Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds
Abstract
1. Introduction
2. Results
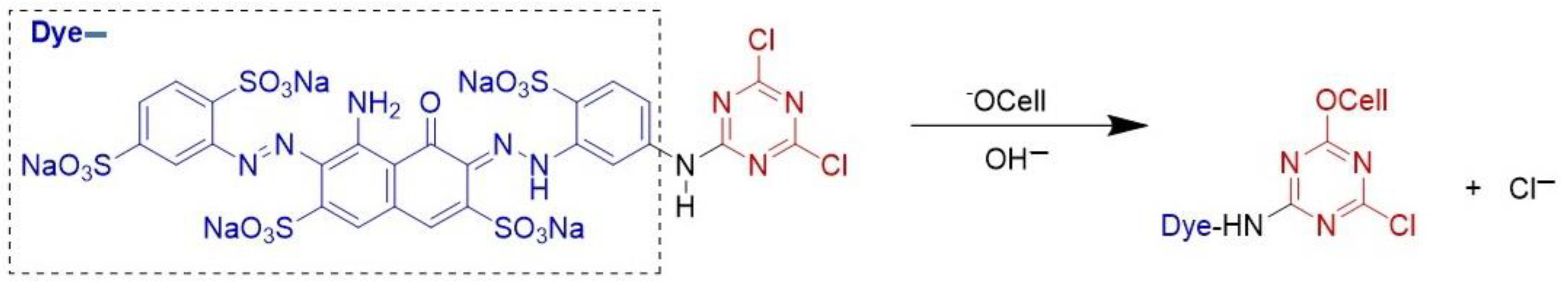
