Physical and Chemical Properties of Vegetable Films Based on Pumpkin Purée and Biopolymers of Plant and Animal Origin
Abstract
1. Introduction
2. Results
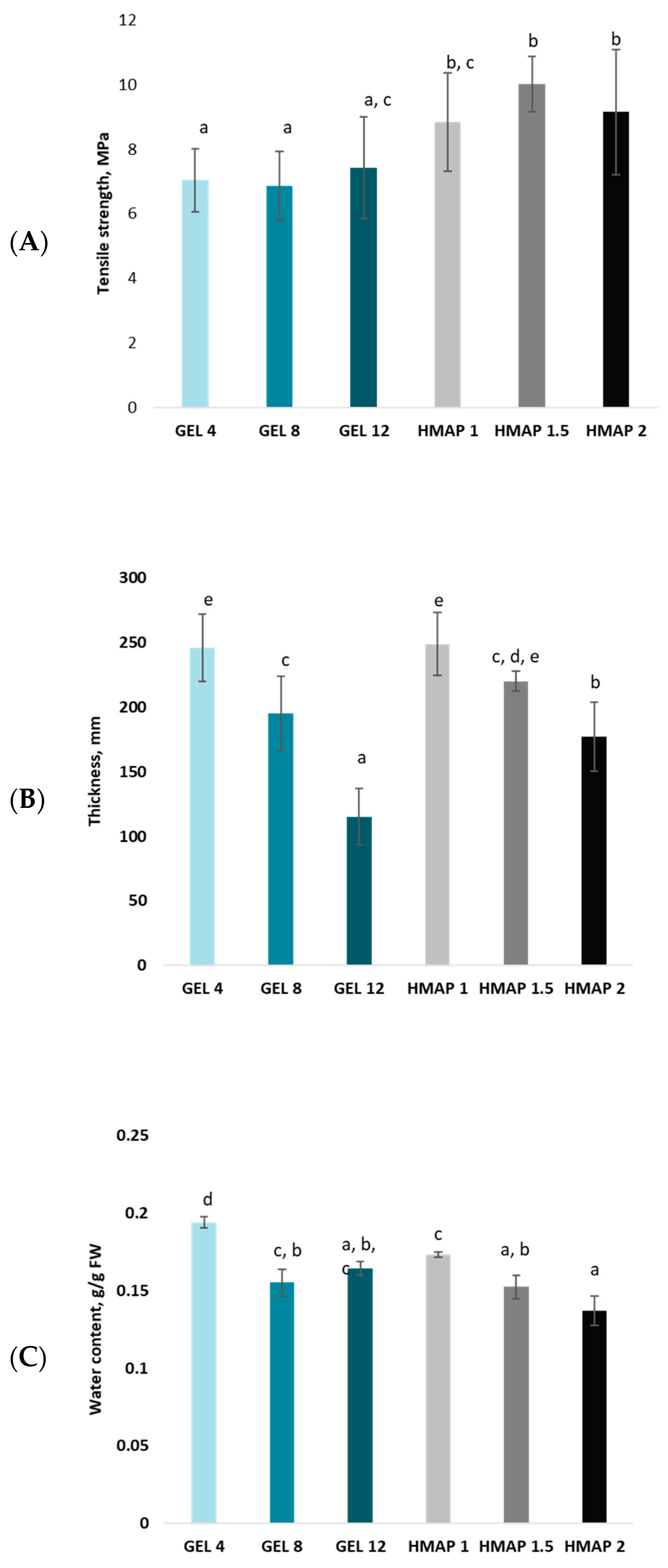
2.1. Selected Properties of Composite Films Determined by the Composition and Properties of Film-Forming Solutions Based on Pumpkin Purée and Selected Hydrocolloids
2.2. Physicochemical Characteristics of Composite Film-Forming Solutions
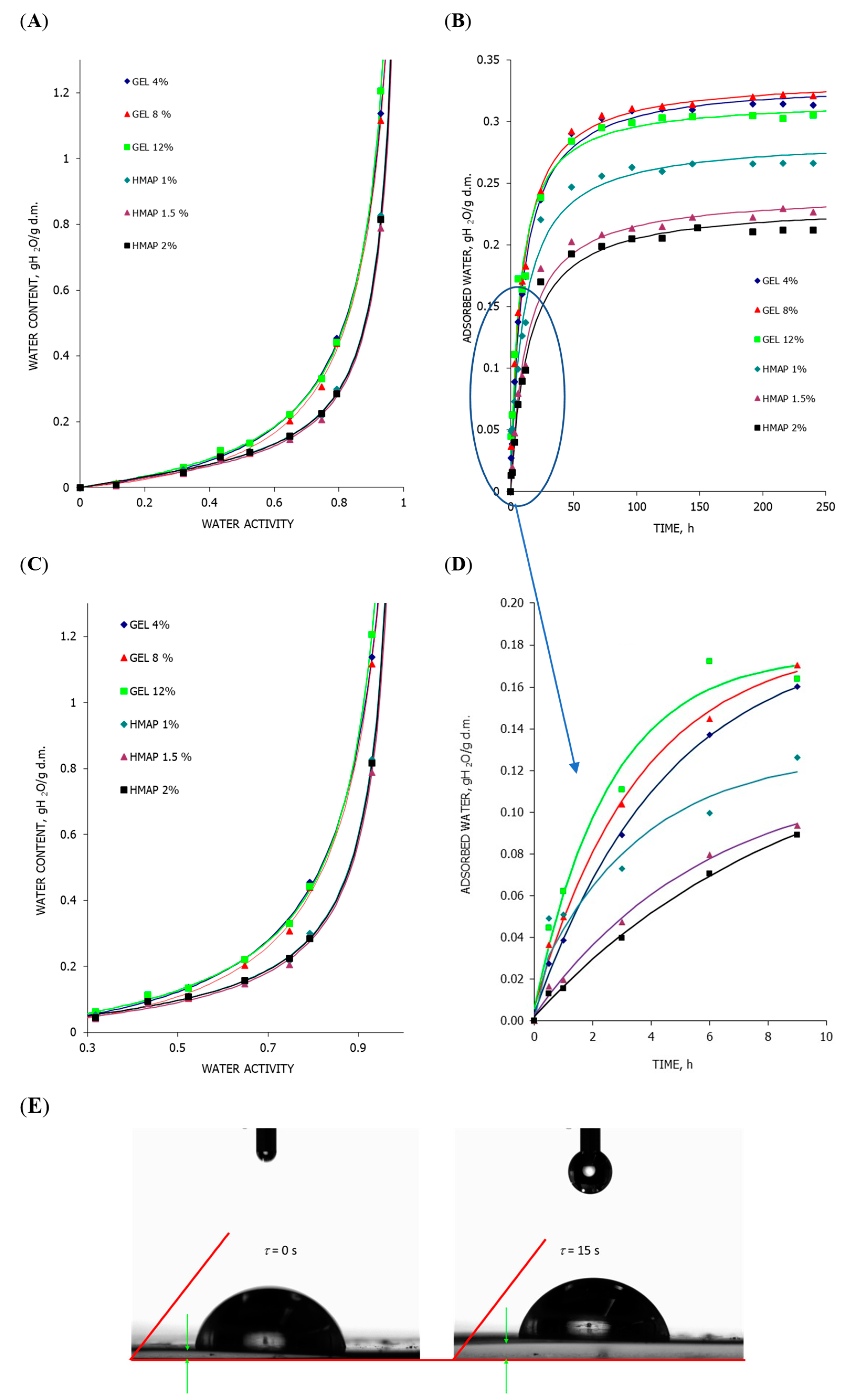
2.3. Analysis of the State of Water in the Polymer Matrix of Composite Gelatin and Pectin Films with the Addition of Pumpkin Purée
2.4. Analysis of the Mechanical Parameters of the Polymer Matrix of Composite Gelatin–Pectin Films with the Addition of Pumpkin Purée
2.5. Analysis of the Thermal Properties of the Polymer Matrix of Composite Gelatin–Pectin Films with the Addition of Pumpkin Purée
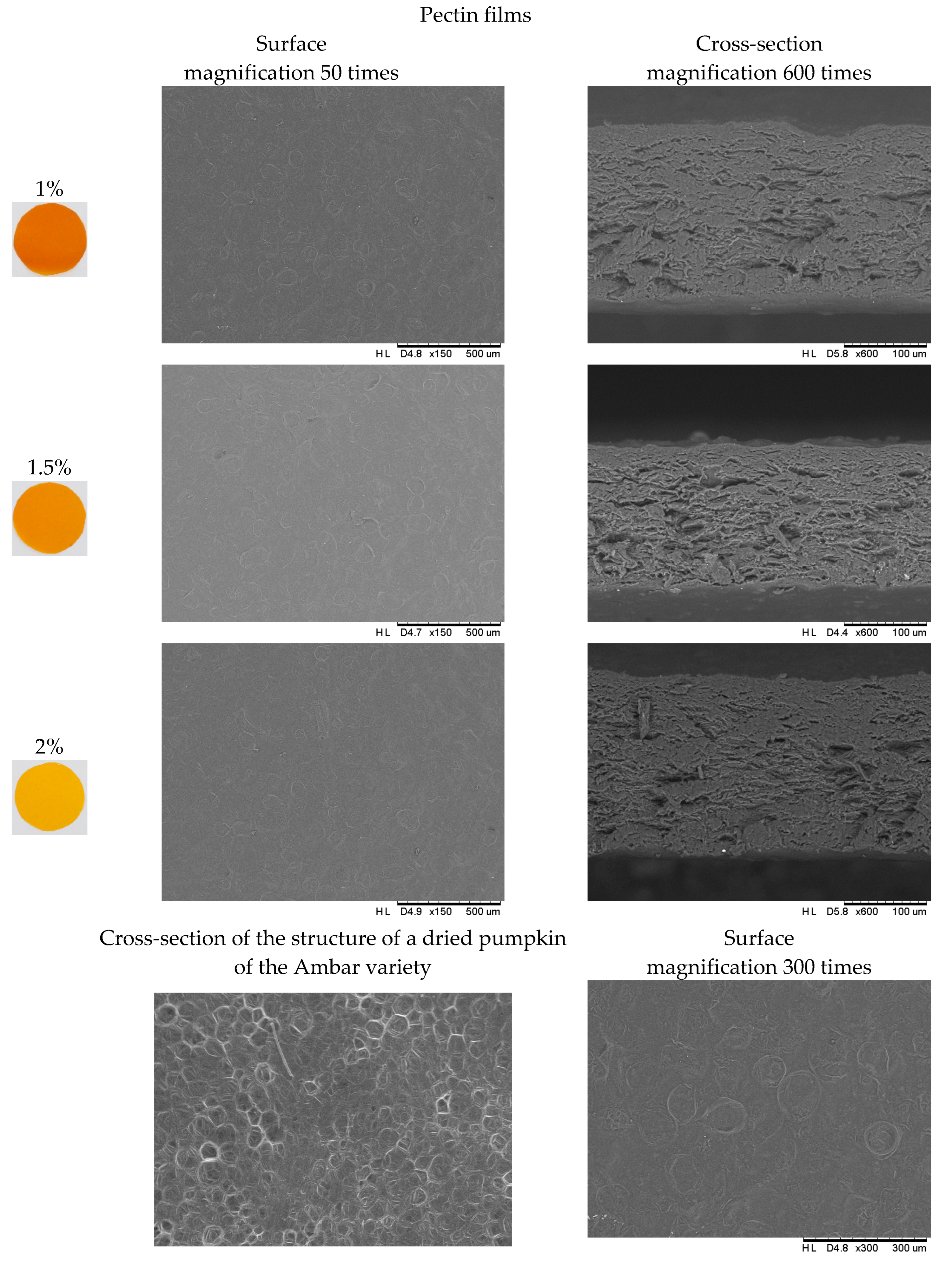
2.6. Analysis of the Structure of the Polymer Matrix of Composite Gelatin–Pectin Films with the Addition of Pumpkin Purée
3. Materials and Methods
3.1. Materials
3.2. Characteristics of Pumpkin Purée
3.2.1. Water Content
3.2.2. Total and Active Acidity
3.2.3. Extract
3.2.4. Chemical Composition
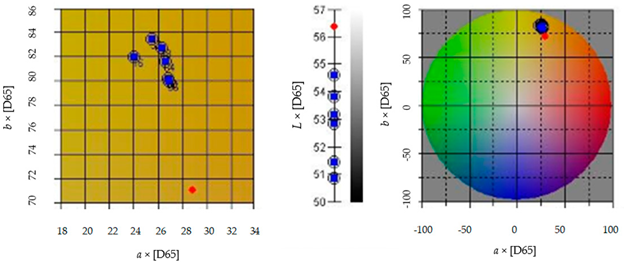
3.2.5. Color
3.3. Preparation of Film-Forming Solutions
3.4. Particle Size Distribution in Film-Forming Solutions
3.5. Film Formation
3.6. Water Vapor Sorption Isotherms and Kinetics
3.7. Mechanical Properties
3.8. Thermal Properties
3.9. Microstructure
