were mixed 3 times thoroughly and 54 µL was immediately removed for the 0 min point. Then 44 µL NADPH cofactor was added to the incubation plate, and shaken at 37 °C for 120 min. At 15, 30, 60, 90, and 120 min, a 60 µL sample was transferred to the quenching solution and centrifuged at 4000 rpm for 20 min at 4 °C [
6]. Then, 80 µL supernatant was transferred to the HPLC water and shaken for 10 min before LC-MS/MS analysis.
3.5. Metabolic Stability in Hepatocytes
Cryopreserved hepatocytes from male SD rats and humans were both purchased from Bioreclamation-IVT. The human cryopreserved hepatocytes were pooled from 10 human donors with a viability of 77.9% tested by trypan blue. The rats’ cryopreserved hepatocytes were pooled from 12 male SD (Sprague Dawley) rats, and the cell viability was 80.9%. The hepatocytes were diluted to 0.5 × 10
6/mL cell suspension with pre-warmed incubation medium (ultra-pure water). For the T0 samples, the hepatocytes and AN11251 stock solution were mixed, and 25 µL of each sample was immediately transferred into a well containing 125 µL ice-cold stop solution. The hepatocytes were incubated with the AN11251 solution (20 µg/mL) in Williams’ Medium E at 37°C in a 95% humidified incubator at 5% CO
2 to start the reactions with constant shaking at about 600 rpm. At 15, 30, 60, and 90 min, the samples were mixed and then 25 μL of each sample was transferred at each time point to a well containing 125 μL of ice-cold stop solution (acetonitrile containing 200 ng/mL tolbutamide and labetalol as internal standards) followed by mixing. The samples were vortexed on a plate shaker at 500 rpm for 10 min, and centrifuged at 3220×
g for 20 min at 4 °C. The supernatants were transferred to ultra-pure water and analyzed by LC-MS/MS. The equation of first-order kinetics was used to calculate t
1/2 and CL
int:
3.6. Pharmacokinetic Studies in Rats
The pharmacokinetic studies of AN11251 were performed in the formulation of PEG 400:PG:water = 55:25:20 as a clear solution. Animal husbandry procedures in this study were in compliance with the Animal Welfare Act, the National Research Council Guide for the Care and Use of Laboratory Animals (8th edition), and National Laboratory Animal Management Regulations (2017). The animals fasted overnight before administration. Each group had three male SD rats (Charles River Laboratories, bodyweight between 240 g and 250 g). For the intravenous (IV) group, a dose of 3 mg/kg of AN11251 was administered to the male rats by bolus infection. Blood samples were collected at the time points of 0.083 h, 0.25 h, 1 h, 2 h, 4 h, 8 h, 24 h, and 28 h. The oral (PO) group was dosed with AN11251 at 10 mg/kg in the same formulation as the IV group. Blood samples were collected at the time points of 0.25 h, 0.5 h, 1 h, 2 h, 4 h, 8 h, 24 h, and 28 h after dosing [
13]. All the blood samples were centrifugated at 1000×
g for 15 min and stored at a −80 °C refrigerator for LC-MS/MS analysis.
3.7. Pharmacokinetic Analysis
All the pharmacokinetic parameters were calculated from the drug plasma concentration–time data using Phoenix WinNonlin 6.3 and a non-compartmental model. The IV parameters included elimination half-time (T
1/2), the volume of distribution steady state (Vdss), system clearance (Cl), mean residence time (MRT), the area under the plasma concentration–time curve from time 0 to infinity (AUC
0-inf), and the AUC from time last extrapolated to infinity given as a percentage of AUC
0-inf (AUC
extra). The PO parameters included the maximum concentration in plasma (C
max), the time of maximum concentration in plasma (t
max), and bioavailability (F). All the pharmacokinetic parameters were established using the mean plasma concentration data. If the adjusted rsq (linear regression coefficient of the concentration value on the terminal phase) was less than 0.9, T1/2 might not be accurately estimated. If the % AUC
Extra > 20%, AUC
0-inf, Cl, MRT
0-inf, and Vd
ss might not be accurately estimated. The oral bioavailability F in rats was determined using the following equation:
3.8. Bacterial Cell Culture
A total of twenty-four bacterial strains were chosen for testing in this study. The Gram-positive bacterial strains were Staphylococcus aureus ATCC 29213 (MSSA), S. aureus ATCC 700698 (MRSA), S. aureus SAU-0167 (MRSA, resistant to β-lactams, aminoglycosides, fluoroquinolones, and nitrofurantoin), S. aureus SAU-9922 (MRSA, resistant to β-lactams and fluoroquinolones), Staphylococcus epidermidis SEP-1024 (resistant to Aztreonam), Streptococcus pneumoniae SPN-1169 (resistant to Aztreonam, Cefoxitin, and Amikacin, etc.), Streptococcus pyogenes SPY-0253 (resistant to Aztreonam and Amikacin), Enterococcus faecium EFA-0221 (VRE, resistant to β-lactams, aminoglycosides, fluoroquinolones, and nitrofurantoin), Enterococcus faecalis EFA-9212 (resistant to some β-lactams including cephalosporins, aminoglycosides, and nitrofurantoin), and E. faecalis ATCC 29212. The strains with an ATCC code were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA) and the others were stored in HD Biosciences (Shanghai, China). Gram-negative strains were Haemophilus influenzae ATCC 49247, Acinetobacter baumannii ATCC BAA-1605, Stenotrophomonas maltophilia STM-0001 (resistant to β-lactams, aminoglycosides, and nitrofurantoin, from HD Bio), Klebsiella pneumoniae ATCC BAA-1705, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, and Acinetobacter baumannii ATCC 17978. Mycobacterial strains used were Mycobacterium bovis BCG Pasteur 1173P2, Mycolicibacterium smegmatis mc2155 ATCC 700084, Mycobacterium tuberculosis H37Rv ATCC 27294 (in Beijing Chest Hospital), Mycobacterium abscessus-GDI, and three clinical isolates: M. abscessus C16, C28, C58 (Beijing Children’s Hospital).