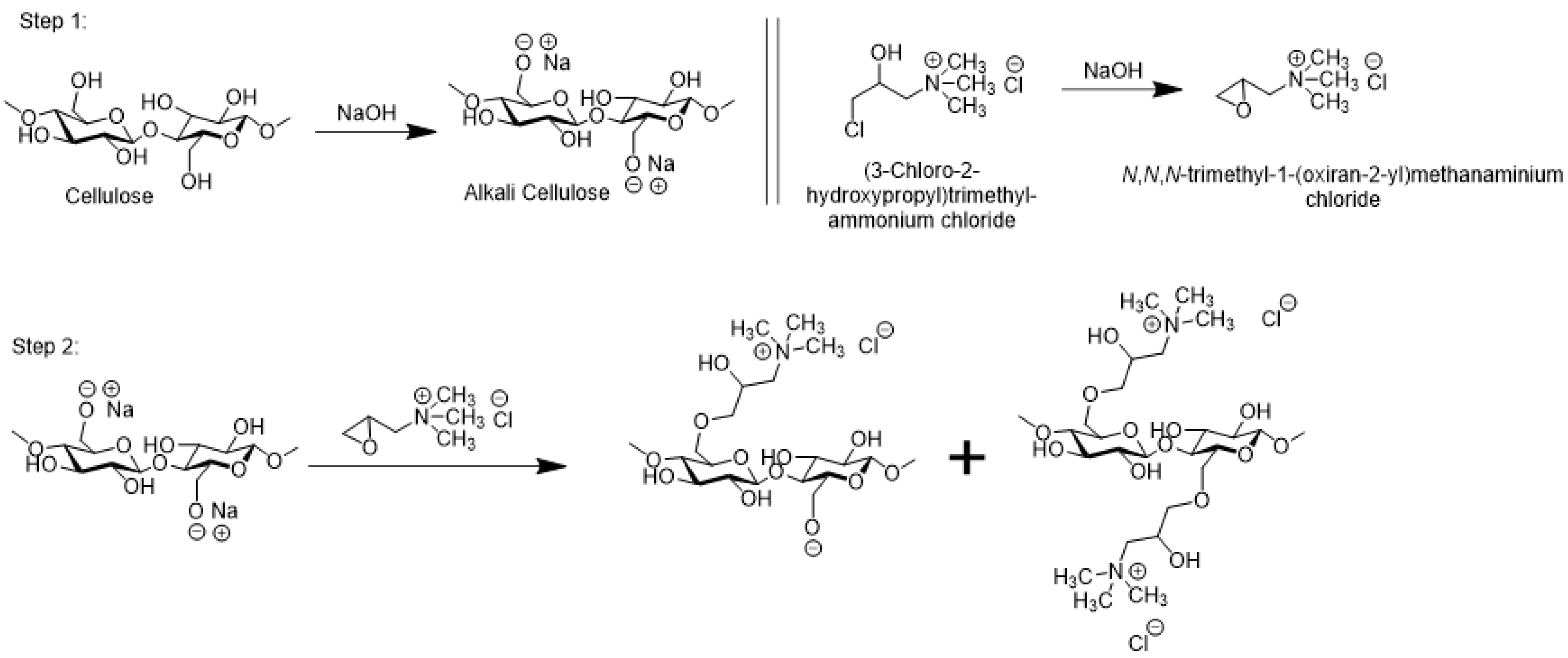
2.1. Fabric Cationization
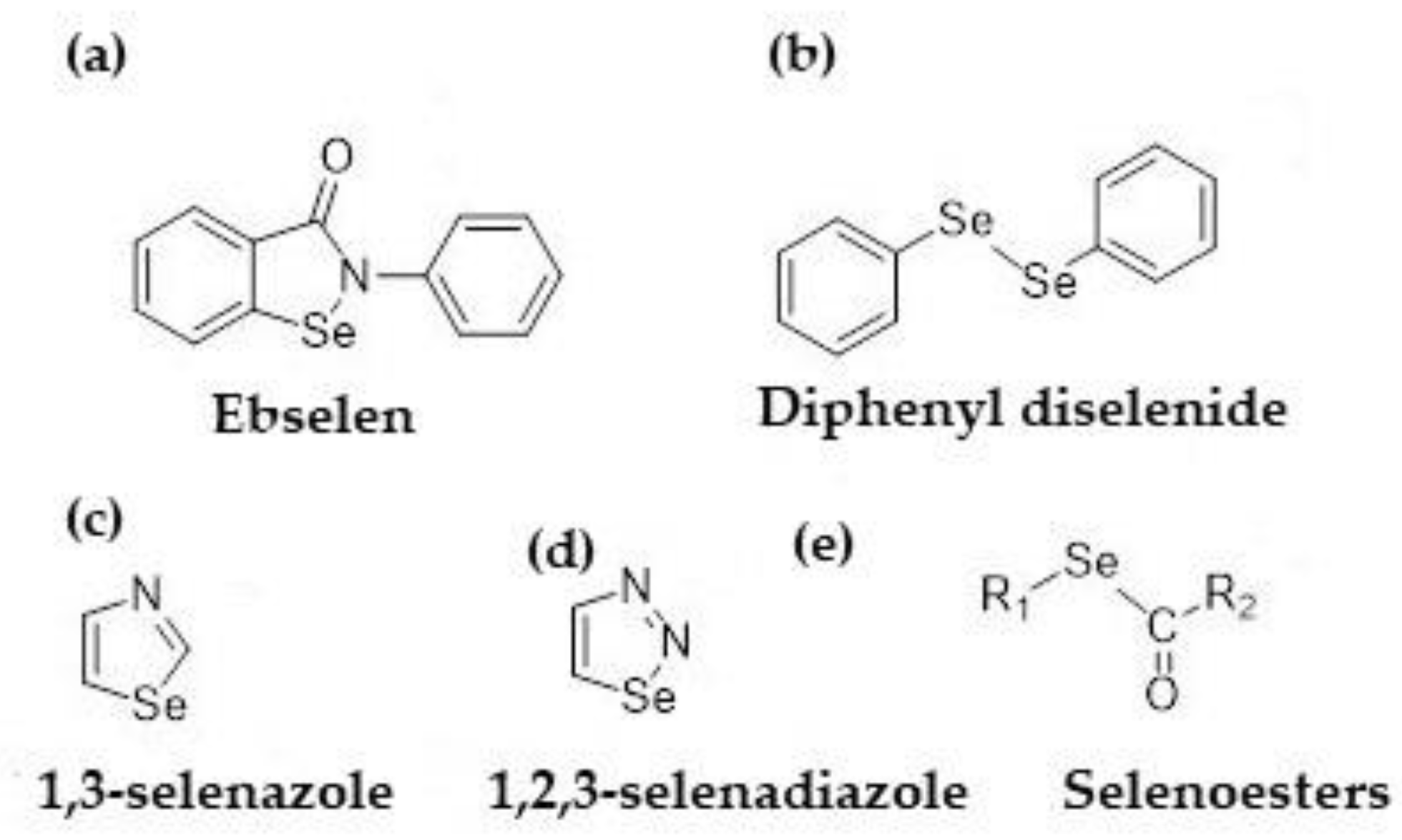
