Simulation Study for the Adsorption of Carbon Disulfide on Hydroxyl Modified Activated Carbon
Abstract
1. Introduction
2. Model Construction and Simulation Details
2.1. Model Construction
2.2. Simulation Details
3. Results and Discussion
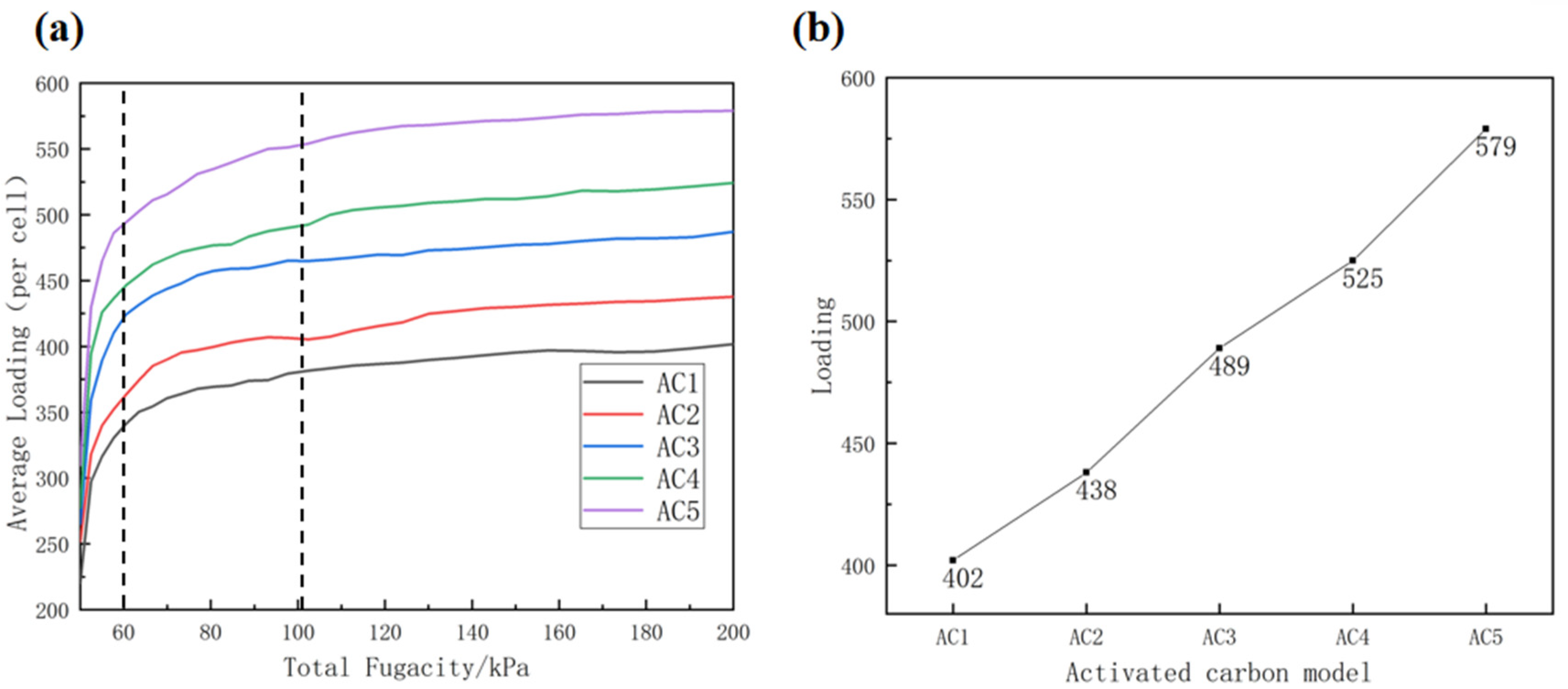
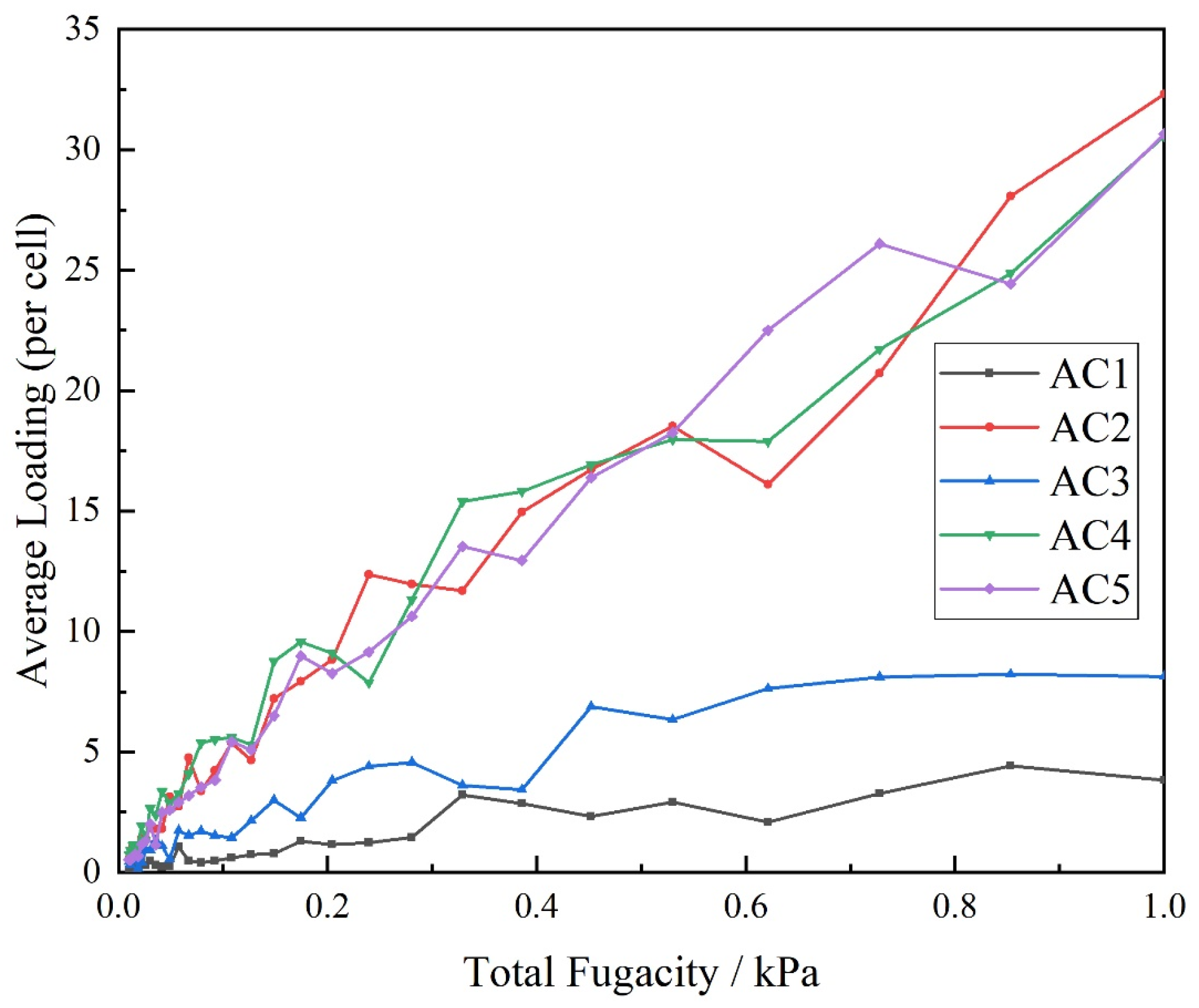
3.1. Effect of Activated Carbon Models with Different Hydroxyl Contents on the Adsorption Isotherm of Carbon Disulfide at Atmospheric Pressure
