Adhesive and Flame-Retardant Properties of Starch/Ca2+ Gels with Different Amylose Contents
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Information of Starches
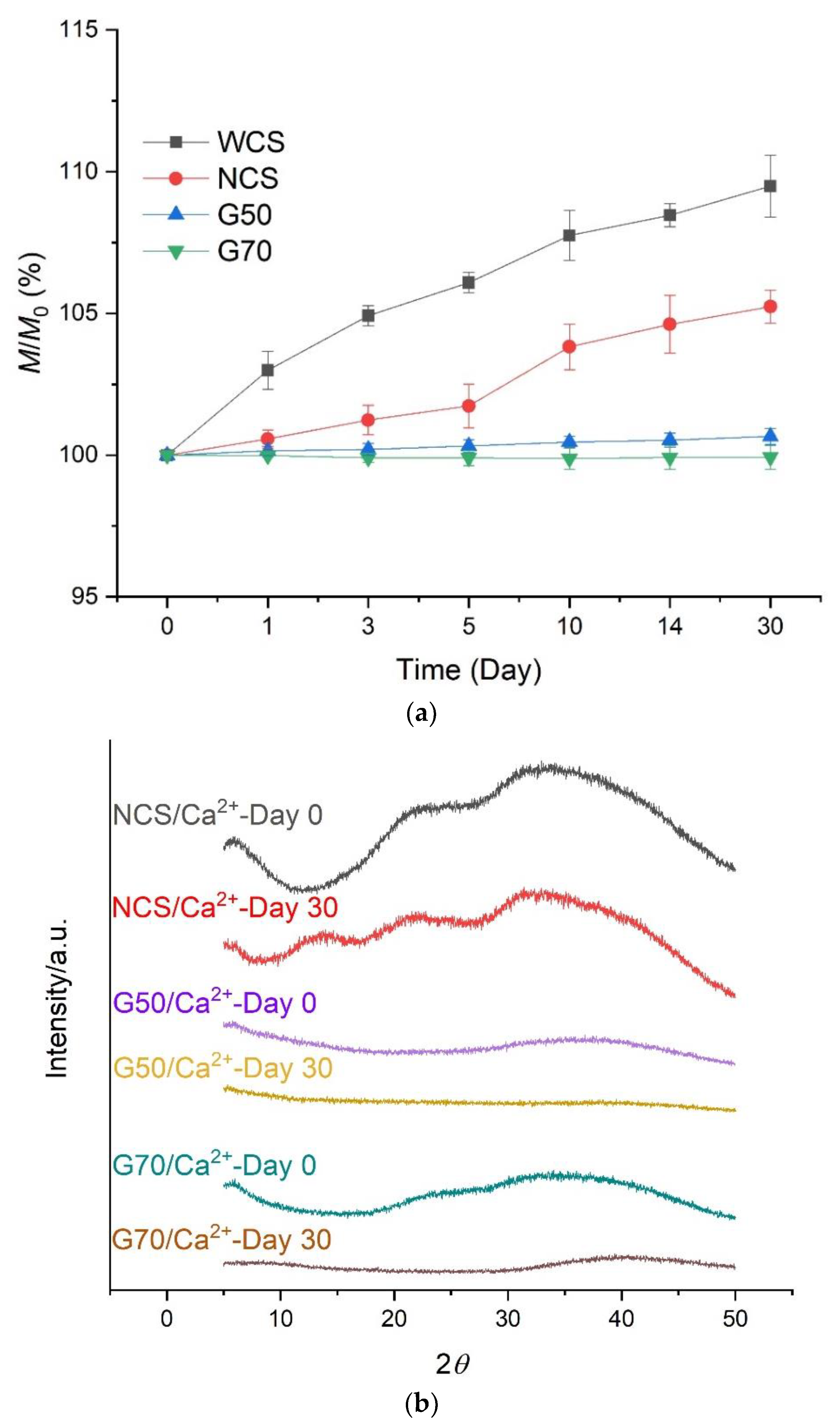
2.2. Mass and Crystalline Structure Changes of Starch/Ca2+ Gels
2.3. Rheological Properties of Starch/Ca2+ Gels
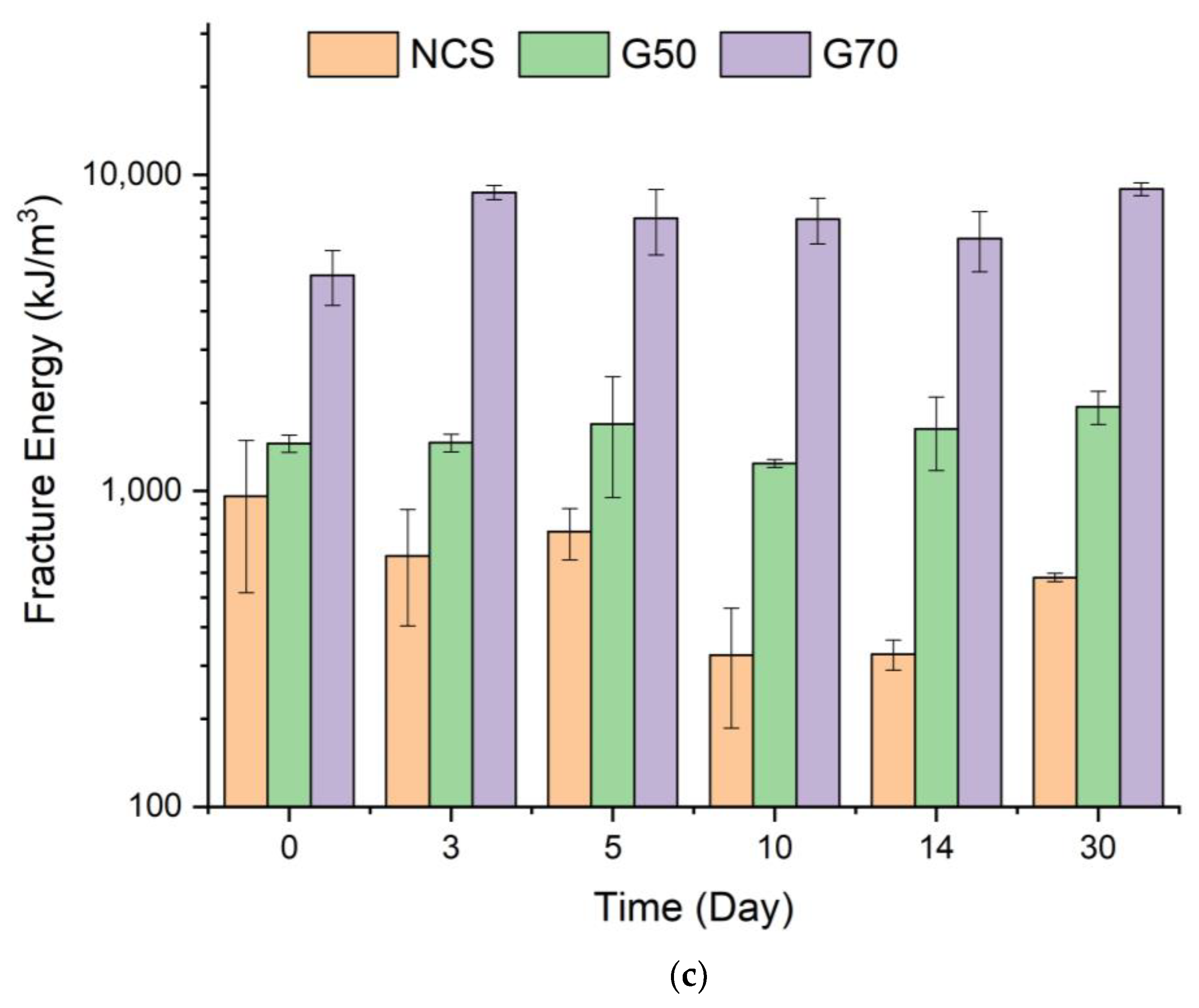
2.4. Tensile Properties of Starch/Ca2+ Gels
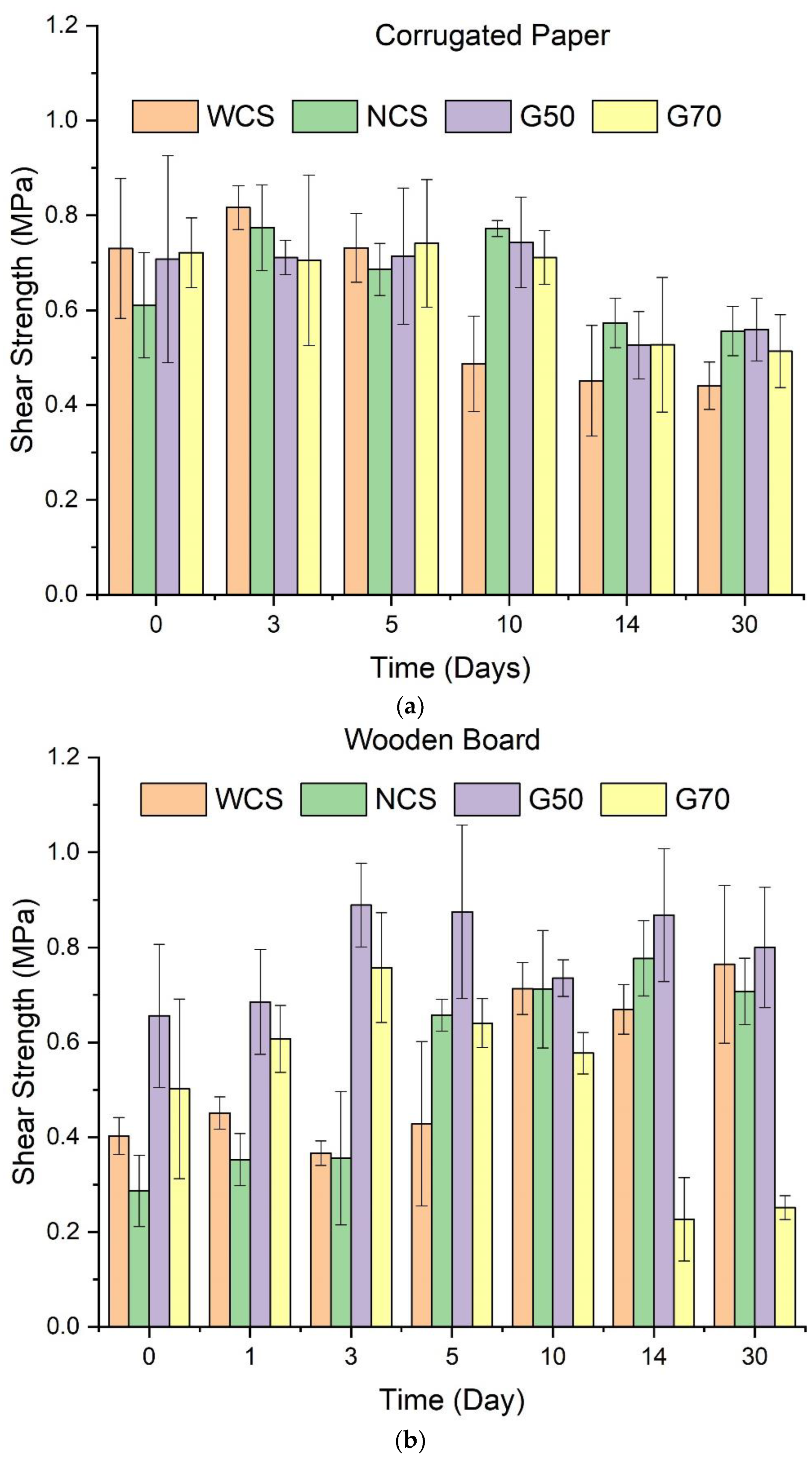
2.5. Adhesive Ability of Starch/Ca2+ Gels
2.6. Flame-Retardant Properties of Starch/Ca2+ Gels
3. Materials and Methods
3.1. Materials
3.2. Molecular Information of Starches
3.3. Amylose Contents in Starch Granules
3.4. Preparation of Starch/Ca2+ Gels
3.5. Mass and Crystalline Structure Changes of Starch/Ca2+ Gels during Storage
3.5.1. Water Retention Property
3.5.2. X-ray Diffraction (XRD)
3.6. Rheological Properties of Starch/Ca2+ Gels
3.6.1. Oscillatory Frequency–Sweep Test
3.6.2. Steady–Shear Test
3.7. Tensile Properties of Starch/Ca2+ Gels
3.8. Adhesive Properties of Starch/Ca2+ Gels
3.9. Morphology of the Section of Bonded Wooden Boards
3.10. Limiting Oxygen Index of Starch/Ca2+ Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ai, Y.; Jane, J.-l. Gelatinization and rheological properties of starch. Starch-Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Zhu, H.Y.; Liang, W.X.; Li, X.; Liu, L.S.; Zhang, X.D.; Yue, H.F.; Xue, J.Q.; Liu, X.X.; Guo, D.W. High-amylose starch as a new ingredient to balance nutrition and texture of food. J. Cereal Sci. 2018, 81, 8–14. