Validation of Broth Macrodilution Volatilization Method for Testing of Essential Oils in Liquid and Vapor Phase: Chemical Composition, Cytotoxicity, and Antibacterial Effect of Indian Medicinal Plants against Pneumonia-Causing Pathogens
Abstract
1. Introduction
2. Results
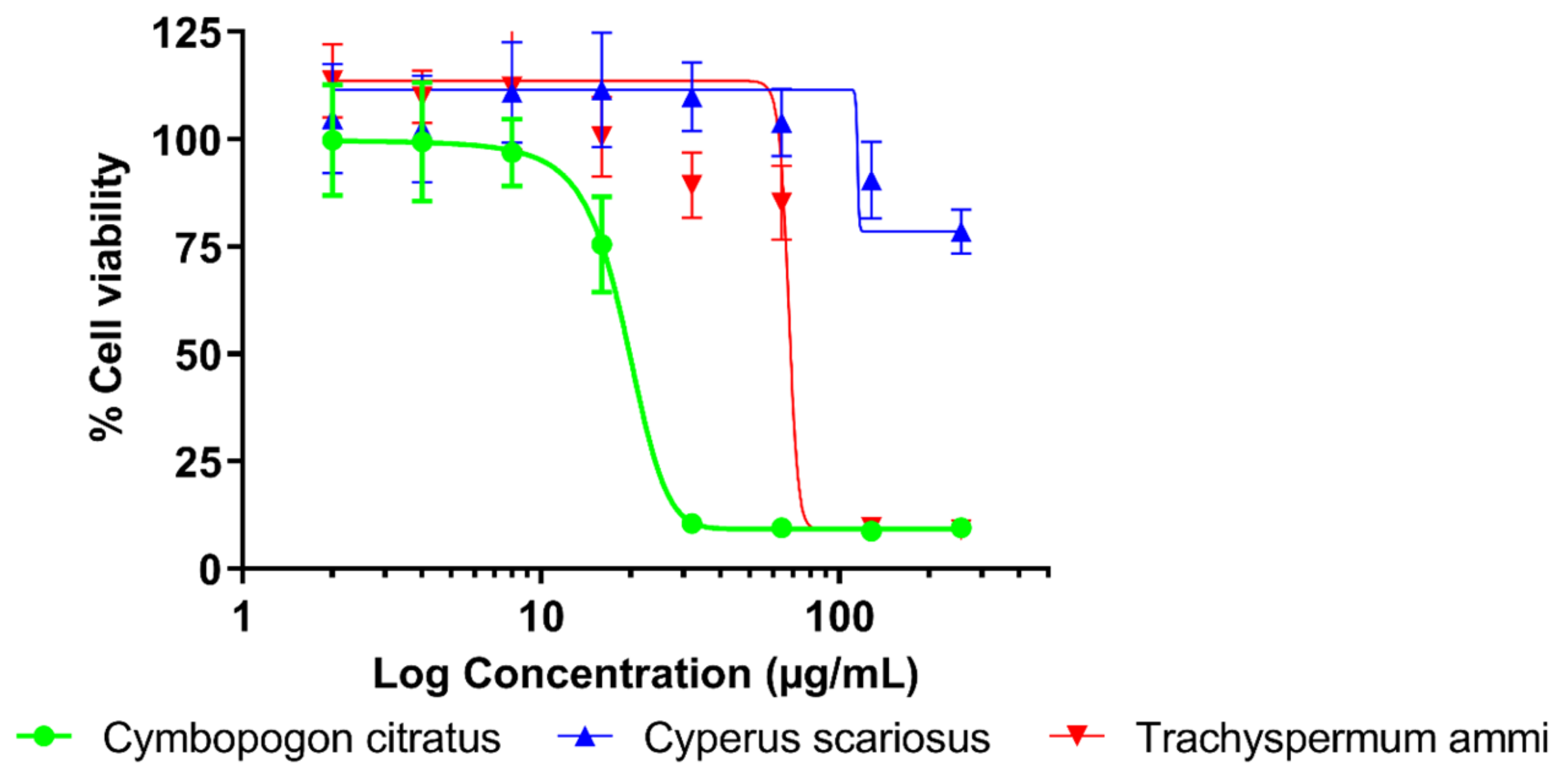
2.1. Antibacterial Activity and Cytotoxicity
2.2. Chemical Composition
2.3. Chemical Composition of T. ammi EO Vapors
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Sample Preparation
4.3. Bacterial Strains and Culture Media
4.4. Antimicrobial Assay
4.5. Cell Cultures
4.6. Cytotoxicity Assay
4.7. Chemical Analysis of EOs’ Liquid Phase
4.8. Chemical Analysis of EOs’ Vapor Phase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grossman, T.H.; Fyfe, C.; O’Brien, W.; Hackel, M.; Minyard, M.B.; Waites, K.B.; Dubois, J.; Murphy, T.M.; Slee, A.M.; Weiss, W.J.; et al. Fluorocycline TP-271 is potent against complicated community-acquired bacterial pneumonia pathogens. mSphere 2017, 2, e00004-17. [Google Scholar] [CrossRef]
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menendez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Eshwara, V.K.; Mukhopadhyay, C.; Rello, J. Community-acquired bacterial pneumonia in adults: An update. Indian J. Med. Res. 2020, 151, 288. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled therapy in respiratory disease: The complex interplay of pulmonary kinetic processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef]
- Quon, B.S.; Goss, C.H.; Ramsey, B.W. Inhaled antibiotics for lower airway infections. Ann. Am. Thorac. Soc. 2014, 11, 425–427. [Google Scholar] [CrossRef]
- Tiddens, H.A.W.M.; Bos, A.C.; Mouton, J.W.; Devadason, S.; Janssens, H.M. Inhaled antibiotics: Dry or wet? Eur. Clin. Respir. J. 2014, 44, 1308–1318. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Urbanova, K.; Rondevaldova, J.; Damme, P.; Kokoska, L. In vitro antimicrobial combinatory effect of Cinnamomum cassia essential oil with 8-hydroxyquinoline against Staphylococcus aureus in liquid and vapour phase. J. Appl. Microbiol. 2020, 129, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Horvath, G.; Acs, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 339–340. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khaneghah, A.M.; Sant’Ana, A.S. Essential Oils in Food Processing: Chemistry, Safety and Applications, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Ross, C.F. Headspace analysis. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 27–50. [Google Scholar]