Antibacterial susceptibility tests were performed according to the Performance Standards for Antimicrobial Susceptibility Testing (M100, 29th Edition, 2019) published by the Clinical Laboratory Standards Institute (CLSI). The bacterial strains were first recovered from a −80 °C frozen stock on appropriate medium agar plates. Specifically, blood TSB agar plates were used for Streptococcus spp. and Enterococcus spp., chocolate agar plates for H. influenzae, and TSB agar plates for other strains. Plates were incubated at 37 °C with (H. influenzae, Streptococcus spp. and Enterococcus spp.) or without 5% CO2 for 24 h. Mycobacterial strains were recovered by thawing the frozen stock with fresh 7H9 broth medium supplemented with 10% OADC, 0.2% glycerol, and 0.05% tyloxapol and then shaking at 100 rpm at 37 °C.
3.9. Minimum Inhibitory Concentration (MIC) and Cytotoxicity Determination
Assay plates were prepared by dispensing stock solution of AN11251 or positive controls in a two-fold serial dilution starting from 32 µg/mL into 96-well cell plates. For anti-mycobacterial tests, the starting concentration of AN11251 was 20 µg/mL. The final volume in each well was made up to 2 µL with DMSO. CAMHB medium for Gram bacterial strains was prepared and sterilized. Inoculation suspension was made by resuspending fresh colonies in sterile saline (0.9% NaCl) and diluting to OD600 about 0.15. Then the suspension was further diluted 1:300 with each bacterial optimum medium to achieve ~2 × 105 CFU/mL. Specifically, H. influenzae was with HTM broth, Streptococcus spp. and Enterococcus spp. with CAMHB supplemented with sheep blood, and CAMHB broth for other bacterial strains. An amount of 98 µL of each diluted bacterial inoculation was dispensed to the assay plates that had the compound. Medium containing 2% DMSO served as a negative control, while media containing Rifampicin or Ciprofloxacin served as positive controls. The plates were incubated for 20 h at 37 °C. The lowest concentration of the essential inhibited any increases visible to the naked eye, which were noted as MIC. Determination of antimycobacterial MIC with Microplate Alamar Blue Assay (MABA) followed the Mycobacteria Protocols (2015, 3rd Ed. T Parish). Recovered mycobacterial strains were transferred to fresh 7H9 broth medium and grown to the log phase OD600 ~0.6–0.8. The mycobacterial culture was further diluted with 7H9 broth medium until OD600 0.01. An amount of 200 µL diluted inoculation was dispensed into each well of the 96-well assay plates. The 7H9 medium with 1% DMSO served as a negative control and Rifampicin and Isoniazid as the positive controls. Assay plates with M. smegmatis and M. abscessus were incubated at 37 °C for 72 h, while M. bovis BCG and M. tuberculosis H37Rv were incubated for 7 days. Afterward, 50 µL Alamar Blue reagent prepared with the commercial stock and 10% Tween 80 in a volume ratio of 1:1 was added to each well, followed by 20 h of incubation at 37 °C. Fluorescence at excitation 544 nm and emission 590 nm was measured. The cytotoxicity of AN11251 was assayed against the Vero6 cell line by a 10-point series with 2-fold dilution of the compound in the range of 40 to 0.078 µM in triplicate in a 96-well microplate. The luminescence generated with CellTiter-Glo kit (Promega) was recorded after 48 h incubation and the cytotoxic concentration (CC50) was determined. Data were analyzed with GraphPad Prism 9.5.