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, P.; Li, Y.; Li, J.; Li, X.; Yang, J.; Ji, M.; Li, F.; Zhang, C. Recent advances and future challenges of the starch-based bio-composites for engineering applications. Carbohydr. Polym. 2023, 307, 120627. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Budarin, V.; Clark, J.H. Green power—“molten” starch adhesives. J. Mater. Chem. 2009, 19, 8589–8593. [Google Scholar] [CrossRef]
- Tratnik, N.; Kuo, P.Y.; Tanguy, N.R.; Gnanasekar, P.; Yan, N. Biobased Epoxidized Starch Wood Adhesives: Effect of Amylopectin and Amylose Content on Adhesion Properties. ACS Sustain. Chem. Eng. 2020, 8, 17997–18005. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, J.P.; Wang, Z.J. Preparation and Properties of Cassava Starch-based Wood Adhesives. Bioresources 2016, 11, 6756–6767. [Google Scholar] [CrossRef]
- Chen, X.J.; Yao, W.R.; Gao, F.F.; Zheng, D.Y.; Wang, Q.; Cao, J.; Tan, H.Y.; Zhang, Y.H. Physicochemical Properties Comparative Analysis of Corn Starch and Cassava Starch, and Comparative Analysis as Adhesive. J. Renew. Mater. 2021, 9, 979–992. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, L.L.; Gu, J.Y.; Tan, H.Y.; Zhu, L.B. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Tang, H.; Fan, S.; Li, Y.; Dong, S. Amylose: Acetylation, Optimization, and Characterization. J. Food Sci. 2019, 84, 738–745. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Halley, P.J.; Avérous, L. Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 2012, 37, 595–623. [Google Scholar] [CrossRef]