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Braga, M.E.M. Edible films and coatings based on agrifood residues: A new trend in the food packaging research. Curr. Opin. Food Sci. 2023, 50, 101006. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Singh, G.P.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Sothornvit, R. Effect of banana and plasticizer types on mechanical, water barrier, and heat sealability of plasticized banana-based films. J. Food Process. Preserv. 2018, 42, e13380. [Google Scholar] [CrossRef]
- Tulamandi, S.; Rangarajan, V.; Rizvi, S.S.; Singhal, R.S.; Chattopadhyay, S.K.; Saha, N.C. A biodegradable and edible packaging film based on papaya puree, gelatin, and defatted soy protein. Food Packag. Shelf Life 2016, 10, 60–71. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Orsuwan, A.; Shankar, S.; Wang, L.-F.; Sothornvit, R.; Rhim, J.-W. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016, 60, 476–485. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The Effect of Whey Protein-Based Edible Coatings Incorporated with Lemon and Lemongrass Essential Oils on the Quality Attributes of Fresh-Cut Pears during Storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Nandi, D.; Parameswaranpillai, J.; Siengchin, S. Recent innovations in bionanocomposites-based food packaging films—A comprehensive review. Food Packag. Shelf Life 2022, 33, 100877. [Google Scholar] [CrossRef]
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabrò, V. Stability of Film-Forming Dispersions: Affects the Morphology and Optical Properties of Polymeric Films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef]
- De Paola, M.G.; Andreoli, T.; Lopresto, C.G.; Calabrò, V. Starch/pectin-biobased films: How initial dispersions could affect their performances. J. Appl. Polym. Sci. 2022, 139, 52032. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de F.F. Soares, N.