3.2. Effect of Activated Carbon Models with Different Hydroxyl Contents on the Adsorption Isotherm of Carbon Disulfide at Low Pressure
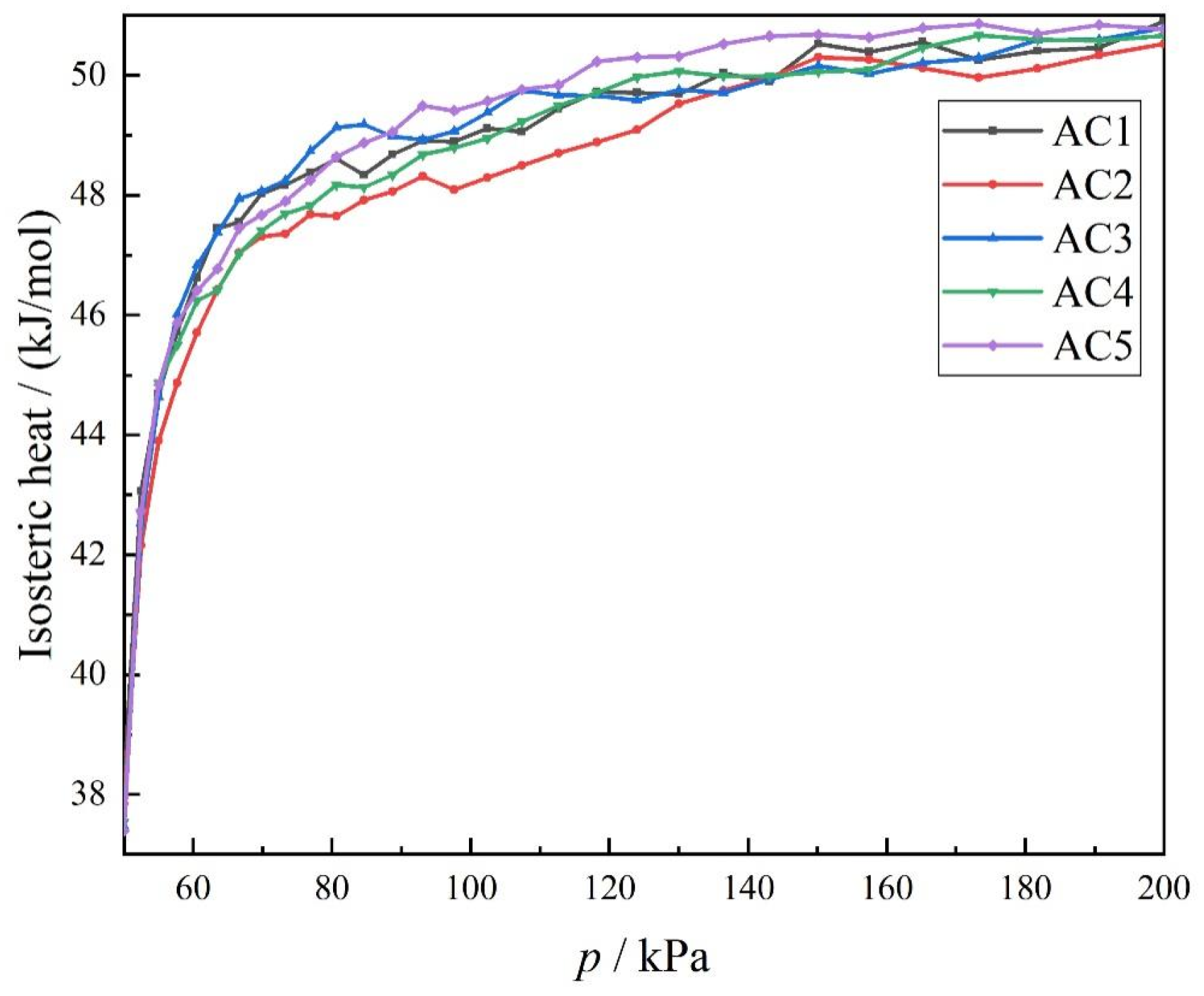
3.3. Effect of Different Hydroxyl Content on the Equivalent Adsorption Heat of Carbon Disulfide by Activated Carbon Models
3.4. Diffusion Coefficient of Carbon Disulfide in Activated Carbon Models with Different Hydroxyl Contents
3.5. Effect of the AC3 Activated Carbon Model on Adsorption of Carbon Disulfide at Different Temperatures