- Ma, C.; Xie, F.; Wei, L.; Zheng, C.; Liu, X.; Wang, L.; Liu, P. All-Starch-Based Hydrogel for Flexible Electronics: Strain-Sensitive Batteries and Self-Powered Sensors. ACS Sustain. Chem. Eng. 2022, 10, 6724–6735. [Google Scholar] [CrossRef]
- Lin, L.S.; Guo, D.W.; Zhao, L.X.; Zhang, X.D.; Wang, J.; Zhang, F.M.; Wei, C.X. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. High-Amylose Starches to Bridge the “Fiber Gap”: Development, Structure, and Nutritional Functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xie, F.; Wen, W.; Chen, L.; Shang, X.; Liu, P. Understanding the structural features of high-amylose maize starch through hydrothermal treatment. Int. J. Biol. Macromol. 2016, 84, 268–274. [Google Scholar] [CrossRef]
- Yang, M.; Chen, T.; Tan, L.; Ji, Q. Investigation on Flame Retardancy of Starch Modified with Metal Ions. Mater. Sci. 2019, 9, 157–163. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, P.; Chen, Y.; Huang, T.; Liu, Y.; Ding, D.; Zhang, G. A bio-based macromolecular phosphorus-containing active cotton flame retardant synthesized from starch. Carbohydr. Polym. 2022, 298, 120076. [Google Scholar] [CrossRef]