- Schweitzer, B.; Balazs, V.L.; Molnar, S.; Szogi-Tatar, B.; Boszormenyi, A.; Palkovics, T.; Horvath, G.; Schneider, G. Antibacterial effect of lemongrass (Cymbopogon citratus) against the aetiological agents of pitted keratolyis. Molecules 2022, 27, 1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Q.; Tang, J.; Zhang, Z. Component analysis of volatile oil from fruits of Trachyspermum ammi by headspace solid-phase microextraction GC-MS. Chin. J. Pharm. Anal. 2013, 33, 607–610. [Google Scholar]
- Upasani, S.V.; Beldar, V.G.; Tatiya, A.U.; Upasani, M.S.; Surana, S.J.; Patil, D.S. Ethnomedicinal plants used for snakebite in India: A brief overview. Integr. Med. Res. 2017, 6, 114–115. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhattacharya, S.; Chaudhuri, S. Conservation and documentation of the medicinal plant resources of India. Biodivers. Conserv. 2006, 15, 2705–2717. [Google Scholar] [CrossRef]
- Vaidya, V.N.; Tatiya, A.U.; Elango, A.; Kukkupuni, S.K.; Vishnuprasad, C.N. Need for comprehensive standardization strategies for marketed Ayurveda formulations. J. Ayurveda Integr. Med. 2018, 9, 312–315. [Google Scholar] [CrossRef]
- Ningthoujam, S.S.; Talukdar, A.D.; Potsangbam, K.S.; Choudhury, M.D. Traditional uses of herbal vapour therapy in Manipur, Northeast India: An ethnobotanical survey. J. Ethnopharmacol. 2013, 147, 136–147. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, R.; Sodha, R.S.; Rajawat, B.S. Trachyspermum ammi. Pharmacogn. Rev. 2012, 6, 56–60. [Google Scholar] [CrossRef]
- Singh, V.; Ali, M.; Negi, A.; Sultana, S. Analysis and antimicrobial activity of the essential oil of Cyperus rotundus L. rhizomes. J. Med. Plants Stud. 2018, 6, 101–105. [Google Scholar]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Baines, D.; Alexander, T.W. A Vapour phase assay for evaluating the antimicrobial activities of essential oils against bovine respiratory bacterial pathogens. Lett. Appl. Microbiol. 2017, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Houdkova, M.; Kokoska, L. Volatile antimicrobial agents and in vitro methods for evaluating their activity in the vapour phase: A review. Planta Med. 2020, 86, 822–857. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Toukourou, H.; Gbaguidi, F.; Quetin-Leclercq, J. Phytochemical composition, antibacterial activity against sore throat pathogens and toxicological evaluation of Cymbopogon citratus essential oil from Benin. J. Pharmacogn. Phytochem. 2019, 8, 3258–3263. [Google Scholar]
- Special Programme for Research and Training in Tropical Diseases. Available online: http://www.who.int/tdr/grants/workplans/en/cytotoxicity_invitro.pdf (accessed on 21 December 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Publishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- National Institute of Standards and Technology. Available online: https://www.nist.gov/ (accessed on 27 March 2023).