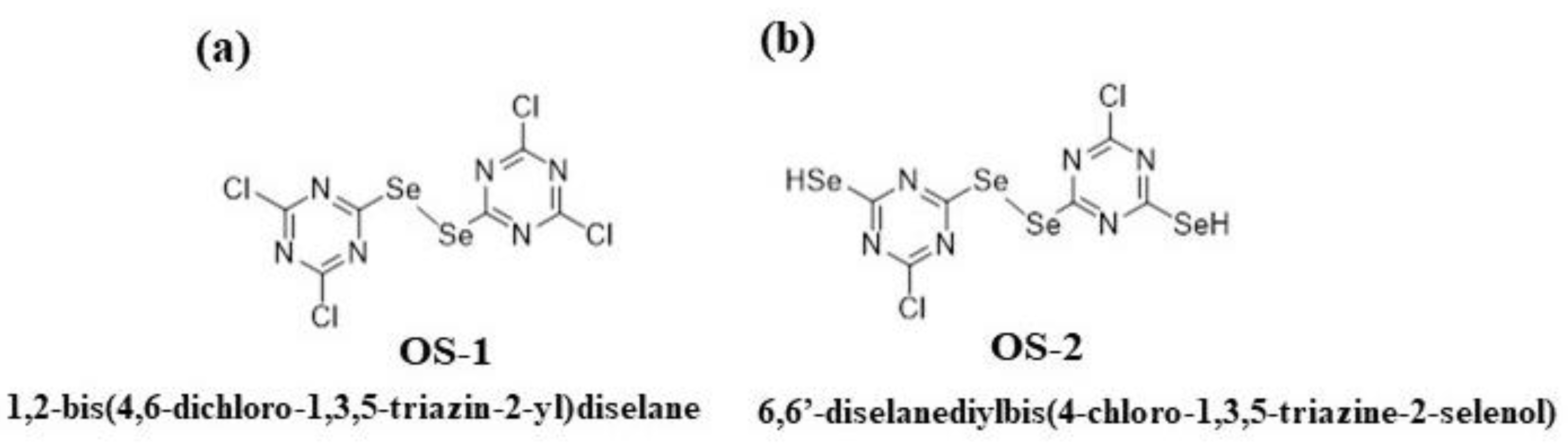
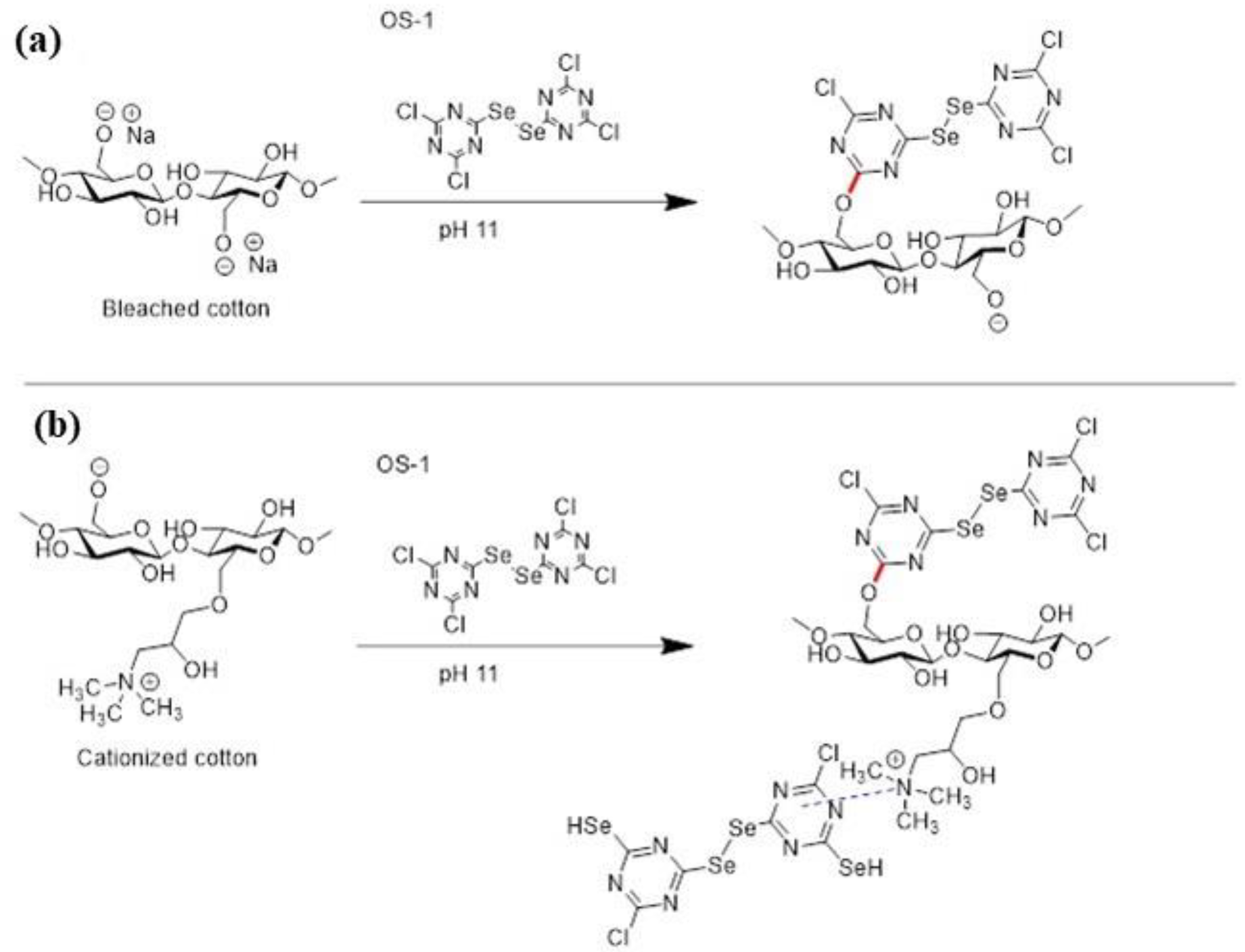
2.2. Preparation of