- Shi, R.; Tan, L.; Zong, L.; Ji, Q.; Li, X.; Zhang, K.; Cheng, L.; Xia, Y. Influence of Na+ and Ca2+ on flame retardancy, thermal degradation, and pyrolysis behavior of cellulose fibers. Carbohydr. Polym. 2017, 157, 1594–1603. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibres. Polym. Degrad. Stab. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Lin, M.; Shang, X.; Liu, P.; Xie, F.; Chen, X.; Sun, Y.; Wan, J. Zinc chloride aqueous solution as a solvent for starch. Carbohydr. Polym. 2015, 136, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, P.; Ma, C.; Zhang, N.; Shang, X.; Wang, L.; Xie, F. Structural Disorganization and Chain Aggregation of High-Amylose Starch in Different Chloride Salt Solutions. ACS Sustain. Chem. Eng. 2020, 8, 4838–4847. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Alvarez-Ramirez, J.; Agama-Acevedo, E. Structural characterization of aroid starches by means of chromatographic techniques. Food Hydrocoll. 2017, 69, 97–102. [Google Scholar] [CrossRef]
- Castanha, N.; Miano, A.C.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H.; Augusto, P.E.D. Starch modification by ozone: Correlating molecular structure and gel properties in different starch sources. Food Hydrocoll. 2020, 108, 106027. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Wang, X.; Li, D.; Chen, L.; Li, X. Glass transition temperature of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2010, 51, 388–391. [Google Scholar] [CrossRef]