- Paul, S.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725–731. [Google Scholar] [CrossRef]
- Jebelli Javan, A.; Salimiraad, S.; Khorshidpour, B. Combined effect of Trachyspermum ammi essential oil and propolis ethanolic extract on some foodborne pathogenic bacteria. Vet. Res. Forum 2019, 10, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Gardener, A.C.; Trifan, A.; Spac, A.; Brebu, M.; Miron, A.; Aprotosoaie, A.C. Antibacterial activity of traditional spices against lower respiratory tract pathogens: Combinatorial effects of Trachyspermum ammi essential oil with conventional antibiotics. Lett. Appl. Microbiol. 2018, 67, 449–457. [Google Scholar] [CrossRef]
- Vazirian, M.; Hekmati, D.; Ostad, S.; Manayi, A. Toxicity evaluation of essential oil of Trachyspermum ammi in acute and sub-chronic toxicity experiments. J. Med. Plants 2019, 18, 70–77. [Google Scholar]
- ECHA. European Chemicals Agency. Available online: https://echa.europa.eu/ (accessed on 26 February 2023).
- Xie, K.; Tashkin, D.P.; Luo, M.Z.; Zhang, J.Y. Chronic toxicity of inhaled thymol in lungs and respiratory tracts in mouse model. Pharmacol. Res. Perspect. 2019, 7, e00516. [Google Scholar] [CrossRef]
- Howyzeh, M.S.; Noori, S.A.S.; Shariati, J.V.; Niazian, M. Essential oil chemotype of Iranian Ajowan (Trachyspermum ammi L.). J. Essent. Oil-Bear. Plants 2018, 21, 273–276. [Google Scholar] [CrossRef]
- Modareskia, M.; Fattahi, M.; Mirjalili, M.H. Thymol screening, phenolic contents, antioxidant and antibacterial activities of Iranian populations of Trachyspermum ammi (L.) Sprague (Apiaceae). Sci. Rep. 2022, 12, 15645. [Google Scholar] [CrossRef]
- Antih, J.; Houdkova, M.; Urbanova, K.; Kokoska, L. Antibacterial activity of Thymus vulgaris L. essential oil vapours and their GC/MS analysis using solid-phase microextraction and syringe headspace sampling techniques. Molecules 2021, 26, 6553. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Rodrigues, F.; Caldeira, M.; Câmara, J.S. A powerful approach to explore the potential of medicinal plants as a natural source of odor and antioxidant compounds. J. Food Sci. Technol. 2016, 53, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Chialva, F.; Gabri, G.; Liddle, P.A.P.; Ulian, F. Qualitative evaluation of aromatic herbs by direct headspace GC analysis. Applications of the method and comparison with the traditional analysis of essential oils. J. High. Resolut. Chromatogr. 1982, 5, 182–188. [Google Scholar] [CrossRef]
- Laird, K.; Phillips, C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012, 54, 169–174. [Google Scholar] [CrossRef]
- Manvitha, K.; Bidya, B. Review on pharmacological activity of Cymbopogon citratus. Int. J. Herb. Med. 2014, 6, 5–7. [Google Scholar]
