Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. C. verum EO Yield and Chemical Composition
2.2. Determination of Significant Factors by Plackett–Burman Design
2.3. Biological Activity Determination
- Anti-candida Activity
- 2.
- Antibacterial Activity
- 3.
- Anti-leishmanial activity
- 4.
- Cytotoxicity against Raw 264.7
2.4. Invitro Release of C. verum EO
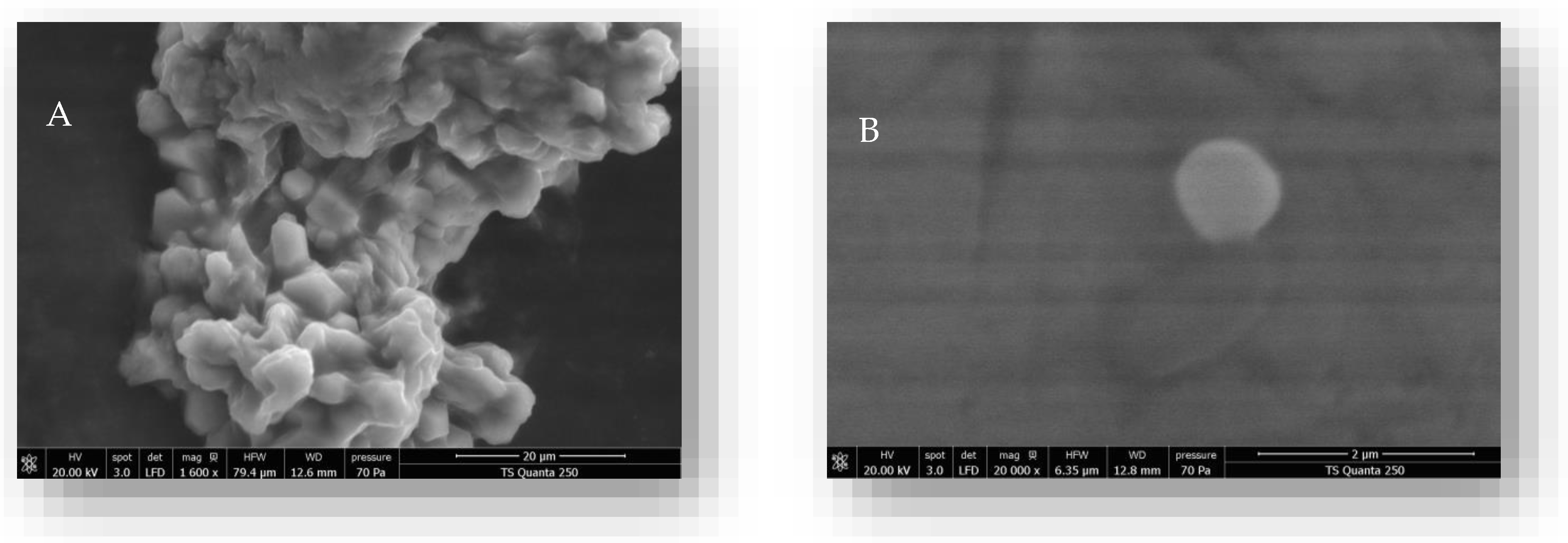
2.5. Particle Size and Zeta Potential Determination
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
3. Conclusions
4. Material and Methods
4.1. Materials
4.2. Essential Oil Extraction
4.3. GC-MS Analysis of C. verum EO
4.4. Preparation of C. verum-EO/CN-NPs
4.5. Experimental Design
4.5.1. Plackett–Burman Screening Design Methodology
4.5.2. Box–Behnken Design (BBD) Response Surface Methodology (RSM)
4.5.3. Multi-Response Optimization Using Desirability
4.6. Anti-Candida Assay
4.7. Antibacterial Activity
Well Diffusion Method
- 5.