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An overview of fruit and vegetable edible packaging materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Liu, H.; Li, M.; Ma, Z. Barrier and mechanical properties of carrot puree films. Food Bioprod. Process. 2011, 89, 149–156. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Moreira, F.K.; Bresolin, J.D.; Bernardo, M.P.; de Sousa, C.P.; Mattoso, L.H.C. Sustainable Production and In vitro Biodegradability of Edible Films from Yellow Passion Fruit Coproducts via Continuous Casting. ACS Sustain. Chem. Eng. 2018, 6, 9883–9892. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, S.R.; Sabolović, M.B.; Žlabur, J.; Marović, R.; Brnčić, M. Carotenoid Content and Profiles of Pumpkin Products and By-Products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef] [PubMed]
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of protein and pectin from pumpkin industry by-products and their utilization for developing edible film. J. Food Sci. Technol. 2020, 57, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Giacomazza, D.; Bulone, D.; Biagio, P.L.S.; Marino, R.; Lapasin, R. The role of sucrose concentration in self-assembly kinetics of high methoxyl pectin. Int. J. Biol. Macromol. 2018, 112, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, L.; Chen, D.; Guo, X.; Liu, F.; Pan, S. Unexpected gelation behavior of citrus pectin induced by monovalent cations under alkaline conditions. Carbohydr. Polym. 2019, 212, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Abboud, K.Y.; Iacomini, M.; Simas, F.F.; Cordeiro, L.M. High methoxyl pectin from the soluble dietary fiber of passion fruit peel forms weak gel without the requirement of sugar addition. Carbohydr. Polym. 