- Valkova, V.; Duranova, H.; Galovicova, L.; Borotova, P.; Vukovic, N.L.; Vukic, M.; Kacaniova, M. Cymbopogon citratus essential oil: Its application as an antimicrobial agent in food preservation. Agronomy 2022, 12, 155. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Houdkova, M.; Albarico, G.; Doskocil, I.; Tauchen, J.; Urbanova, K.; Tulin, E.E.; Kokoska, L. Vapors of volatile plant-derived products significantly affect the results of antimicrobial, antioxidative and cytotoxicity microplate-based assays. Molecules 2020, 25, 6004. [Google Scholar] [CrossRef] [PubMed]
- Gaworski, C.L.; Vollmuth, T.A.; York, R.G.; Heck, J.D.; Aranyi, C. Developmental toxicity evaluation of inhaled citral in Sprague-Dawley Rats. Food Chem. Toxicol. 1992, 30, 269–275. [Google Scholar] [CrossRef]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Mekonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub-acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef]
- El-Kased, R.F.; El-Kersh, D.M. GC–MS profiling of naturally extracted essential oils: Antimicrobial and beverage preservative actions. Life 2022, 12, 1587. [Google Scholar] [CrossRef]
- Mohamed Hanaa, A.R.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef] [PubMed]
- Bhawna, K.; Sharma, S.K.; Singh, L.; Mohapatra, S.; Singh, T. Cyperus scariosus: A potential herb. Int. Res. J. Pharm. 2013, 4, 17–20. [Google Scholar] [CrossRef]
- Jha, V.; Patel, R.; Devkar, S.; Shaikh, M.A.; Rai, D.; Walunj, S.; Koli, J.; Jain, T.; Jadhav, N.; Shruti Narvekar, S.; et al. Chemical composition, bioactive potential, and thermal behaviour of Cyperus scariosus essential oil. Chem. Sci. Int. J. 2022, 31, 1–14. [Google Scholar] [CrossRef]
- Clery, R.A.; Cason, J.R.L.; Zelenay, V. Constituents of cypriol oil (Cyperus scariosus R. Br.): N-containing molecules and key aroma components. J. Agric. Food Chem. 2016, 64, 4566–4573. [Google Scholar] [CrossRef]
- Kumar, A.; Niranjan, A.; Lehri, A.; Srivastava, R.K.; Tewari, S.K. Effect of geographical climatic conditions on yield, chemical composition and carbon isotope composition of nagarmotha (Cyperus scariosus R. Br.) essential oil. J. Essent. Oil-Bear. Plants 2016, 19, 368–373. [Google Scholar] [CrossRef]
- Houdkova, M.; Chaure, A.; Doskocil, I.; Havlik, J.; Kokoska, L. New broth macrodilution volatilization method for antibacterial susceptibility testing of volatile agents and evaluation of their toxicity using modified MTT assay in vitro. Molecules 2021, 26, 4179. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Approved Standard, CLSI Document M02-A11; CLSI: Wayne, PA, USA, 2012; p. 32. ISBN 1-56238-782-0. [Google Scholar]