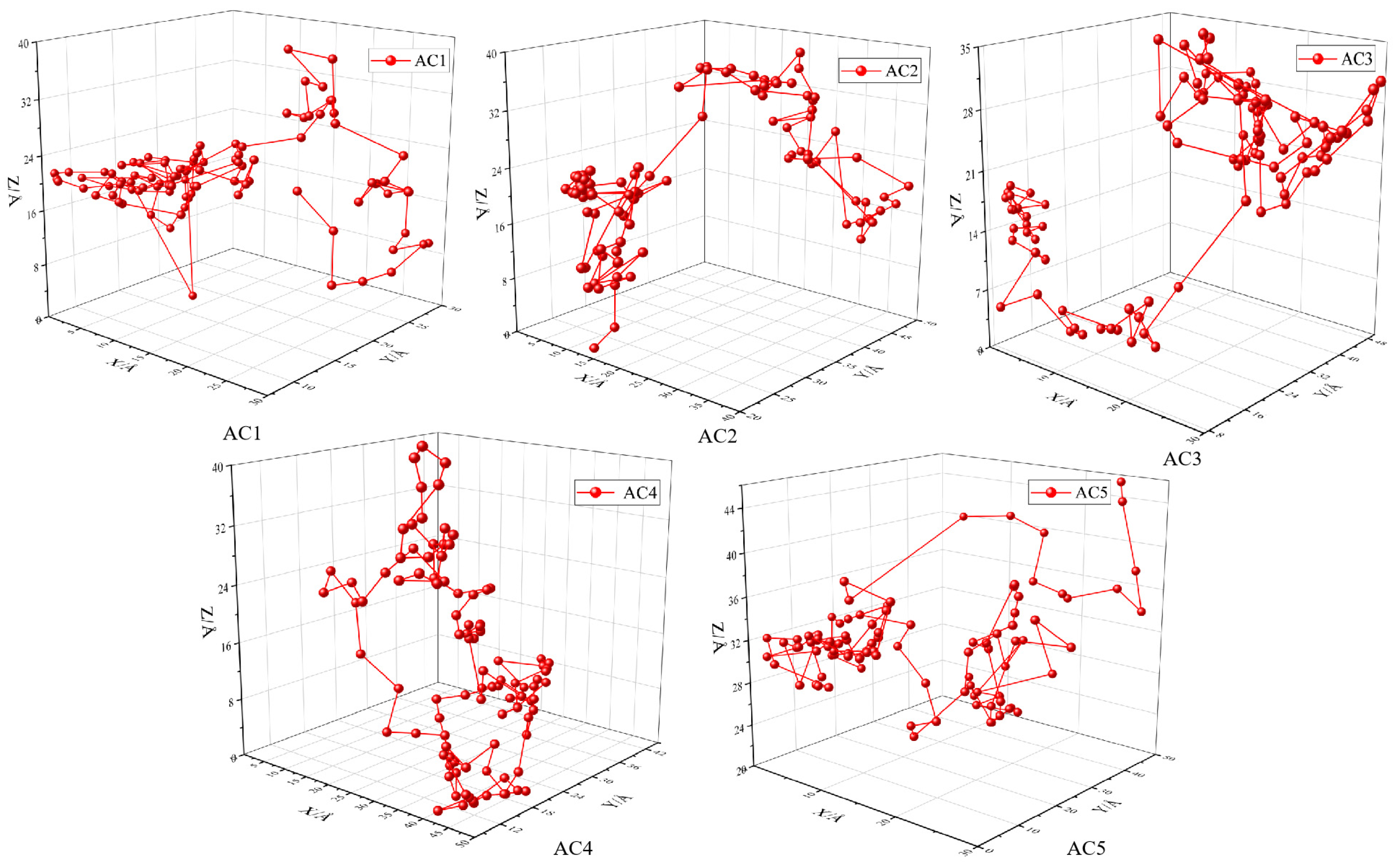
3.6. Trajectory of a Single Carbon Disulfide Molecule in Activated Carbon Models with Different Hydroxyl Contents
4. Conclusions
- (1)
- Using Materials Studio 2018 (MS 2018) software, an activated carbon model that is similar to reality can be constructed, and all the models and simulation methods in this work can be used to provide ideas for exploring issues related to activated carbon in the future.