- Minimum inhibitory concentration determination (MIC):
4.8. Leishmaniacidal Activity
4.9. Cytotoxicity Assay and Selectivity Index
4.10. Determination of the Encapsulation Efficiency (EE%)
4.11. In-Vitro Release Studies of C. verum EO
4.12. Characterization of C. verum EO/CN-NPs
4.12.1. Fourier TransformInfrared Spectroscopy (FTIR)
4.12.2. Zeta Potential and Particle Size Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruhnke, M. Antifungal stewardship in invasive Candida infections. Clin. Microbiol. Infect. 2014, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- Essid, R.; Hammami, M.; Gharbi, D.; Karkouch, I.; Ben Hamouda, T.; Elkahoui, S.; Limam, F.; Tabbene, O. Antifungal mechanism of the combination of Cinnamomum verum and Pelargonium graveolens essential oils with fluconazole against pathogenic Candida strains. Appl. Microbiol. Biotechnol. 2017, 101, 6993–7006. [Google Scholar] [CrossRef] [PubMed]
- Alasaad, S. War diseases revealed by the social media: Massive leishmaniasis outbreak in the Syrian Spring. Parasites Vectors 2013, 6, 94. [Google Scholar] [CrossRef]
- de Oliveira, D.P.; de Almeida, L.; Marques, M.J.; de Carvalho, R.R.; Dias, A.L.T.; da Silva, G.A.; de Pádua, R.M.; Braga, F.C.; da Silva, M.A. Exploring the bioactivity potential of Leonotis nepetifolia: Phytochemical composition, antimicrobial and antileishmanial activities of extracts from different anatomical parts. Nat. Prod. Res. 2019, 35, 3120–3125. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Li, Y.; Kam, S.-L.; Wang, H.; Wong, E.Y.L.; Ooi, V.E.C. Antimicrobial Activities of Cinnamon Oil and Cinnamaldehyde from the Chinese Medicinal Herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2019, 148, 1280–1289. [Google Scholar] [CrossRef]
- Al-Dbass, A.M.; Al Daihan, S.; Al-Nasser, A.A.; Al-Suhaibani, L.S.; Almusallam, J.; Alnwisser, B.I.; Saloum, S.; Alotaibi, R.S.; Alessa, L.A.; Bhat, R.S. Biogenic Silver Nanoparticles from Two Varieties of Agaricus bisporus and Their Antibacterial Activity. Molecules 2022, 27, 7656. [Google Scholar] [CrossRef]
- Alduraihem, N.S.; Bhat, R.S.; Al-Zahrani, S.A.; Elnagar, D.M.; Alobaid, H.M.; Daghestani, M.H. Anticancer and Antimicrobial Activity of Silver Nanoparticles Synthesized from Pods of Acacia nilotica. Processes 2023, 11, 301. [Google Scholar] [CrossRef]
- Keute, M.; Demirezen, M.; Graf, A.; Mueller, N.G.; Zaehle, T. No modulation of pupil size and event-related pupil response by transcutaneous auricularrvagus nerve stimulation (taVNS). Sci. Rep. 2019, 9, 11452. [Google Scholar] [CrossRef]
- Ruiz, Y.P.M.; Campos, L.A.D.A.; Agreles, M.A.A.; Galembeck, A.; Cavalcanti, I.M.F. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics 2023, 12, 104. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.; Gavlighi, H.A.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beefpatties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef]
- Aktug, S.L.; Durdu, S.; Kalkan, S.; Cavusoglu, K.; Usta, M. In vitro biological and antimicrobial properties of chitosan-basedbioceramiccoatings on zirconium. Sci. Rep. 2021, 11, 15104. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.D.S.; Nunes, J.B.; da Silva, B.O.; Ferraz, E.R.; Fernandes, A.; Felzenszwalb, I.; Amaral, A.C.F.; Roullin, V.G.; Falcão, D.Q. PLGA nanoparticles optimized by Box-Behnken for efficient encapsulation of therapeutic Cymbopogoncitratus essential oil. Colloids Surf. B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef]