- AOAC International. Official Methods of Analysis, Official Method 925.10; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012; ISBN 0935584838. [Google Scholar]
- European Pharmacopoeia. Published in Accordance with the Convention on the Elaboration of a European Pharmacopoeia, 7th ed.; European Treaty Series No. 50; Council of Europe: Strasbourg, France, 2013. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 25th Informational Supplement M100-S25; CLSI: Wayne, PA, USA, 2015; ISBN 1-56238-989-0. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kokjohn, K.; Bradley, M.; Griffiths, B.; Ghannoum, M. Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int. J. Dermatol. 2003, 42, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Trevor, A.J.; Katzung, B.G.; Kruidering-Hall, M. Katzung and Trevor’s Pharmacology Examination and Board Review, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2015; p. 20. ISBN 978-0-07-182639-6. [Google Scholar]
| Sample | Bacterium/Growth Medium/Minimum Inhibitory Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Essential Oil | Haemophilus influenzae | Staphylococcus aureus | Streptococcus pneumoniae | Streptococcus pyogenes | x-MIC | ||||
| Broth | Agar | Broth | Agar | Broth | Agar | Broth | Agar | ||
| Cymbopogon citratus | 256 | 256 | 512 | 1024 | 512 | 1024 | 512 | 1024 | 448 |
| Cyperus scariosus. | 1024 | 1024 | 1024 | >1024 | 1024 | >1024 | 1024 | >1024 | 1024 |
| Trachyspermum ammi | 128 | 256 | 512 | 512 | 512 | 1024 | 512 | 1024 | 416 |
| Positive antibiotic control | 1 a | n.d. | 0.5 b | n.d. | 0.25 c | n.d. | 0.25 d | n.d. | - |
| Sample | IC50 ± SD (µg/mL) | IC80 ± SD (µg/mL) | TI |
|---|---|---|---|
| Essential oil | |||
| Cymbopogon citratus | 19.63 ± 1.02 | 29.54 ± 2.18 | 0.065 |
| Cyperus scariosus | >258 | >258 | >0.252 |
| Trachyspermum ammi | 82.04 ± 3.39 | 156.57 ± 13.88 | 0.376 |
| Positive control | |||
| vinorelbine | 0.54 ± 0.26 | >10 | n.a. |
| RI a | Compound | Cl. b | Column | ||||
|---|---|---|---|---|---|---|---|
| Content c (%) | Identification f | ||||||
| Obs. | Lit. | HP-5MS | DB-WAX | HP-5MS | DB-WAX | ||
| 915 | 926 | Tricyclene | MH | 0.26 ± 0.12 | tr. d | RI, MS, Std, | MS |
| 927 | 939 | α-Pinene | MH | 0.20 ± 0.04 | - e | RI, MS, Std | - |
| 942 | 953 | Camphene | MH | 2.42 ± 0.79 | 1.62 ± 0.34 | RI, MS, Std | MS |
| 979 | 985 | Sulcatone | MO | 0.35 ± 0.18 | - | RI, MS | - |
| 1023 | 1029 | D-Limonene | MH | 0.23 ± 0.03 | - | RI, MS | - |
| 1029 | 1050 | trans-β-Ocimene | MH | 0.31 ± 0.09 | - | RI, MS | - |
| 1039 | 1011 | 3-Carene | MH | 0.13 ± 0.02 | - | RI, MS, Std | - |
| 1063 | 1030 | 4-Nonanone | MO | 1.41 ± 0.38 | 1.55 ± 0.97 | RI, MS | MS |
| 1098 | 1098 | Linalool | MO | 0.46 ± 0.24 | 0.31 ± 0.07 | RI, MS, Std | MS |
| 1178 | 1184.7 | Isogeranial | MO | 0.70 ± 0.01 | 0.55 ± 0.05 | RI, MS | MS |