- (2)
- Hydroxyl-modified activated carbon enhances the adsorption capacity of carbon disulfide molecules, and the hydroxyl content has a great influence on the adsorption of carbon disulfide. With the increase in the basic unit content of hydroxyl-modified hexachlorobenzene, the adsorption capacity of activated carbon also increases, whereas the adsorption efficiency first increases and then decreases. The adsorption capacity for second-rate carbon sulfide molecules is the largest when the basic unit content of hydroxyl-modified hexabenzobenzene is 50%, and when it is 25%, the adsorption efficiency is the highest.
- (3)
- The adsorption sites of carbon disulfide molecules in the activated carbon model change after hydroxyl functional groups are introduced. Carbon disulfide molecules are polar molecules, which easily form hydrogen bonds during the adsorption process. The atoms in the activated carbon have a stronger superposition effect on the charge of carbon disulfide, which is beneficial to adsorption in a low-pressure environment.
- (4)
- With the increase in the basic unit content of hydroxyl-modified hexachlorobenzene, the porosity and solvent-accessible surface area of the activated carbon models increased, while the ultimate diameter and maximum pore diameter of the pores changed to different degrees. The change in the internal pore structure of these activated carbon models also led to a great difference in the diffusion coefficients of carbon disulfide molecules in different hydroxyl-modified activated carbons.
- (5)
- Equivalent adsorption heat and temperature were found to have little effect on the adsorption of carbon disulfide in the hydroxyl-modified activated carbon model.
- (6)
- According to this simulation, at 318 K and atmospheric pressure, the activated carbon model containing 25% hydroxyl-modified activated carbon basic unit has the best adsorption performance and high adsorption efficiency for carbon disulfide molecules, which can reduce energy consumption and production cost when applied to production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, C.; Xu, L.; Yue, L.; Chen, Y.; Chen, J.; Hao, Z. Supported nanometric pd hierarchical catalysts for efficient toluene removal; Catalyst characterization and activity elucidation. Ind. Eng. Chem. Res. 2012, 51, 7211–7222. [Google Scholar] [CrossRef]