Organoselenium Textiles
2.2.1. Preparation
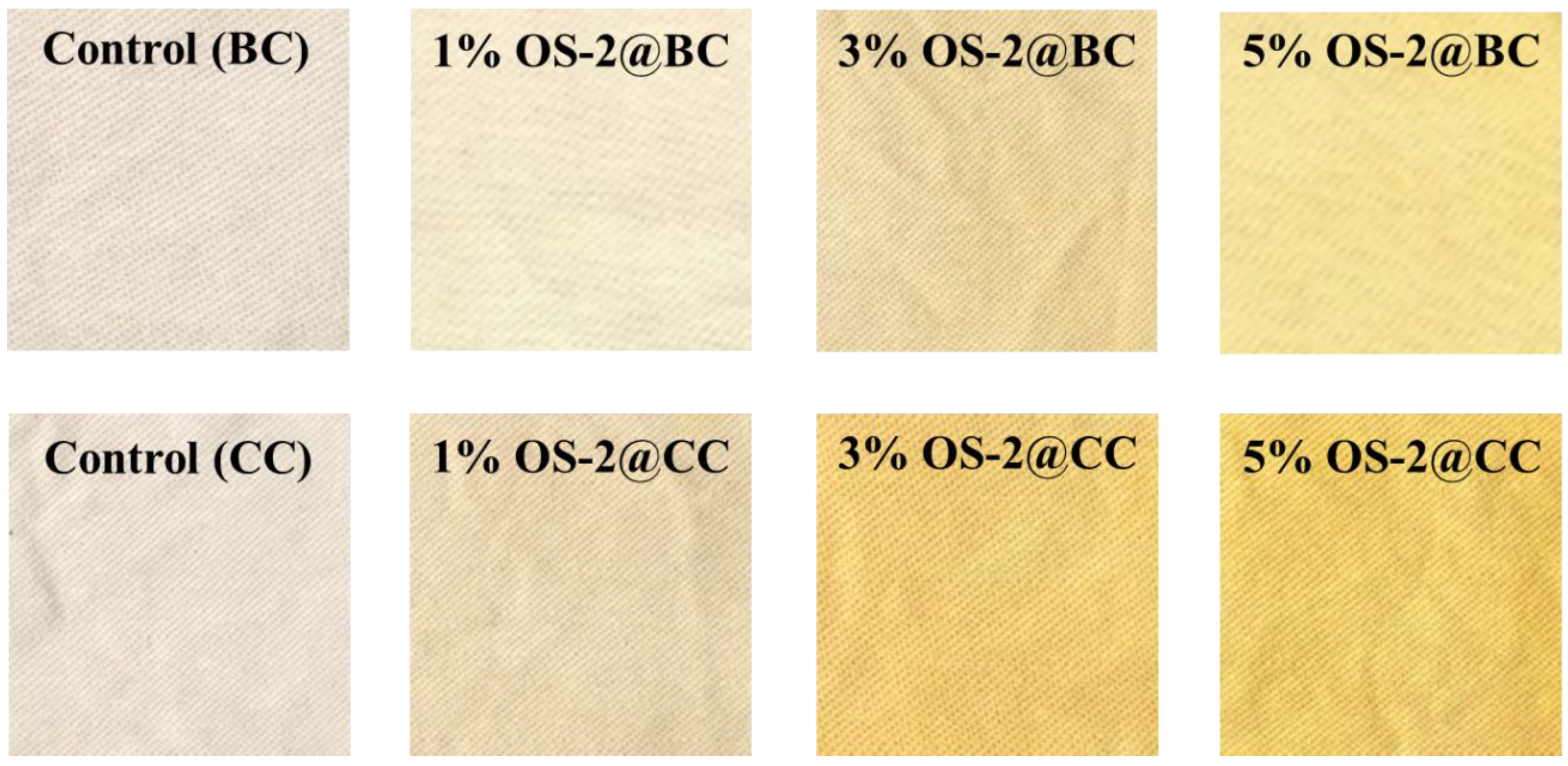
2.2.2. Appearance