3.10. Prediction of the PK Parameters in Humans
The human systemic clearance and volume of distribution were predicted through allometric scaling reported by Johnson & Johnson [
14]. Allometric scaling is empirically based on the similarity of anatomy and physiology in mammals. The body weight, brain weight, and maximum lifespan of different animals all influence the animal’s distribution and elimination. The rat is the preferred species for evaluating the PK profile of drug candidates in early stages. The systemic clearance and the volume of distribution of humans have a strong relationship with those of rats. Based on the investigation of a large and diverse set of drugs, a fixed exponent allometric scaling method could be used to predict human in vivo PK parameters.
We used two approaches to predict the human PK parameters from rat in vivo PK data. Both strategies were consistent to the standard allometric equation:
where Y is the human PK parameter such as systemic clearance and volume of distribution. W is the body weight, a is the allometric coefficient, and b is the allometric exponent.
The first method uses the log–log regression technique to express the relationship between human and rat PK parameters as follows:
where Y is the PK parameter, such as systemic clearance and volume of distribution, α is the slope, and β is the intercept.
The second method assumes the allometric coefficient and exponent are fixed values, and the PK values scaled from rats to human can be expressed as follows:
where Y is the PK parameter systemic clearance and volume of distribution. W
human, the human body weight, is assumed to be 70 kg. W
rat, the rat body weight, is assumed to be 0.25 kg in the calculations. The b allometric exponents were between 0–1 after calculation.
3.11. Systemic Clearance
Using the first method, the unit of the experimental clearance is mL/min/kg. There is a reasonable relationship between human and rat systemic clearance values:
Using the second method, the exponent is defined as 0.67, a human body weight is 70 kg, and a rat body weight is 0.25 kg. The equation is derived as follows:
This equation can be expressed as follows after calculation:
Based on the analysis results, the two methods are equivalent. Thus, we used the following simplified allometric scaling equation to predict human systemic clearance:
3.12. Volume of Distribution
Using the first method, the unit of volume of distribution was L/kg. There was a good correlation between human volume of distribution and rat:
Using the second method, the exponent was fixed to be 0.93, the human body weight was 70 kg, and the rat body weight was 0.25 kg. The equation could be derived as follows:
The equation could be expressed as follows after calculation:
Based on the calculation results, the two prediction methods are equivalent. Thus, we used the simplified allometric scaling equation to predict human volume of distribution:
3.13. Prediction the Human Dose Using PK/PD Modeling
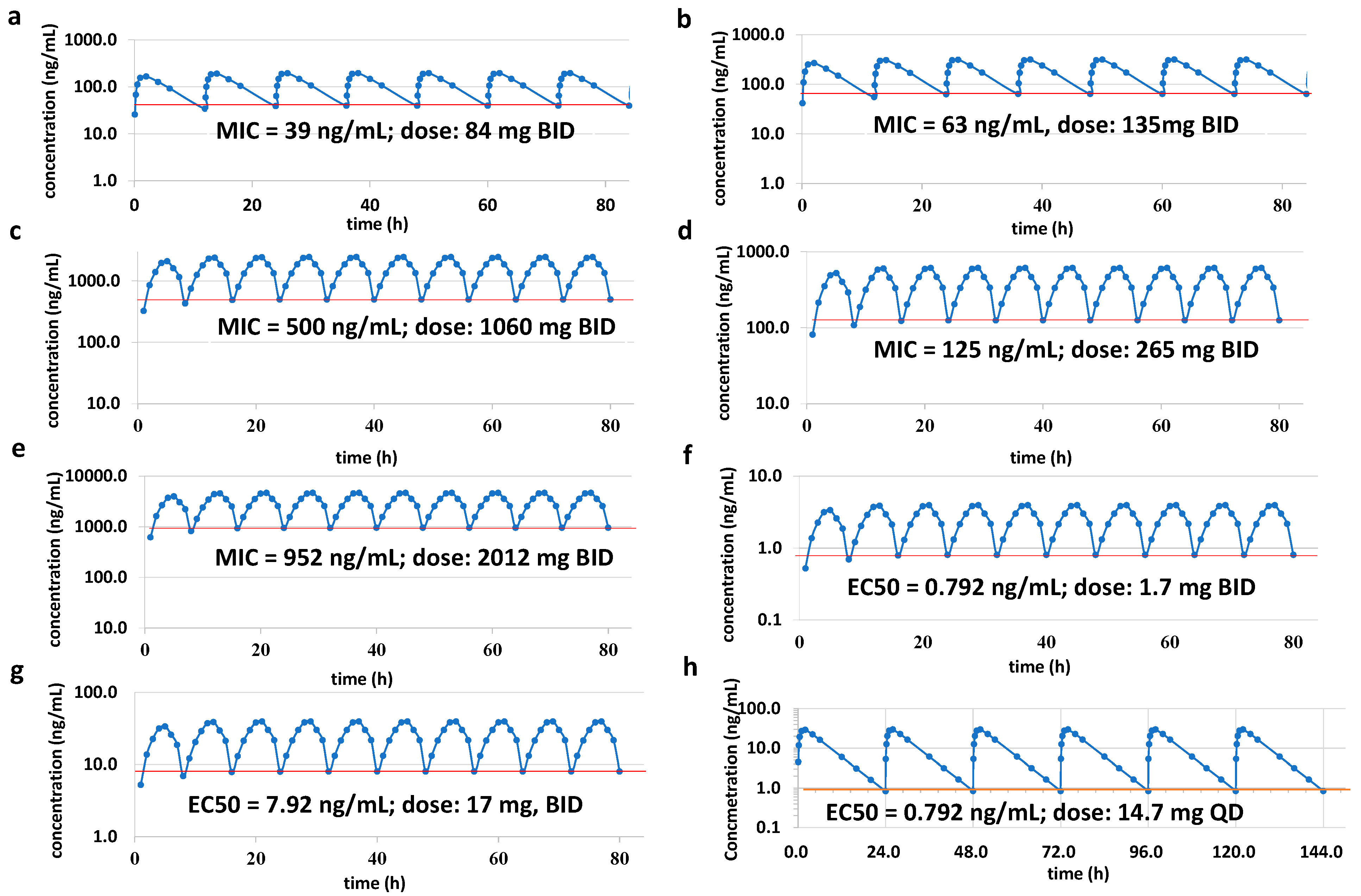
The human dose for efficacy was predicted by one-compartment PK/PD modeling [
15]. The dosing frequency was twice a day (BID) and lasted for four days. The PK/PD modeling predicts the dose using the equation as follows:
Ka: absorption rate constant
F: bioavailability
X0: dose
kel: elimination rate constant
V: apparent distribution volume
At the first day 12 h, the equation is as follows:
4. Conclusions
In conclusion, this work provides a further study on the potency and PK profile of AN11251. Because of the incorporated benzoxaborole at the C14 of pleuromutilin, AN11251 exhibits a good balance on the ADME properties and antibacterial activities [
11]. To our delight, AN11251 has a moderate human hepatic (64.8 µL/min/10
6) and high plasma protein binding (PPB: 0.976), which would generate a good systemic clearance. The high lipophilicity (log P = 4.5) is beneficial to the good permeability (Papp = 14.1 × 10
−6 cm/s) and bioavailability. The high plasma protein binding helps in reducing in vivo metabolism. When AN11251 was administrated to rats by the intravenous route (3 mg/kg), we observed moderate to good clearance (CL = 19.8 mL/min/kg) and good exposure (AUC
0–24 = 2550 ng. h/mL). Its primary metabolite results from the hydroxylation at C (2) site. There have been plenty of modifications for improving the PK properties of pleuromutilin. Substituting C14 with leucine and the incorporation of the carbonyl group at C3 were shown to increase the half-life, but the AUC was very low in the mice PK profile, which made that compound unlikely to be a good anti-TB drug candidate [
23]. Modification of pleuromutilin with pyridine-thiazole at the C14 site could generate a compound with promising anti-MRSA isolate activity, but may result in a poor PK profile as well, such as high systemic clearance and low exposure. Heteroaromatic substitution in the C14 side chain of pleuromutilin presents different in vitro and in vivo properties, indicating that minor structure differences in the substituents on the C14 would have considerable influence on the PK profile [
24].
AN11251 has been described as an anti-
Wolbachia drug candidate [
13], with a low MIC from 0.79 µg/mL to 7.9 µg/mL. Through PK/PD modeling, the human dose is predicted to be only 1.7 mg twice a day, or 14.7 mg once a day based on the EC50 of the
Wolbachia-infected LWD1 cells. The SAR study demonstrated that the optimal activity was obtained by linking benzoxaborole to the pleuromutilin core, which impacts the potency and PK properties. Secondly, leucine modification of pleuromutilin was shown to be effective against replicating and non-replicating TB bacteria [
23]. Here, AN11251 shows anti-TB activity with MIC at 0.952 µg/mL, leading to the human dose predicted to be 2012 mg twice a day. Furthermore, pyridine-thiazole-pleuromutilin has been evaluated against MRSA with MIC from 4 µg/mL to 64 µg/mL [
25]. The anti-Gram-positive activity of AN11251 was also investigated and shown to be good to excellent. The MIC value ranges from less than 0.039 µg/mL to 0.5 µg/mL against a broad spectrum of G+ bacterial species, and the effective human dose could thus be from 84 mg to 2012 mg twice a day. All the results demonstrated that AN11251 has a good PK profile and has the potential to be developed as a preclinical anti-
Wolbachia and anti-Gram-positive candidate.