- Gonzalez-Miquel, M.; Palomar, J.; Rodriguez, F. Selection of ionic liquids for enhancing the gas solubility of volatile organic compounds. J. Phys. Chem. B 2012, 117, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, X.; Zhang, X. Advances in adsorption-photocatalysis for degradation of indoor VOCs. Chem. Ind. Eng. Prog. 2016, 35, 2215–2221. [Google Scholar]
- Sun, W. Safety Technology of Hazardous Chemicals, 3rd ed.; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Spink, E.F.; Muller, K.R. Removal of Contaminations from Gas Streams of Viscose Fibre Anufacture. Patent RF Number 2199376C2; ICI7B01D 53/52, 2003. [Google Scholar]
- Morooka, T. Method of CS2 Removal. Patent Jap. Number 3265589B2; ICI 7B01D 53/86, 2002. [Google Scholar]
- De, E.K. Way of Removal of Sulfurous Compounds from Gas Mixture. EP Number 1116511A1; ICI 7B01D 53/50, 2001. [Google Scholar]
- Shaubyue, P. Method of Fluid Desulfurization. Patent RF Number 2200618C2; ICI 7B01D 53/86, 2003. [Google Scholar]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Bandosz, T.J. Activated Carbon Surfaces in Environmental Remediation; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Dash, R.; Chmiola, J.; Yushin, G.; Gogotsi, Y.; Laudisio, G.; Singer, J.; Fischer, J.; Kucheyev, S. Titanium carbide derived nanoporous carbon for energy-related applications. Carbon 2006, 44, 2489–2497. [Google Scholar] [CrossRef]
- Mochida, I.; Shirahama, N.; Kawano, S.; Korai, Y.; Yasutake, A.; Tanoura, M.; Fujii, S.; Yoshikawa, M. NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area. Fuel Carbon 2000, 79, 1713–1723. [Google Scholar] [CrossRef]
- Primavera, A.; Trovarelli, A.; Andreussi, P.; Dolcetti, G. The effect of water in the low-temperature catalytic oxidation of hydrogen sulfide to sulfur over activated carbon. Appl. Catal. A Gen. 1998, 173, 185–192. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Matatovmeytal, Y.; Sheintuch, M.; Shter, G.; Grader, G. Optimal temperatures for catalytic regeneration of activated carbon. Carbon 1997, 35, 1527–1531. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.J. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Demir, B.; Göktuğ, A.M. Adsorption of perfluorohexane in BAM-P109 type activated carbon via molecular simulation. Adsorpt. Sci. Technol. 2016, 34, 79–92. [Google Scholar] [CrossRef]