- Attallah, O.A.; Shetta, A.; Elshishiny, F.; Mamdouh, W. Essential oil loaded pectin/chitosan nanoparticles preparation and optimization via Box–Behnken design against MCF-7 breast cancer celllines. RSC Adv. 2020, 10, 8703–8708. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Ramadhani, A.M.; Erwa, I.Y.; Ishag, O.A.O.; Saeed, M.B. Phytochemical Screening, Chemical Composition and Antimicrobial Activity of Cinnamon verum Bark. Int. Res. J. Pure Appl. Chem. 2020, 21, 36–43. [Google Scholar] [CrossRef]
- Ainane, T.; Khammour, F.; Merghoub, N.; Elabboubi, M.; Charaf, S.; Ainane, A.; Elkouali, M.H.; Talbi, M.; Abba, E.H.; Cherroud, S. Cosmetic bio-productbased on cinnamon essential oil “Cinnamomum verum” for the treatment of mycoses: Preparation, chemical analysis and antimicrobial activity. MOJ Toxicol. 2019, 5, 5–8. [Google Scholar] [CrossRef]
- Valizadeh, S.; Katiraee, F.; Mahmoudi, R.; Fakheri, T.; Mardani, K. Biological properties of Cinnamomum zeylanicum essential oil: Phytochemical component, antioxidant and antimicrobial activities. Int. J. Food Saf. Nutr. 2015, 6, 174–184. [Google Scholar]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Yue, S. Optimization of preparation conditions of eucalyptus essential oil microcapsules by response surface methodology. J. Food Process. Preserv. 2019, 43, e14188. [Google Scholar] [CrossRef]
- Bangun, H.; Tandiono, S.; Arianto, A. Preparation and evaluation of chitosan-tripolyphosphate nanoparticles sus-pension as an antibacterial agent. J. Appl. Pharm. Sci. 2018, 8, 147–156. [Google Scholar]
- Xu, Y.; Du, Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, G.K.; Maia, F.C.; De Oliveira, T.R.; De Feiria, S.N.B.; Joia, F.; Barbosa, J.P.; Boni, G.C.; Sardi, J.D.C.O.; Rosalen, P.L.; Höfling, J.F. Effect of Cinnamomum verum Leaf Essential Oil on Virulence Factors of Candida Species and Determination of the In-Vivo Toxicity with Galleria mellonella model. Memórias Do Inst. Oswaldo Cruz. 2020, 115, e200349. [Google Scholar] [CrossRef]
- De Castro, R.D.; Lima, E.O. Anti-Candida activity and chemical composition of Cinnamomum zeylanicum blume essential oil. Braz. Arch. Biol. Technol. 2013, 56, 749–755. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Ribeiro, W.L.C.; Macedo, I.T.F.; dos Santos, J.M.L.; de Oliveira, E.F.; Camurça-Vasconcelos, A.L.F.; de Paula, H.C.B.; Bevilaqua, C.M.L. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemon chuscontortus. Exp. Parasitol. 2013, 135, 24–29. [Google Scholar] [CrossRef]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D.; Molina, J.; Souto, A.P.; Fangueiro, R.; Zille, A. Properties and controlled release of chitosan microencapsulated limonene oil. Rev. Bras. Farm. 2014, 24, 691–698. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilledpork. LWT-Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticlesloaded with nettle essential oil. J. Food Meas. Charact. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT—Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive cons-tituents: A novelstrategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Nouri, A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef]
- Nagy, M.; Szemán-Nagy, G.; Kiss, A.; Nagy, Z.L.; Tálas, L.; Rácz, D.; Majoros, L.; Tóth, Z.; Szigeti, Z.M.; Pócsi, I.; et al. Antifungal Activity of an Original Amino-Isocyanon aphthalene (ICAN) Compound Family: Promising Broad Spectrum Antifungals. Molecules 2020, 25, 903. [Google Scholar] [CrossRef]
- Barbarossa, A.; Sblano, S.; Rosato, A.; Carrieri, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Synergistic Action of Cinnamomum verum Essential Oil with Sertraline. Antibiotics 2022, 11, 1617. [Google Scholar] [CrossRef]