| 1190 | 1189 | α-Terpineol | MO | 0.39 ± 0.03 | - | RI, MS | - |
| 1238 | 1240 | β-Citral | MO | 35.8 ± 0.61 | 24.3 ± 8.82 | RI, MS, Std | MS |
| 1268 | 1270 | α-Citral | MO | 48.9 ± 0.55 | 33.2 ± 11.2 | RI, MS, Std | MS |
| 1376 | 1381 | Geranyl acetate | MO | 2.02 ± 0.20 | tr. | RI, MS | MS |
| 1412 | 1418 | Caryophyllene | SH | 0.45 ± 0.31 | 0.75 ± 0.05 | RI, MS | MS |
| 1510 | 1513 | γ-Cadinene | SH | 1.19 ± 0.33 | 1.20 ± 0.23 | RI, MS | MS |
| g | 1797 | Geraniol | MO | - | 0.77 ± 0.07 | - | MS |
| 1582 | 1581 | Caryophyllene oxide | SH | 3.0 ± 1.34 | 2.88 ± 1.40 | RI, MS, Std | MS |
| g | 1430 | α-Cyclocitral | MO | - | 0.45 ± 0.04 | - | MS |
| g | NA | Isoneral | MO | - | 0.36 ± 0.02 | - | MS |
| g | 1669 | Isoborneol | MO | - | 0.83 ± 0.01 | - | MS |
| Total content (%) | 99.62 | 68.77 | |||||
| RI a | Compound | Cl. b | Column | ||||
|---|---|---|---|---|---|---|---|
| Content c (%) | Identification f | ||||||
| Obs. | Lit. | HP-5MS | DB-WAX | HP-5MS | DB-WAX | ||
| 927 | 939 | α-Pinene | MH | 1.34 ± 0.10 | 0.50 ± 0.01 | RI, MS, Std | MS |
| 970 | 980 | β-Pinene | MH | 1.88 ± 0.33 | 0.06 ± 0.00 | RI, MS, Std | MS |
| 1025 | 1032 | Eucalyptol | MO | 0.24 ± 0.16 | - d | RI, MS | - |
| 1137 | 1137 | Pinocarveol | MO | 1.85 ± 0.23 | 0.60 ± 0.01 | RI, MS | MS |
| 1158 | 1165 | Pinocarvone | MO | 0.37 ± 0.32 | 0.09 ± 0.00 | RI, MS | MS |
| 1168 | 1193 | Myrtenal | MO | 0.41 ± 0.27 | 0.13 ± 0.30 | RI, MS | MS |
| 1314 | 1327 | Cyprotene | SH | 0.16 ± 0.05 | tr.e | RI, MS | MS |
| 1344 | 1349 | α-Terpinyl acetate | MO | 1.65 ± 0.18 | tr. | RI, MS | MS |
| 1371 | 1376 | Copaene | SH | 1.46 ± 0.47 | tr. | RI, MS | MS |
| 1394 | 1398 | Cyperene | SH | 9.87 ± 0.59 | 8.5 ± 0.04 | RI, MS | MS |
| 1446 | 1477 | α-Muurolene | SH | 0.16 ± 0.04 | tr. | RI, MS | MS |
| 1456 | 1461 | Rotundene | SH | 1.94 ± 0.07 | 1.25 ± 0.01 | RI, MS | MS |
| 1483 | 1473.7 | γ-Patchoulene | SH | 0.19 ± 0.05 | tr. | RI, MS | MS |
| 1489 | 1491 | Valencene | SH | 0.63 ± 0.08 | 0.57 ± 0.60 | RI, MS | MS |
| 1518 | 1518 | β-Cadinene | SH | 0.31 ± 0.18 | 0.08 ± 0.40 | RI, MS | MS |
| 1528 | 1532 | Cyperene epoxide | SO | 2.65 ± 0.26 | 1.50 ± 0.00 | RI, MS | MS |
| 1541 | 1542 | α-Calacorene | SH | 0.13 ± 0.05 | - | RI, MS | - |
| 1565 | 1579 | Isoaromadendrene epoxide | SO | 0.62 ± 0.07 | 1.03 ± 0.00 | RI, MS | MS |
| 1572 | 1627 | Longiverbenone | SO | 1.33 ± 0.15 | 1.20 ± 0.08 | RI, MS | MS |
| 1582 | 1581 | Caryophyllene oxide | SH | 19.79 ± 0.58 | 17.54 ± 0.12 | RI, MS, Std | MS |
| 1591 | NA | β-Santalol | SO | 0.38 ± 0.12 | - | RI, MS | - |
| 1609 | 1608 | Humulene epoxide 2 | SO | 1.69 ± 0.25 | 2.60 ± 0.10 | RI, MS | MS |
| 1656 | 1604 | Globulol | SO | 0.23 ± 0.04 | 0.39 ± 0.03 | RI, MS | MS |