- Bahamon, D.; Vega, L.F. Pharmaceutical removal from water effluents by adsorption on activated carbons: A Monte Carlo simulation study. Langmuir 2017, 33, 11146–11155. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Jain, S.K.; Jin, Q.; Peng, X. Adsorption and structure of benzene confined in disordered porous carbons: Effect of pore heterogeneity and surface chemistry. Mol. Simul. 2018, 44, 1291–1303. [Google Scholar] [CrossRef]
- Jain, S.K.; Pellenq, R.J.-M.; Pikunic, J.P.; Gubbins, K.E. Molecular modeling of porous carbons using the hybrid reverse Monte Carlo method. Langmuir 2006, 22, 9942–9948. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gubbins, K.; Pellenq, R.J.-M.; Pikunic, J.P. Molecular modeling and adsorption properties of porous carbons. Carbon 2006, 44, 2445–2451. [Google Scholar] [CrossRef]
- Raji, Y.; Nadi, A.; Mechnou, I.; Saadouni, M.; Cherkaoui, O.; Zyade, S. High adsorption capacities of crystal violet dye by low-cost activated carbon prepared from Moroccan Moringa oleifera wastes: Characterization, adsorption and mechanism study. Diam. Relat. Mater. 2023, 135, 0925–9635. [Google Scholar] [CrossRef]
- Mechnou, I.; Meskini, S.; Mourtah, I.; Lebrun, L.; Hlaibi, M. Use of phosphorus-doped microporous carbon from olive mill wastewater for effective removal of Crystal violet and Methylene blue. J. Clean. Prod. 2023, 393, 0959–6526. [Google Scholar] [CrossRef]
- Mechnou, I.; Meskini, S.; El Ayar, D.; Lebrun, L.; Hlaibi, M. Olive mill wastewater from a liquid biological waste to a carbon/oxocalcium composite for selective and efficient removal of methylene blue and paracetamol from aqueous solution. Bioresour. Technol. 2022, 365, 128162. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Juhola, A.J. Iodine adsorption and structure of activated carbons. Carbon 1975, 13, 437–442. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; López-González, J.D.; Berenguer, C. Activated carbons from almond shells—II: Characterization of the pore structure. Carbon 1984, 22, 13–18. [Google Scholar] [CrossRef]
- Dollimore, J.; Ubberton, G.P.; Harrison, B.H. The reactivity and pore structure of a steam activated carbon. Carbon 1973, 11, 683. [Google Scholar] [CrossRef]
- Kutics, K.; Kotsis, L.; Argyelán, J.; Szolcsányi, P. Study of the adsorption characterictics and pore structure of activated carbons. Surf. Technol. 1985, 25, 87–96. [Google Scholar] [CrossRef]
- Radeke, K.H. Characterisation of the microporous structure of activated carbons using immersion calorimetry. Carbon 1984, 22, 473–476. [Google Scholar] [CrossRef]