- Gupta, C.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251. [Google Scholar]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Raafat, D.; Bargen, K.; Haas, A.; Sahl, H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Ghanbariasad, A.; Valizadeh, A.; Ghadimi, S.N.; Fereidouni, Z.; Osanloo, M. Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. J. Drug Deliv. Sci. Technol. 2021, 63, 102436. [Google Scholar] [CrossRef]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, I.; Anjum, S. Applications of Nanomaterials in Leishmaniasis: A Focus on Recent Advances and Challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.S.; Sánchez, A.; Alonso, M.J. Chitosan Nanoparticles as New Ocular Drug Delivery Systems: In Vitro Stability, in Vivo Fate, and Cellular Toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Lu, F.; Deng, J.; Zhang, J.; Fang, P.; Zhou, S.F. A study on the hemocompatibility of dendronizedchitosan derivatives in red blood cells. Drug Des. Dev. Ther. 2015, ume 9, 2635–2645. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Castro, J.I.; Valencia Llano, C.H.; Navia Porras, D.P.; Delgado Ospina, J.; Valencia Zapata, M.E.; Herminsul Mina Hernandez, J.; Chaur, M.N. Synthesis, characterization, and histological evaluation of chitosan-Ruta graveolens essential oil films. Molecules 2020, 25, 1688. [Google Scholar] [CrossRef]
- Quihui-Cota, L.; Morales-Figueroa, G.G.; Valbuena-Gregorio, E.; Campos-García, J.C.; Silva-Beltrán, N.P.; López-Mata, M.A. Membrana de Quitosano con Aceites Esenciales de Romero y Árbol de Té: Potencial como Biomaterial. Rev. Mex. Ing. Biomédica 2017, 38, 255–264. [Google Scholar] [CrossRef]
- Guidotti-Takeuchi, M.; Ribeiro, L.N.D.M.; dos Santos, F.A.L.; Rossi, D.A.; Della Lucia, F.; de Melo, R.T. Essential Oil-Based Nanoparticles as Antimicrobial Agents in the Food Industry. Microorganisms 2022, 10, 1504. [Google Scholar] [CrossRef]
- Xue, T.; Wang, W.; Yang, Z.; Wang, F.; Yang, L.; Li, J.; Meng, Z.; Gu, R.; Gan, H. Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS. Int. J. Mol. Sci. 2022, 23, 8810. [Google Scholar] [CrossRef]
- Zaki, S.S.O.; Ibrahim, M.N.; Katas, H. Particle Size Affects Concentration-Dependent Cytotoxicity of Chitosan Nanoparticlestowards Mouse Hematopoietic Stem Cells. J. Nanotechnol. 2015, 2015, 919658. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, H.; Li, D.; Xiong, X.; Tan, M.; Huang, D.; Guo, X.; Zhang, X.; Yan, H. Self-assembled nanoparticles based on folic acid modified carboxymethyl chitosan conjugated with targeting antibody. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 446–453. [Google Scholar] [CrossRef]
- Qi, L.-F.; Xu, Z.-R.; Li, Y.; Jiang, X.; Han, X.-Y. In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J. Gastroenterol. 2005, 11, 5136. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Amiri, Z.; Guo, Y.-G.; Singh, A.P.; Kenari, R.E. Fabrication of cumin loaded-chitosan particles: Characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem. 2020, 310, 125821. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of encapsulated peppermint essential oil in chitosannanoparticles: Characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Physiol. 2020, 170, 104679. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Subasinghe, U.G.P.P.; Wickramarachchi, S. Encapsulation of cinnamon leaf oil within chitosan: Formulation and characterization. Ceylon J. Sci. 2019, 48, 279. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Akindoyo, J.O. Comparative study between hydrodistillation and microwave-assisted hy-drodistillation for extraction of Cinnamomum cassia oil. ARPN J. Eng. Appl. Sci. 2016, 11, 2647–2652. [Google Scholar] [CrossRef]