| 1664 | 1663 | Patchouli alcohol | SO | 0.50 ± 0.05 | - | RI, MS | - |
| 1677 | 1676 | Mustakone | SO | 6.26 ± 0.26 | 3.67 ± 0.30 | RI, MS | MS |
| 1697 | 1694 | Cyperotundone | SO | 29.1 ± 1.11 | 28.91 ± 0.72 | RI, MS | MS |
| 1750 | 1752 | Aristolone | SO | 3.17 ± 0.77 | 3.73 ± 0.10 | RI, MS | MS |
| 1808 | 1807 | Nootkatone | SO | 2.17 ± 0.49 | 2.03 ± 0.40 | RI, MS | MS |
| g | NA | β-Pinone | MO | - | tr. | - | MS |
| g | 1586 | β-Elemene | SH | - | tr. | - | MS |
| g | 1652 | cis-Verbenol | MO | - | tr. | - | MS |
| g | NA | Aristolochene | SH | - | tr. | - | MS |
| g | 1680 | α-Terpineol | tr. | - | MS | ||
| g | NA | α-Maaliene | SH | - | tr. | - | MS |
| g | 1784 | Myrtenol | MO | - | tr. | - | MS |
| g | 2063 | Cubenol | SO | - | 0.49 ± 0.00 | - | MS |
| g | 1978 | α-Cedrene epoxide | SO | - | 0.40 ± 0.10 | - | MS |
| g | NA | Aromadendrene oxide-(1) | SO | - | 1.35 ± 0.40 | - | MS |
| g | NA | Calarene epoxide | SO | - | 0.52 ± 0.02 | - | MS |
| g | NA | Diepicedrene-1-oxide | SO | - | 0.05 ± 0.00 | - | MS |
| Total content (%) | 91.48 | 77.17 | |||||
| RI a | Compound | Cl. b | Column | ||||
|---|---|---|---|---|---|---|---|
| Content c (%) | Identification e | ||||||
| Obs. | Lit. | HP-5MS | DB-WAX | HP-5MS | DB-WAX | ||
| 920 | 917 | β-Thujene | MH | 0.30 ± 0.03 | 0.17 ± 0.02 | RI, MS | MS |
| 964 | 925 | α-Pinene | MH | 0.19 ± 0.01 | 0.42 ± 0.13 | RI, MS, Std | MS |
| 981 | 971 | β-Pinene | MH | 1.87 ± 0.25 | 2.13 ± 0.62 | RI, MS, Std | MS |
| 994 | 984 | β-Myrcene | MH | 0.21 ± 0.06 | 0.38 ± 0.03 | RI, MS | MS |
| 1007 | 974 | 2-Carene | MH | 0.20 ± 0.02 | - d | RI, MS | MS |
| 1050 | 1031 | β-Cymene | MH | 22.6 ± 0.89 | 17.1 ± 3.99 | RI, MS, Std | MS |
| 1079 | 1065 | γ-Terpinene | MH | 21.5 ± 0.86 | 17.6 ± 0.99 | RI, MS, Std | MS |
| 1175 | 1086 | Isoterpinolene | MH | 0.08 ± 0.06 | - | RI, MS | MS |
| 1315 | 1290 | Thymol | MO | 51.2 ± 1.25 | 45.8 ± 4.41 | RI, MS, Std | MS |
| f | 1172 | α-Terpinene | MH | - | 0.12 ± 0.01 | - | MS |
| f | 1244 | D-Limonene | MH | - | 0.10 ± 0.01 | - | MS |
| f | 1195 | β-Phellandrene | MH | - | 0.08 ± 0.03 | - | MS |
| f | NA | trans-2-Caren-4-ol | MO | - | 0.18 ± 0.02 | - | MS |
| f | 1680 | Terpineol | MO | - | 0.06 ± 0.01 | - | MS |
| f | 1635 | Terpinen-4-ol | MO | - | 0.20 ± 0.04 | - | MS |
| Total content (%) | 99.47 | 84.26 | |||||
| RI a | Compound | Extraction Method/Time (h)/Content b (%) | Ident. e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid Phase Microextraction | Gas Tight Syringe Extraction | ||||||||||||
| Obs. | Lit. | 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | ||
| 925 | 939 | α-Pinene | 1.27 ± 0.10 | 1.46 ± 0.00 | 1.42 ± 0.01 | 1.41 ± 0.00 | 1.25 ± 0.01 | tr. c | tr. | tr. | tr. | tr. | RI, MS |