- Leonard, A.J.; Eric, B.S. An hypothesis concerning the relationship between the adsorption kinetics and the structure of activated carbons. Carbon 1981, 19, 231–232. [Google Scholar]
- Wyckoff, R.W.G. Crystal Structures. Nature 1949, 163, 622. [Google Scholar]
- Palmer, J.; Brennan, J.; Hurley, M.; Balboa, A.; Gubbins, K. Detailed structural models for activated carbons from molecular simulation. Carbon 2009, 47, 2904–2913. [Google Scholar] [CrossRef]
- Petkov, V.; Difrancesco, R.G.; Billinge, S.J.L.; Acharya, M.; Foley, F.C. Local structure of nanoporous carbons. Philos. Mag. B 1999, 79, 1519–1530. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Linares-Solano, A. Effect of carbon support and mean Pt particle size on hydrogen chemisorption by carbon-supported Pt ctalysts. J. Catal. 1991, 128, 0021–9571. [Google Scholar]
- Xiao, B.; Cao, Q.; Ma, P.; Bi, H.; Li, P. Study on the mechanism of adsorption of toluene on activated carbon modified by hydroxyl group based on molecular dynamics simulation. Chin. J. Process Eng. 2022, 22, 660–670. [Google Scholar]
- Kumar, K.V.; Salih, A.; Lu, L.; Müller, E.A.; Rodriguez-Reinoso, F. Molecular Simulation of Hydrogen Physisorption and Chemisorption in Nanoporous Carbon Structures. Adsorpt. Sci. Technol. 2011, 29, 799–817. [Google Scholar] [CrossRef]
- Di, B.E.; Sarkisov, L. Systematic development of predictive molecular models of high surface area activated carbons for adsorption applications. Carbon 2013, 64, 262–280. [Google Scholar]
- Birkett, G.R.; Do, D.D. Simulation study of water adsorption on carbon black:The effect of graphite water interaction strength. J. Phys. Chem. C 2007, 111, 5735–5742. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Sun, X.; Wang, C.; Ma, Y.; Song, X.; Li, K.; Ning, P.; He, H. Single atom Fe in favor of carbon disulfide (CS2) adsorption and thus the removal efficiency. Sep. Purif. Technol. 2021, 258, 1383–5866. [Google Scholar] [CrossRef]
- Sibari, A.; Kerrami, Z.; Benaissa, M.; Kara, A. Coverage-dependent adsorption of small gas molecules on black phosphorene: A DFT study. Surf. Sci. 2021, 710, 0039–6028. [Google Scholar]
- Li, X.; Song, K.; Zhao, D.; Yu, L.; Li, S. Molecular simulation of effect of surface functional groups on benzene adsorption properties of activated carbon. Acta Pet. Sin. Pet. Process. 2021, 37, 1078–1085. [Google Scholar]
- Morris, J.R.; Contescu, C.I.; Chisholm, M.F.; Cooper, V.R.; Guo, J.; He, L.; Ihm, Y.; Mamontov, E.; Melnichenko, Y.B.; Olsen, R.J.; et al. Modern approaches to studying gas dsorption in nanoporous carbon. J. Mater. Chem. A 2013, 1, 9341–9350. [Google Scholar] [CrossRef]