- Zhong, Y.; Tai, L.; Blennow, A.; Ding, L.; Herburger, K.; Qu, J.; Xin, A.; Guo, D.; Hebelstrup, K.H.; Liu, X. High-amylose starch: Structure, functionality and applications. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Liu, P.; Ma, C.; Li, Y.; Wang, L.; Wei, L.; Yan, Y.; Xie, F. Facile Preparation of Eco-Friendly, Flexible Starch-Based Materials with Ionic Conductivity and Strain-Responsiveness. ACS Sustain. Chem. Eng. 2020, 8, 19117–19128. [Google Scholar] [CrossRef]
- Koshevoy, E.I.; Samsonenko, D.G.; Berezin, A.S.; Fedin, V.P. Metal-Organic Coordination Polymers Formed from γ-Cyclodextrin and Divalent Metal Ions. Eur. J. Inorg. Chem. 2019, 2019, 4321–4327. [Google Scholar] [CrossRef]
- Long, Y.; Christie, G. Microstructure and mechanical properties of orientated thermoplastic starches. J. Mater. Sci. 2005, 40, 111–116. [Google Scholar]
- Gao, Y.; Gu, S.; Jia, F.; Gao, G. A skin-matchable, recyclable and biofriendly strain sensor based on a hydrolyzed keratin-containing hydrogel. J. Mater. Chem. A 2020, 8, 24175–24183. [Google Scholar] [CrossRef]
- Owczarz, P.; Orczykowska, M.; Ryl, A.; Ziolkowski, P. The effects of sucrose on the sol-gel phase transition and viscoelastic properties of potato starch solutions. Food Chem. 2019, 271, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Teyssandier, F.; Cassagnau, P.; Gérard, J.F.; Mignard, N. Sol–gel transition and gelatinization kinetics of wheat starch. Carbohydr. Polym. 2011, 83, 400–406. [Google Scholar] [CrossRef]