| 961 | 1011 | 2-Carene | 0.64 ± 0.00 | 0.44 ± 0.00 | 0.62 ± 0.01 | 0.65 ± 0.02 | 0.72. ±0.01 | - d | - | - | - | - | RI, MS |
| 971 | 980 | β-Pinene | 1.91 ± 0.01 | 2.80 ± 0.05 | 2.36 ± 0.00 | 2.31 ± 0.01 | 1.79 ± 0.20 | 6.57 ± 0.20 | 4.93 ± 0.40 | 3.89 ± 0.10 | 4.11 ± 0.01 | 2.02 ± 0.30 | RI, MS |
| 1031 | 1030 | β-Cymene | 49.14 ± 1.00 | 48.00 ± 1.10 | 46.57 ± 0.80 | 45.97 ± 1.80 | 43.17 ± 0.90 | 52.00 ± 3.50 | 49.18 ± 2.90 | 48.67 ± 2.50 | 46.32 ± 1.40 | 45.60 ± 0.60 | RI, MS |
| 1065 | 1062 | γ-Terpinene | 39.36 ± 0.70 | 35.41 ± 0.50 | 35.11 ± 0.90 | 33.26 ± 0.60 | 31.01 ± 0.20 | 35.00 ± 2.50 | 32.23 ± 0.09 | 31.60 ± 0.50 | 32.22 ± 1.30 | 28.20 ± 2.90 | RI, MS |
| 1306 | 1290 | Thymol | 4.96 ± 0.01 | 5.66 ± 0.40 | 9.82 ± 0.08 | 11.90 ± 0.93 | 12.10 ± 0.80 | tr. | 1.23 ± 0.10 | 2.21 ± 0.20 | 2.07 ± 0.03 | tr. | RI, MS |
| Total content (%) | 97.25 | 94.21 | 95.9 | 95.5 | 90.66 | 93.40 | 88.57 | 87.98 | 85.72 | 79.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaure, A.; Houdkova, M.; Antih, J.; Urbanova, K.; Doskocil, I.; Naik, M.L.; Patel, K.S.; Kokoska, L. Validation of Broth Macrodilution Volatilization Method for Testing of Essential Oils in Liquid and Vapor Phase: Chemical Composition, Cytotoxicity, and Antibacterial Effect of Indian Medicinal Plants against Pneumonia-Causing Pathogens. Molecules 2023, 28, 4625. https://doi.org/10.3390/molecules28124625
Chaure A, Houdkova M, Antih J, Urbanova K, Doskocil I, Naik ML, Patel KS, Kokoska L. Validation of Broth Macrodilution Volatilization Method for Testing of Essential Oils in Liquid and Vapor Phase: Chemical Composition, Cytotoxicity, and Antibacterial Effect of Indian Medicinal Plants against Pneumonia-Causing Pathogens. Molecules. 2023; 28(12):4625. https://doi.org/10.3390/molecules28124625
Chicago/Turabian StyleChaure, Aishwarya, Marketa Houdkova, Julien Antih, Klara Urbanova, Ivo Doskocil, Mukund Lal Naik, Khageshwar Singh Patel, and Ladislav Kokoska. 2023. "Validation of Broth Macrodilution Volatilization Method for Testing of Essential Oils in Liquid and Vapor Phase: Chemical Composition, Cytotoxicity, and Antibacterial Effect of Indian Medicinal Plants against Pneumonia-Causing Pathogens" Molecules 28, no. 12: 4625. https://doi.org/10.3390/molecules28124625
APA StyleChaure, A., Houdkova, M., Antih, J., Urbanova, K., Doskocil, I., Naik, M. L., Patel, K. S., & Kokoska, L. (2023). Validation of Broth Macrodilution Volatilization Method for Testing of Essential Oils in Liquid and Vapor Phase: Chemical Composition, Cytotoxicity, and Antibacterial Effect of Indian Medicinal Plants against Pneumonia-Causing Pathogens. Molecules, 28(12), 4625. https://doi.org/10.3390/molecules28124625