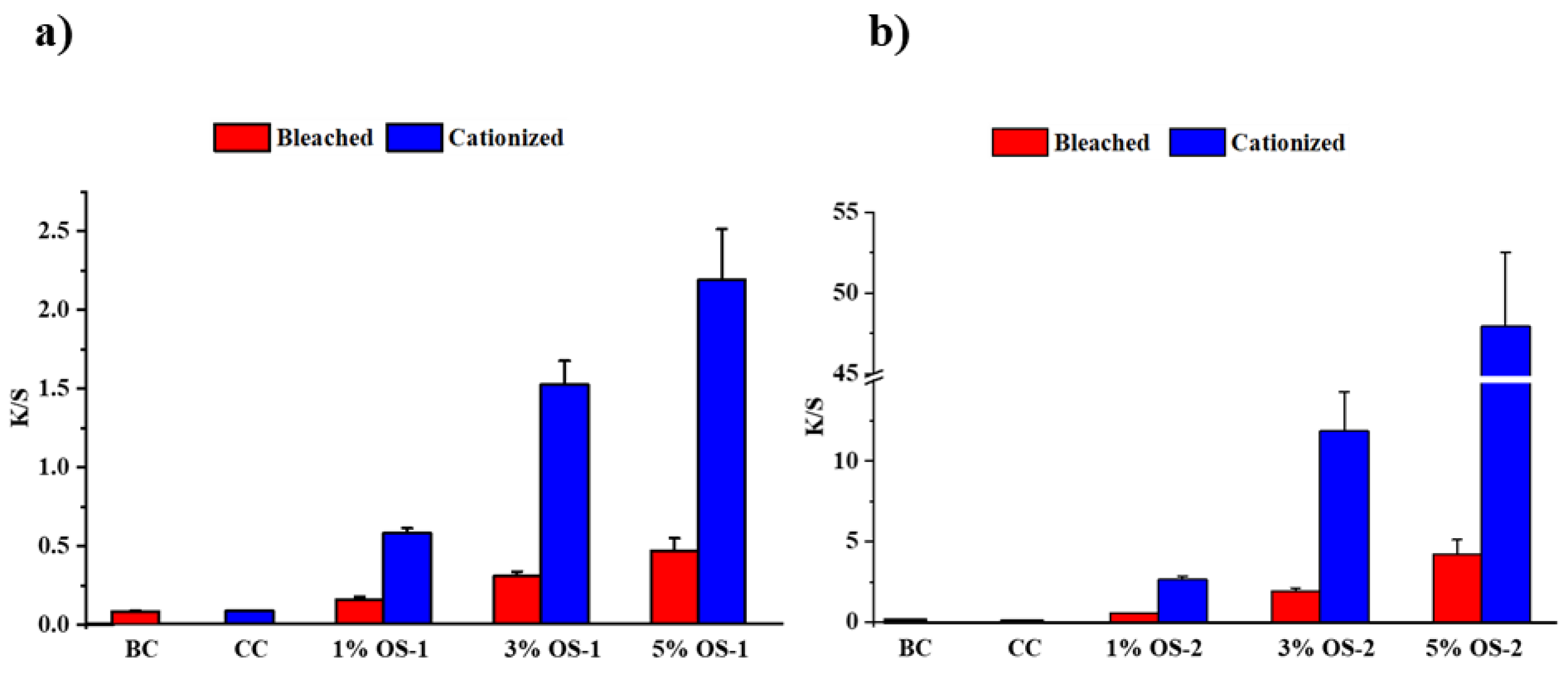
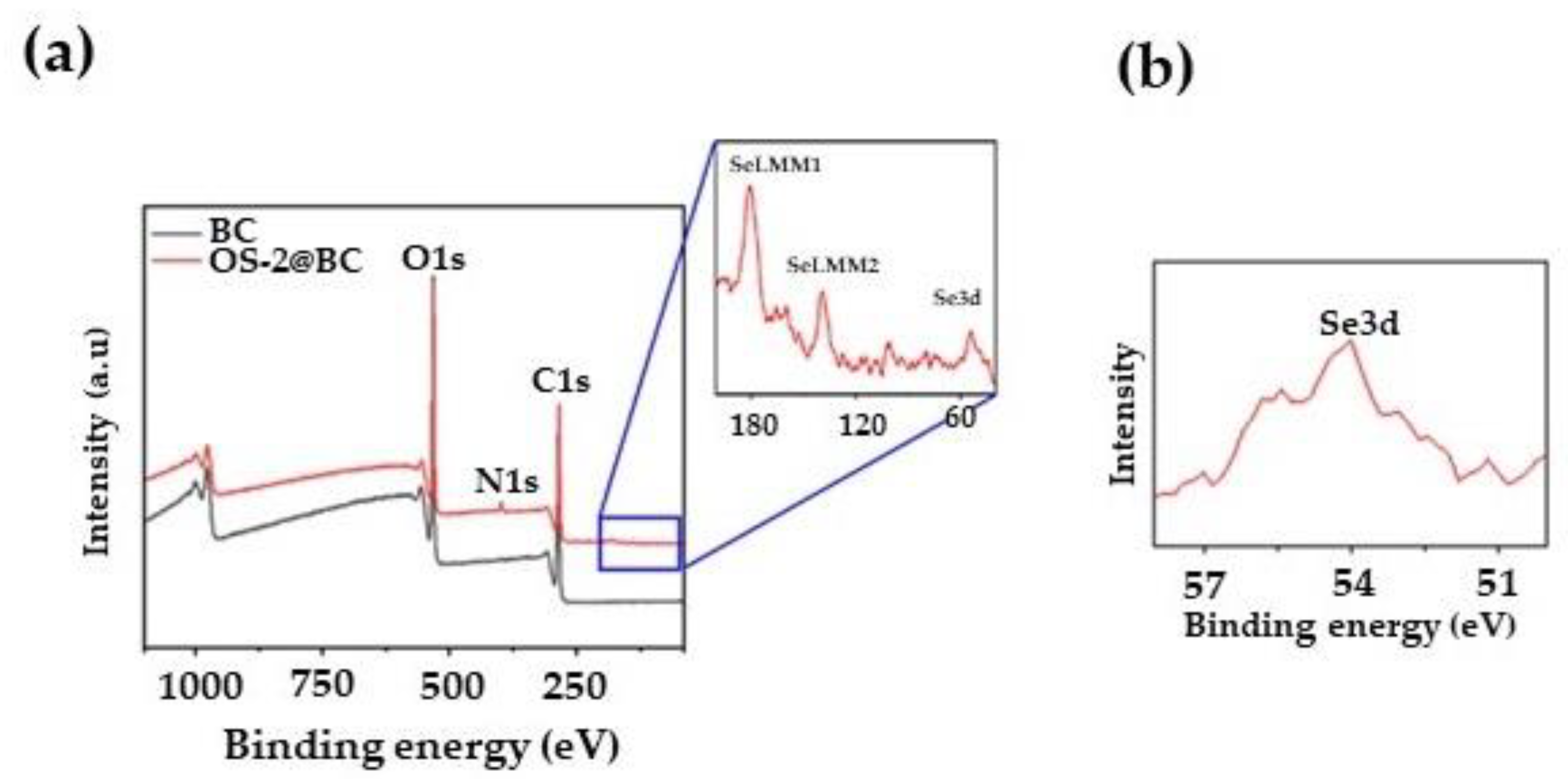
2.3. Textile Characterization