- Hegetschweiler, K.; Hancock, R.D.; Ghisletta, M.; Kradolfer, T.; Gramlich, V.; Schmalle, H.W. 1,3,5-Triamino-1,3,5-trideoxy-cis-inositol, a ligand with a remarkable versatility for metal ions. 5. Complex formation with magnesium(II), calcium(II), strontium(II), barium(II), and cadmium(II). Inorg. Chem. 1993, 32, 5273–5284. [Google Scholar] [CrossRef]
- Hussin, M.H.; Abd Latif, N.H.; Hamidon, T.S.; Idris, N.N.; Hashim, R.; Appaturi, J.N.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Fatriasari, W.; et al. Latest advancements in high-performance bio-based wood adhesives: A critical review. J. Mater. Res. Technol. 2022, 21, 3909–3946. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion processing and characterization of edible starch films with different amylose contents. J. Food Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Donmez Cavdar, A. Effect of various wood preservatives on limiting oxygen index levels of fir wood. Measurement 2014, 50, 279–284. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Shang, X.; Xie, F.; Jiang, H.; Wang, J. Investigation of rheological properties and conformation of cassava starch in zinc chloride solution. Starch-Stärke 2017, 69, 1600384. [Google Scholar] [CrossRef]
- Xie, W.L.; Shao, L. Phosphorylation of corn starch in an ionic liquid. Starch-Stärke 2009, 61, 702–708. [Google Scholar] [CrossRef]
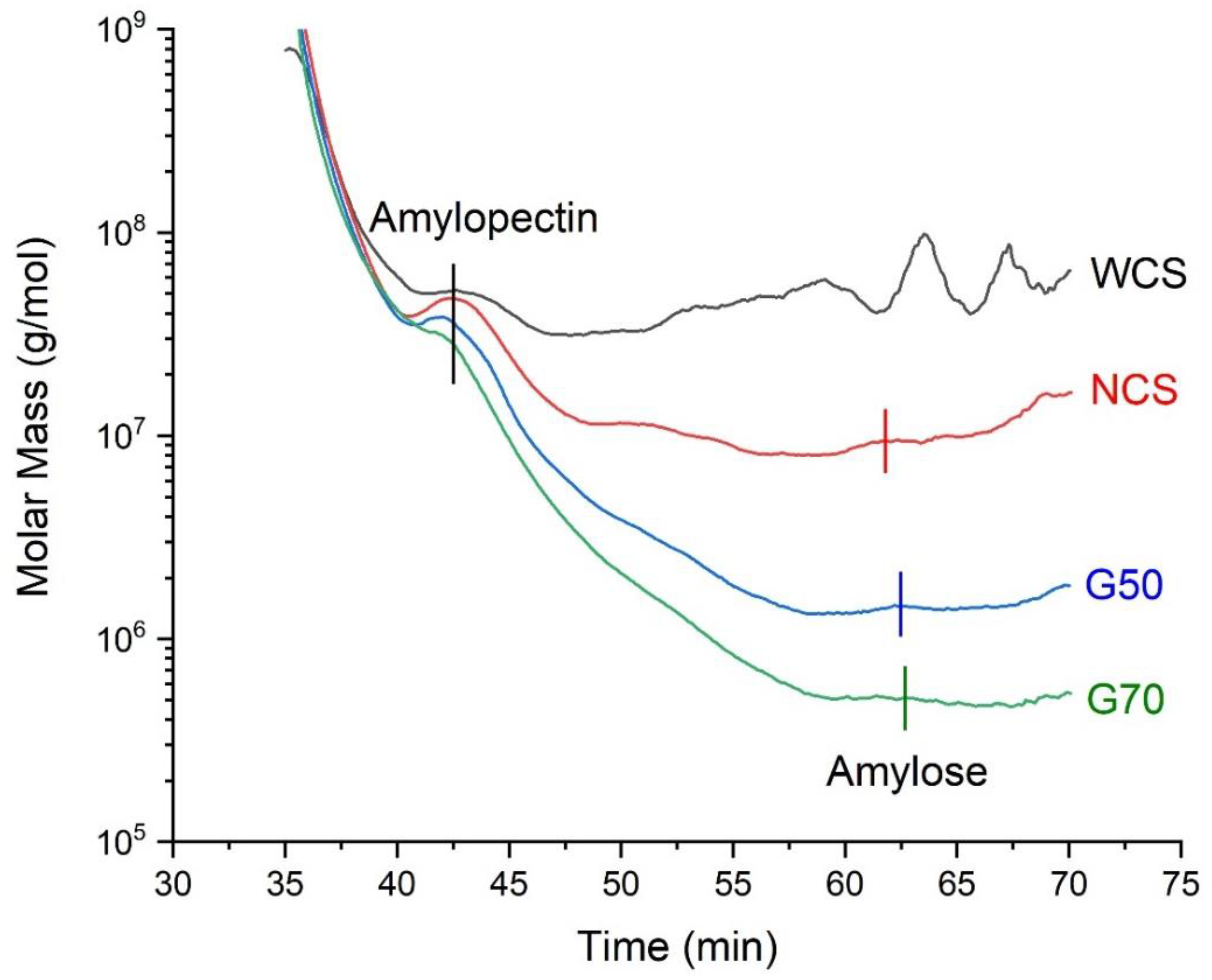