- Essid, R.; Gharbi, D.; Abid, G.; Karkouch, I.; Ben Hamouda, T.; Fares, N.; Trabelsi, D.; Mhadhbi, H.; Elkahoui, S.; Limam, F.; et al. Combined effect of Thymus capitatus and Cinnamomum verum essential oils with conventional drugs against Candida albicans biofilm formation and elucidation of the molecular mechanism of action. Ind. Crop. Prod. 2019, 140, 111720. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Jaurès, A.K.N.; Jackson, A.S.; Aimé, G.F.; Igor, K.V.; Simplice, B.T.; Antoine, H.L.N.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Compl. Altern. Med. 2013, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crop. Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Yang, K.; Liu, A.; Hu, A.; Li, J.; Zen, Z.; Liu, Y.; Tang, S.; Li, C. Preparation and characterization of cinnamon essential oil nanocapsules and comparison of volatile components and antibacterial ability of cinnamon essential oil before and after encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.; Rani, V.D.; Menon, D.; Nair, S.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextransulphate–chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS ONE 2018, 13, e0190976. [Google Scholar] [CrossRef]


| C. albicans MIC ± SD (µg mL−1) | |
|---|---|
| CN-NPs | >2000 |
| C. verum EO | 62.5 ± 1.45 |
| C. verum EO/CN-NPs opt | 125 ± 0.66 |
| AMB | 2 ± 0.03 |
| IZ (mm) /MIC (µg mL−1) | |||||||
|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | C. verum E0 | CN-NPs | C. verum EO/CN-NPs | Tetracycline IZ (mm) | |||
| Staphylococcus aureus ATCC 6538 | 14 | 125 | 10 | 1000 | 16 | 62.5 | 34 ± 0.0 |
| Listeria monocytogenes ATCC 19115 | 14 | 125 | 10 | 1000 | 16 | 62.5 | 37 ± 0.0 |
| Gram-negative bacteria | |||||||
| Escherichia coli ATCC 25922 | 12 | 250 | 8 | 2000 | 14 | 125 | 30 ± 0.0 |
| Salmonella enteritidis DMB 560 | 12 | 250 | 8 | 2000 | 14 | 125 | ND |
| Samples | IC50 ± SD (µg mL−1) | CC50 ± SD (µg mL−1) | SI | ||
|---|---|---|---|---|---|
| L. tropica | L. major | Raw 264.7 | L. tropica | L. major | |
| CN-NPs | NT | NT | >2000 | NT | NT |
| C. verum EO | 14.11 ± 1.22 | 17.32 ± 1.22 | 46.67±0.31 | 3.30 | 2.69 |
| C. verum EO/CN-NPs opt | 10.47± 1.24 | 15.09± 1.66 | 1000 ± 2.65 | 95.5 | 66.26 |
| AMB | 0.34 ± 0.12 | 0.97 ± 0.08 | 10.62 ± 0.58 | 31.23 | 10.94 |
| Samples | Particle Size (nm ± SD) | Polydispersity | Zeta Potential (mV ± SD) |
|---|---|---|---|
| CN-NPs | 210 ± 10.32 | 0.32 | 42.57 ± 0.87 |
| C. verum EO/CN-NPs opt | 480 ± 14.55 | 0.21 | 35.64 ± 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essid, R.; Ayed, A.; Djebali, K.; Saad, H.; Srasra, M.; Othmani, Y.; Fares, N.; Jallouli, S.; Abid, I.; Alothman, M.R.; et al. Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles. Molecules 2023, 28, 5681. https://doi.org/10.3390/molecules28155681
Essid R, Ayed A, Djebali K, Saad H, Srasra M, Othmani Y, Fares N, Jallouli S, Abid I, Alothman MR, et al. Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles. Molecules. 2023; 28(15):5681. https://doi.org/10.3390/molecules28155681
Chicago/Turabian StyleEssid, Rym, Ameni Ayed, Kais Djebali, Houda Saad, Mondher Srasra, Yasmine Othmani, Nadia Fares, Selim Jallouli, Islem Abid, Monerah Rashed Alothman, and et al. 2023. "Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles" Molecules 28, no. 15: 5681. https://doi.org/10.3390/molecules28155681
APA StyleEssid, R., Ayed, A., Djebali, K., Saad, H., Srasra, M., Othmani, Y., Fares, N., Jallouli, S., Abid, I., Alothman, M. R., Limam, F., & Tabbene, O. (2023). Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles. Molecules, 28(15), 5681. https://doi.org/10.3390/molecules28155681