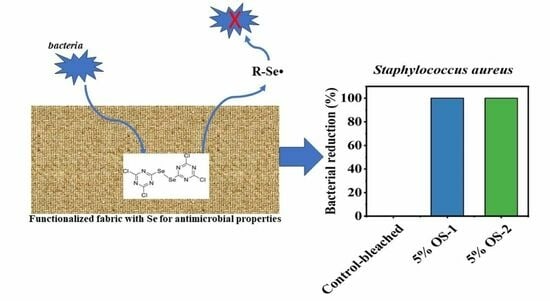
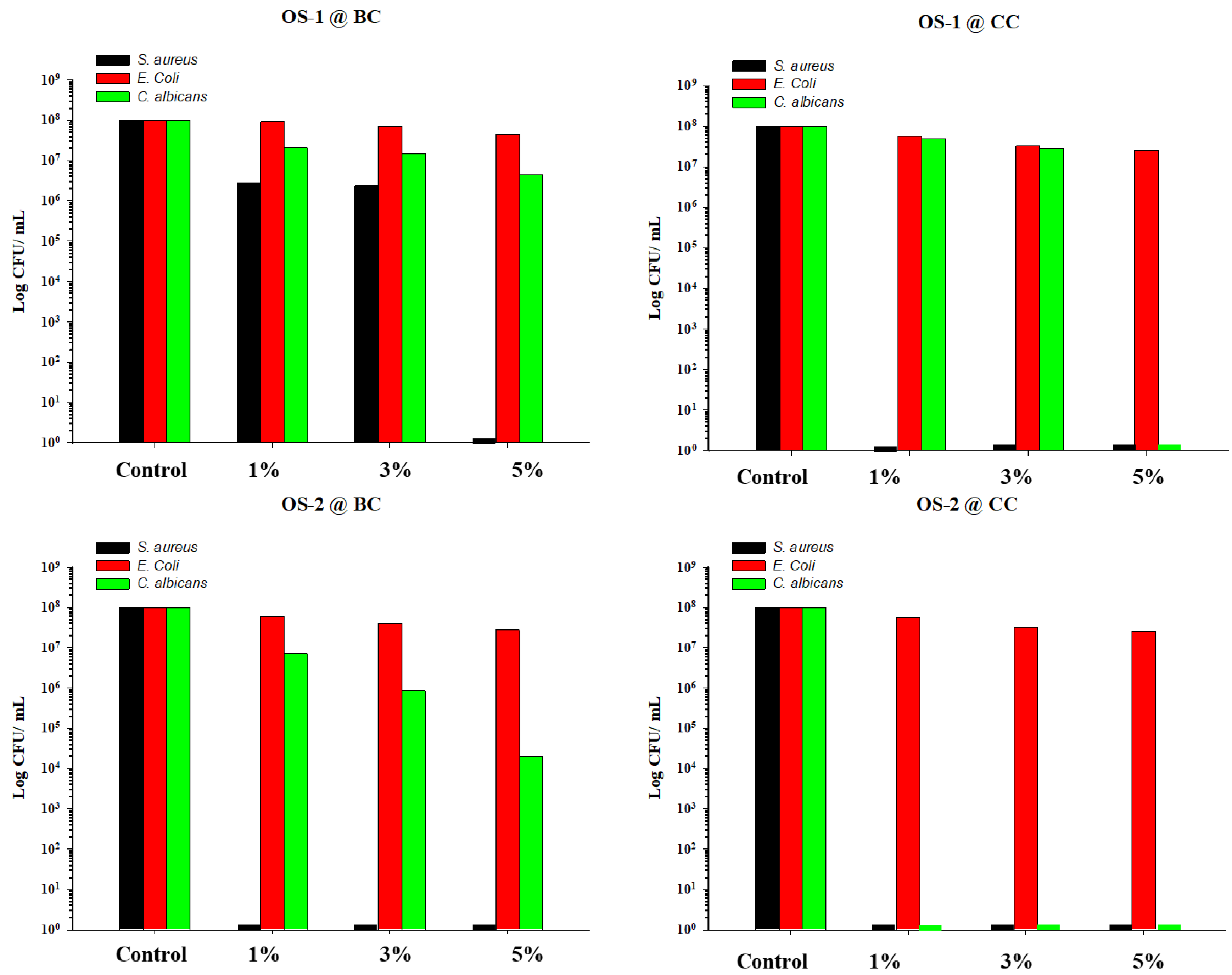
2.4. Antimicrobial Assays
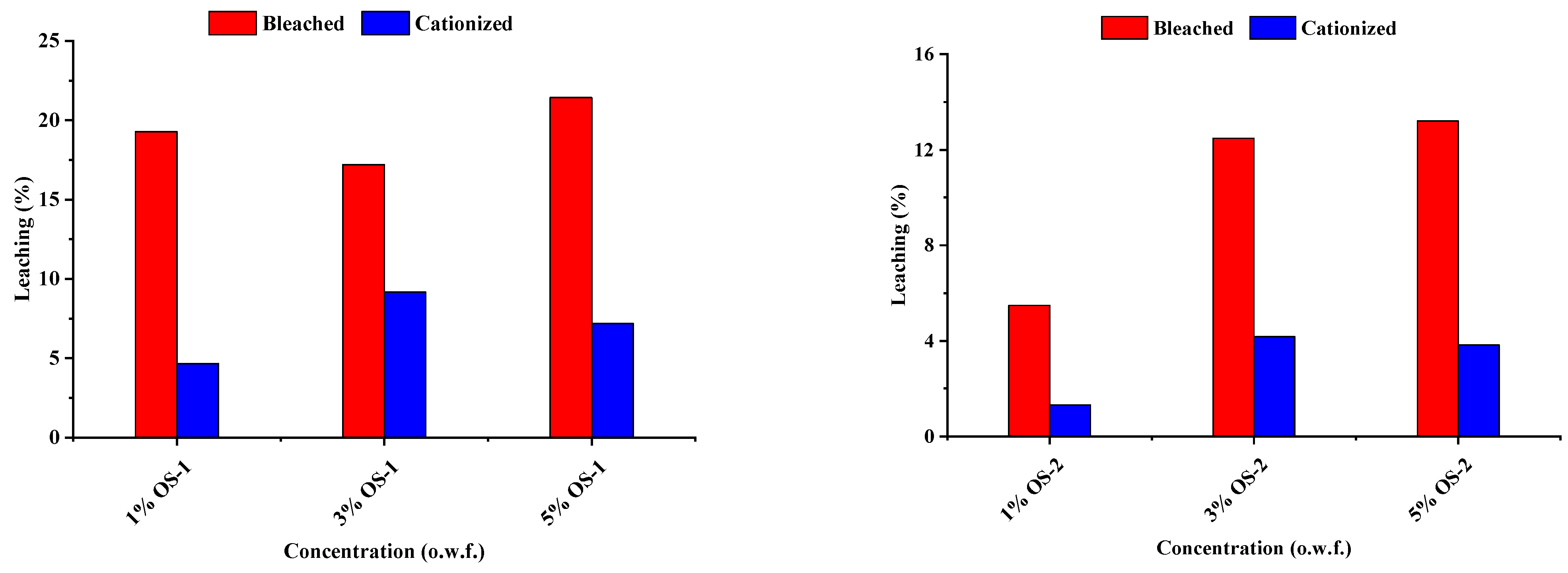