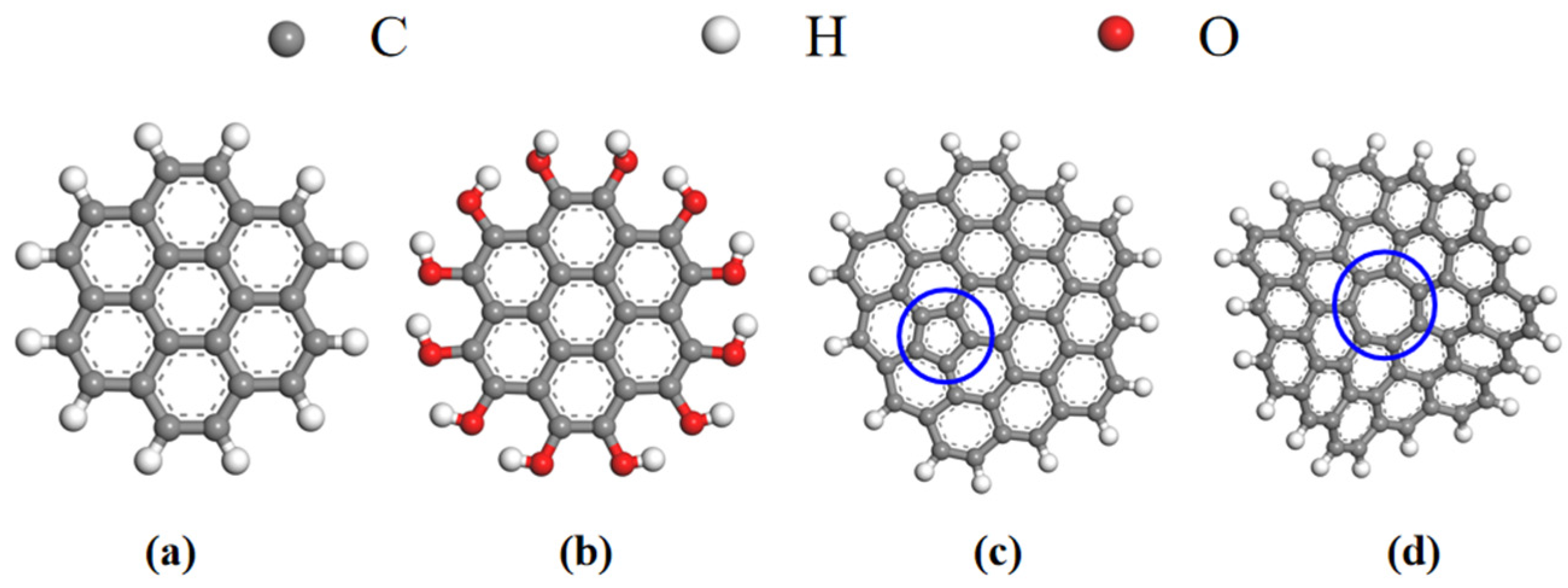
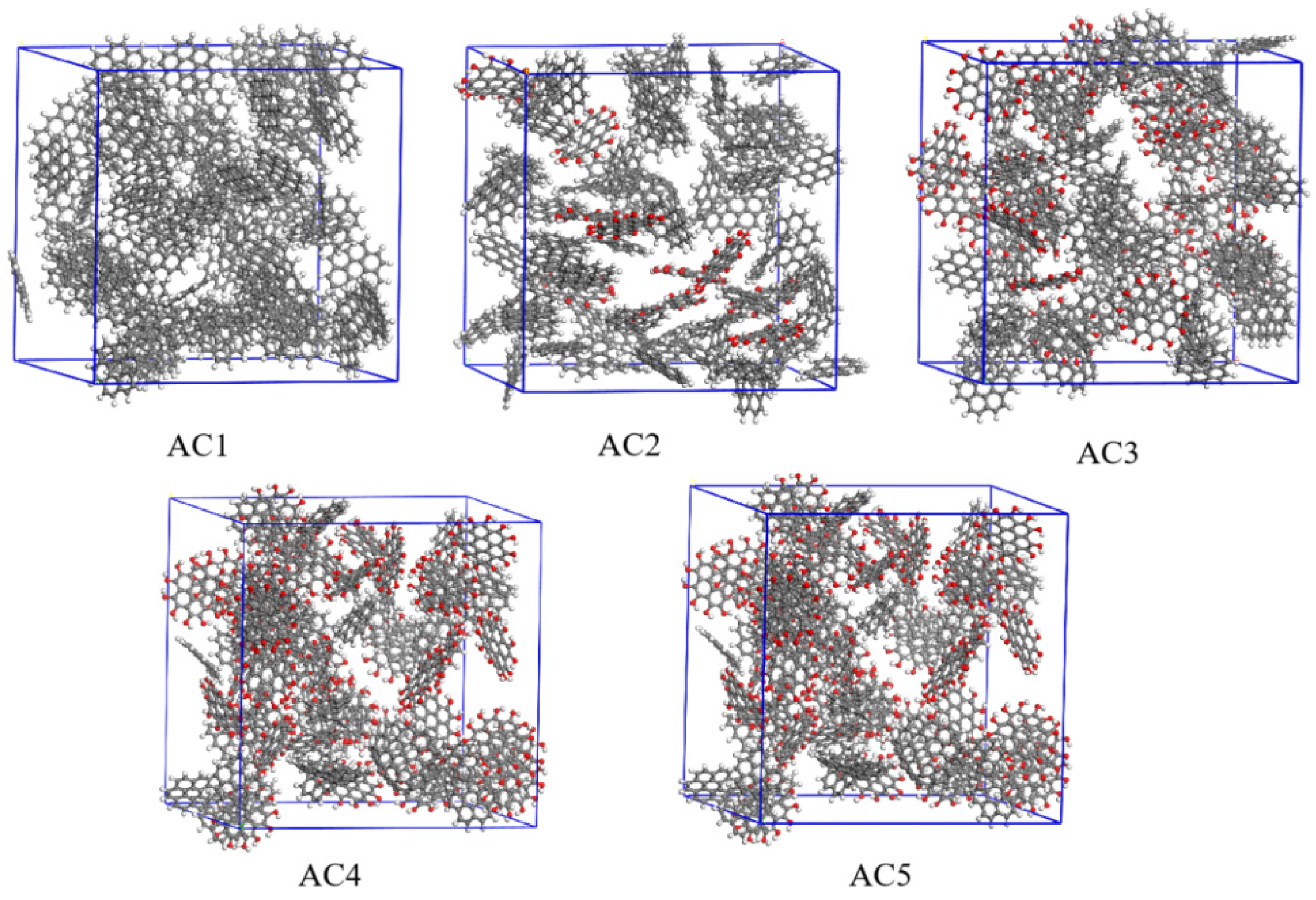

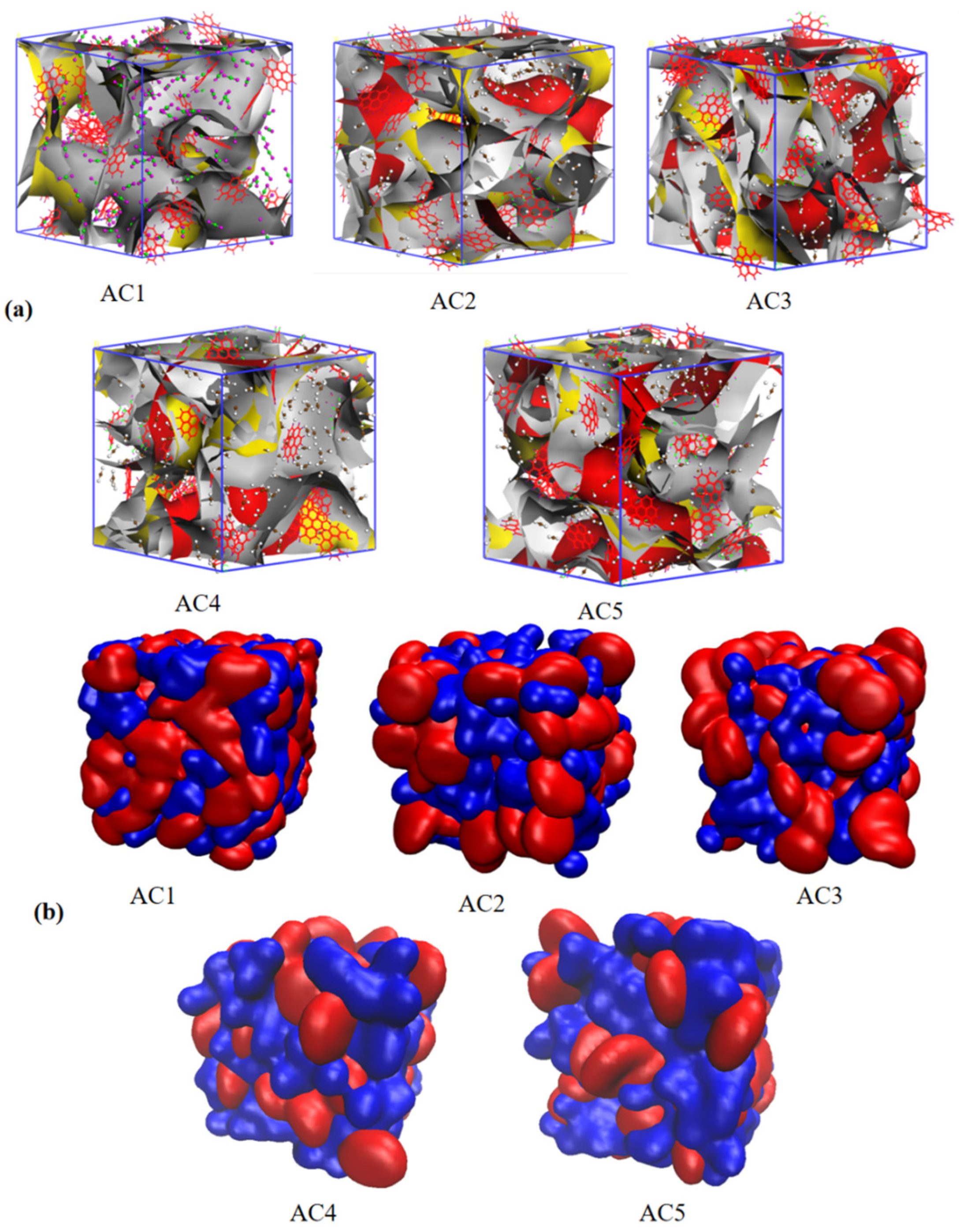


| Model | AC1 | AC2 | AC3 | AC4 | AC5 |
|---|---|---|---|---|---|
| Contains basic units/number | AC: 76 AC-5: 2 AC-7: 2 | AC: 66 AC-OH: 10 AC-5: 2 AC-7: 2 | AC: 56 AC-OH: 20 AC-5: 2 AC-7: 2 | AC: 48 AC-OH: 28 AC-5: 2 AC-7: 2 | AC: 36 AC-OH: 40 AC-5: 2 AC-7: 2 |
| Temperature/K | 315 | 315 | 315 | 315 | 315 |
| Box size/nm × nm × nm | 4.26 × 4.26 × 4.26 | 4.36 × 4.36 × 4.36 | 4.46 × 4.46 × 4.46 | 4.54 × 4.54 × 4.54 | 4.65 × 4.65 × 4.65 |
| Porosity P/% | 69.93 | 71.11 | 73.62 | 73.48 | 75.53 |
| Solvent can reach the surface area S/Å2 | 12,745.85 | 13,718.14 | 15,811.51 | 16,129.28 | 18,414.77 |
| The limiting diameter of the hole DL/Å | 6.15 | 8.21 | 8.03 | 7.44 | 7.78 |
| Maximum aperture DM/Å | 11.85 | 16.06 | 15.14 | 14.34 | 14.87 |
| Hydroxy-modified hexaphenol content/% | 0 | 12.5 | 25 | 35 | 50 |
| Adsorption capacity at simulated end point/number | 402 | 438 | 489 | 525 | 579 |
| The average amount of carbon disulfide adsorbed by 1% hydroxyl/number | --- | (438 − 402)/12.5 = 2.88 | (489 − 438)/12.5 = 4.08 | (525 − 489)/10 = 3.6 | (579 − 525)/15 = 3.6 |
| Model | AC1 | AC2 | AC3 | AC4 | AC5 |
|---|---|---|---|---|---|
| Diffusion coefficient (×10−5cm2/s) | 1.125 | 1.037 | 1.368 | 1.224 | 1.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Li, P.; Hu, B.; Yang, T.; Fu, H.; Chen, S.; Zhang, X. Simulation Study for the Adsorption of Carbon Disulfide on Hydroxyl Modified Activated Carbon. Molecules 2023, 28, 4627. https://doi.org/10.3390/molecules28124627
Cui X, Li P, Hu B, Yang T, Fu H, Chen S, Zhang X. Simulation Study for the Adsorption of Carbon Disulfide on Hydroxyl Modified Activated Carbon. Molecules. 2023; 28(12):4627. https://doi.org/10.3390/molecules28124627
Chicago/Turabian StyleCui, Xiangyu, Penghui Li, Baohua Hu, Teng Yang, Haichao Fu, Shuai Chen, and Xiaolai Zhang. 2023. "Simulation Study for the Adsorption of Carbon Disulfide on Hydroxyl Modified Activated Carbon" Molecules 28, no. 12: 4627. https://doi.org/10.3390/molecules28124627
APA StyleCui, X., Li, P., Hu, B., Yang, T., Fu, H., Chen, S., & Zhang, X. (2023). Simulation Study for the Adsorption of Carbon Disulfide on Hydroxyl Modified Activated Carbon. Molecules, 28(12), 4627. https://doi.org/10.3390/molecules28124627