2020, 246, 116616. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ratcliffe, I.; Williams, P.A.; Luo, S.; Chen, J.; Liu, C. The influence of pH and monovalent ions on the gelation of pectin from the fruit seeds of the creeping fig plant. Food Hydrocoll. 2021, 111, 106219. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Dhiman, A.K.; Sharma, K.D.; Attri, S. Functional constituents and processing of pumpkin: A review. J. Food Sci. Technol.-Mysore 2009, 46, 411–417. [Google Scholar]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2019, 50, 384–401. [Google Scholar] [CrossRef]
- Arifin, N.; Izyan, S.N.; Huda-Faujan, N. Physical properties and consumer acceptability of basic muffin made from pumpkin puree as butter replacer. Food Res. 2019, 3, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Contini, L.R.F.; Yoshida, C.M.P.; Venturini, A.C. Chapter 21—Application of edible biopolymer coatings on meats, poultry, and seafood. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 515–533. [Google Scholar]
- Paramita, V.D.; Kasapis, S. Molecular dynamics of the diffusion of natural bioactive compounds from high-solid biopolymer matrices for the design of functional foods. Food Hydrocoll. 2019, 88, 301–319. [Google Scholar] [CrossRef]
- Granados, A.E.A.; Kawai, K. Effect of cellulose powder content on the water sorption, glass transition, mechanical relaxation, and caking of freeze-dried carbohydrate blend and food powders. LWT 2021, 148, 111798. [Google Scholar] [CrossRef]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum and, Persian gum. LWT 2020, 117, 108617. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture Sensitivity, Optical, Mechanical and Structural Properties of Whey Protein-Based Edible Films Incorporated with Rapeseed Oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Effect of protein concentration on kinetics of water vapour adsorption by coatings prepared on the basis of whey protein isolate. Food Sci. Technol. Qual. 2011, 4, 66–73. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–106. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Kumar, C.; Balasubramanian, M.; Roy, I. Thermal, Structural, and Physical Properties of Freeze Dried Tropical Fruit Powder. J. Food Process. 2014, 2014, 524705. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.; Alvarez-Salas, C.; Esneider-Alcalá, M.; Toxqui-Terán, A.; Pérez-García, S.; Ruiz-Cabrera, M. Towards an improved calorimetric methodology for glass transition temperature determination in amorphous sugars. CyTA J. Food 2012, 10, 258–267. [Google Scholar] [CrossRef]
- Yu, H.; Yang, S.; Yuan, C.; Hu, Q.; Li, Y.; Chen, S.; Hu, Y. Application of biopolymers for improving the glass transition temperature of hairtail fish meat. J. Sci. Food Agric. 2018, 98, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Roos, Y.H. Water Activity and Glass Transition. In Water Activity in Foods; Wiley: New York, NY, USA, 2020; pp. 27–43. [Google Scholar]
- Chang, Y.P.; Cheah, P.; Seow, C. Plasticizing—Antiplasticizing Effects of Water on Physical Properties of Tapioca Starch Films in the Glassy State. J. Food Sci. 2000, 65, 445–451. [Google Scholar] [CrossRef]
- Lewicki, P.P. Water sorption isotherms and their estimation in food model mechanical mixtures. J. Food Eng. 1997, 32, 47–68. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 1995.