| Amylopectin | Amylose | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Content (%) | Mw * (kDa) | Polydispersity (Mw/Mn) | Rz (nm) | Content (%) | Mw (kDa) | Polydispersity (Mw/Mn) | Rz (nm) |
| WCS | 100% | 77,068.05 | 1.553 | 130.51 | 0% | ------ | ------ | ------ |
| NCS | 73% | 61,194.34 | 2.417 | 117.95 | 27% | 9068.29 | 1.011 | 126.56 |
| G50 | 45% | 55,962.61 | 1.452 | 107.28 | 55% | 2060.63 | 1.714 | 92.919 |
| G70 | 32% | 35,458.19 | 1.363 | 105.72 | 68% | 721.32 | 2.076 | 84.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Ling, J.; Mao, T.; Liu, F.; Zhou, W.; Zhang, G.; Xie, F. Adhesive and Flame-Retardant Properties of Starch/Ca2+ Gels with Different Amylose Contents. Molecules 2023, 28, 4543. https://doi.org/10.3390/molecules28114543
Liu P, Ling J, Mao T, Liu F, Zhou W, Zhang G, Xie F. Adhesive and Flame-Retardant Properties of Starch/Ca2+ Gels with Different Amylose Contents. Molecules. 2023; 28(11):4543. https://doi.org/10.3390/molecules28114543
Chicago/Turabian StyleLiu, Peng, Jiandi Ling, Taoyan Mao, Feng Liu, Wenzhi Zhou, Guojie Zhang, and Fengwei Xie. 2023. "Adhesive and Flame-Retardant Properties of Starch/Ca2+ Gels with Different Amylose Contents" Molecules 28, no. 11: 4543. https://doi.org/10.3390/molecules28114543
APA StyleLiu, P., Ling, J., Mao, T., Liu, F., Zhou, W., Zhang, G., & Xie, F. (2023). Adhesive and Flame-Retardant Properties of Starch/Ca2+ Gels with Different Amylose Contents. Molecules, 28(11), 4543. https://doi.org/10.3390/molecules28114543