2.5. Leaching Studies
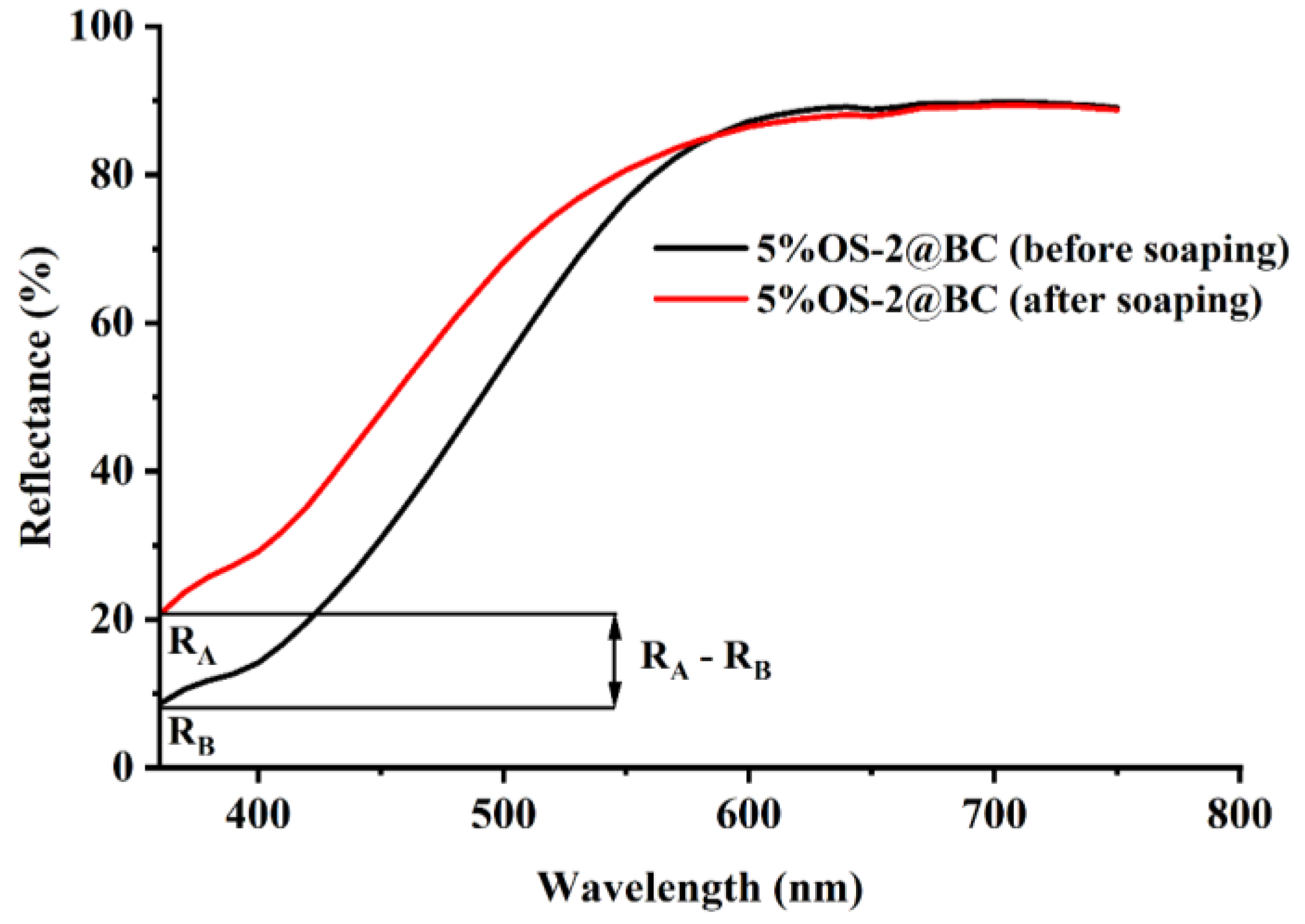
2.5.1. Leaching (%) of OS from