| Parameter | Symbol/Unit | Value |
|---|---|---|
| Particle size distribution | D4.3 [μm] | 80.47 ± 1.49 |
| D3.2 [μm] | 52.69 ± 1.70 | |
| DV10 [μm] | 26.30 ± 1.63 | |
| DV50 [μm] | 80.28 ± 1.47 | |
| DV90 [μm] | 125.07 ± 3.31 | |
Color | L [−] | 54.61 ± 0.07 |
| a [−] | 25.47 ± 0.04 | |
| b [−] | 83.40 ± 0.14 | |
 | ||
| Water content | u [g H2O/g d.m] [%] | 8.52 ± 0.27 89.5 ± 0.3 |
| Total soluble solids | E [°Brix] | 8.3 ± 0.1 |
| pH | pH [−] | 7.3 ± 0.2 |
| Pectins soluble in water | g/100 g fresh mass | 0.23 ± 0.01 |
| Starch | % | 5.54 ± 1.91 |
| Cellulose | % | 4.6 ± 0.3 |
| Hemicellulose | % | 8.5 ± 0.6 |
| Lignin | % | 13.1 ± 0.4 |
| Fiber | % | 1.96 ± 0.36 |
| Protein | % | 1.38 ± 0.16 |
| L-ascorbic acid | mg/100 g fresh mass | 4.47 ± 0.01 |
| Total carotenoids | mg/100 g fresh mass | 15.96 ± 0.52 |
| Β-carotene | mg/100 g fresh mass | 4.77 ± 0.39 |
| Total sugars | % | 3.71 ± 0.46 |
| Reducing sugars | % | 2.23 ± 0.10 |
| Glucose | g/100 g fresh mass | 1.38 ± 0.20 |
| Fructose | g/100 g fresh mass | 1.08 ± 0.10 |
| Saccharose | g/100 g fresh mass | 1.24 ± 0.05 |
| Sample | Tm1 [°C] | Tm2 [°C] | Tm3 [°C] | ΔH [J/g] | Tg | Total Mass Loss in the Temperature Range of 50–300 °C [%] |
|---|---|---|---|---|---|---|
| GEL4 | 66.86 ± 0.91 2 | 150.89 ± 7.52 a,2 | 177.85 ± 10.15 | 214.35 ± 20.44 | −39.99 ± 0.99 ** | 42.38 ± 0.58 * |
| GEL8 | 57.80 ± 1.61 1 | 146.99 ± 0.04 a,1 | 175.76 ± 14.80 | 201.40 ± 13.08 | −46.45 ± 0.66 *** | 45.33 ± 0.08 ** |
| GEL12 | 59.16 ± 5.53 1 | 158.97 ± 6.35 a,2 | 189.42 ± 15.72 | 176.13 ± 62.39 | −45.58 ± 0.81 *** | 41.97 ± 0.08 * |
| HMAP 1 | 145.38 ± 0.11 ACa | 177.02 ± 1.73 | 248.40 ± 0.57 | −34.64 ± 0.27 * | 46.04 ± 0.43 *** | |
| 152.31 ± 0.66 BCa | ||||||
| HMAP 1.5 | 148.63 ± 1.82 ACa | 179.49 ± 1.27 | 262.75 ± 2.05 | −34.81 ± 0.59 * | 46.27 ± 0.29 *** | |
| 154.17 ± 0.98 BCa | ||||||
| HMAP 2 | 149.99 ± 1.02 ACa | 180.34 ± 1.70 | 245.70 ± 1.05 | −39.41 ± 0.08 ** | 47.25 ± 0.12 **** | |
| 156.05 ± 0.96 BCa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowicz, M.; Kadzińska, J.; Bryś, J.; Ciurzyńska, A.; Karwacka, M.; Galus, S. Physical and Chemical Properties of Vegetable Films Based on Pumpkin Purée and Biopolymers of Plant and Animal Origin. Molecules 2023, 28, 4626. https://doi.org/10.3390/molecules28124626
Janowicz M, Kadzińska J, Bryś J, Ciurzyńska A, Karwacka M, Galus S. Physical and Chemical Properties of Vegetable Films Based on Pumpkin Purée and Biopolymers of Plant and Animal Origin. Molecules. 2023; 28(12):4626. https://doi.org/10.3390/molecules28124626
Chicago/Turabian StyleJanowicz, Monika, Justyna Kadzińska, Joanna Bryś, Agnieszka Ciurzyńska, Magdalena Karwacka, and Sabina Galus. 2023. "Physical and Chemical Properties of Vegetable Films Based on Pumpkin Purée and Biopolymers of Plant and Animal Origin" Molecules 28, no. 12: 4626. https://doi.org/10.3390/molecules28124626
APA StyleJanowicz, M., Kadzińska, J., Bryś, J., Ciurzyńska, A., Karwacka, M., & Galus, S. (2023). Physical and Chemical Properties of Vegetable Films Based on Pumpkin Purée and Biopolymers of Plant and Animal Origin. Molecules, 28(12), 4626. https://doi.org/10.3390/molecules28124626