Treated Textile Due to Soaping
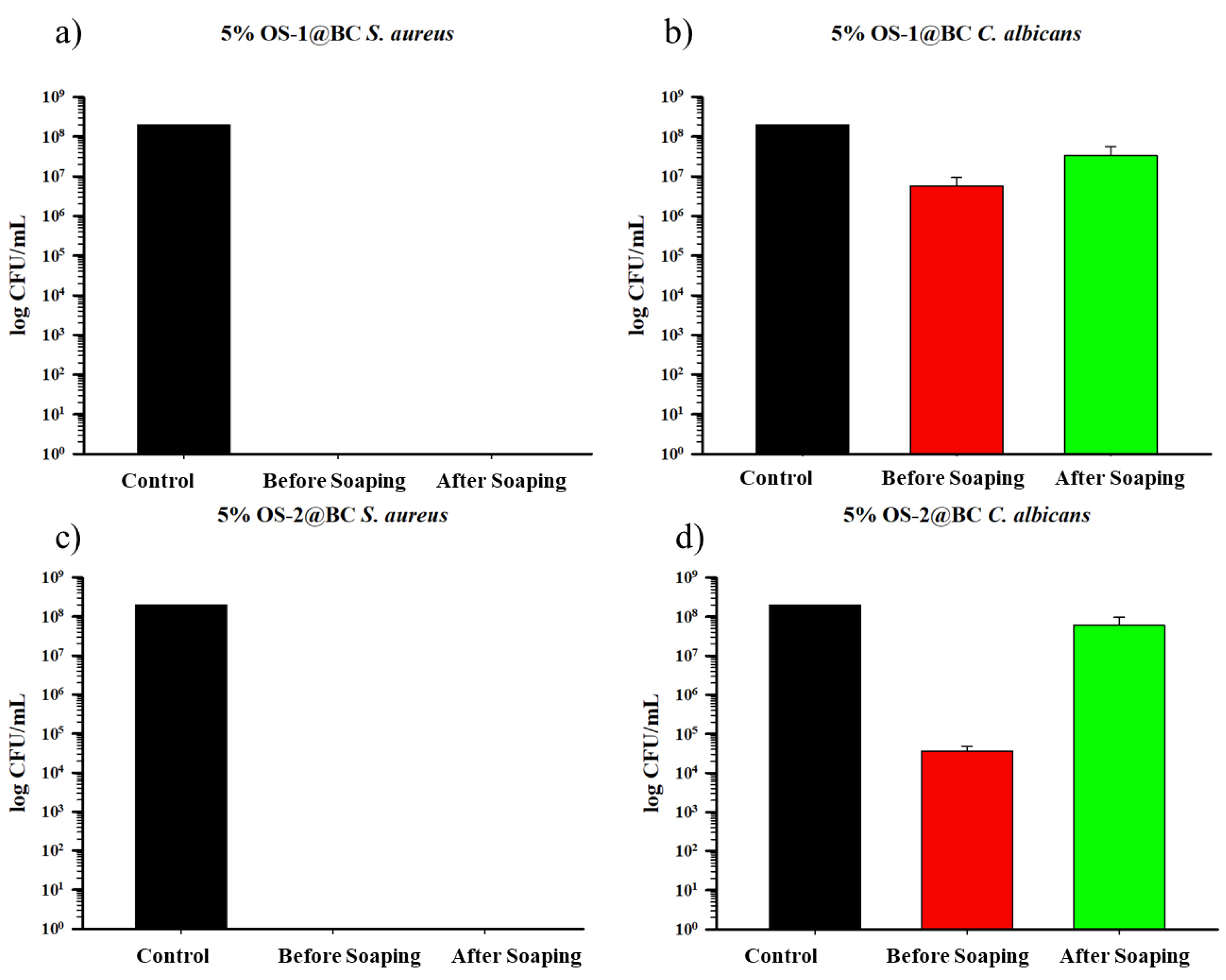
2.5.2. Durability of Antimicrobial Activity
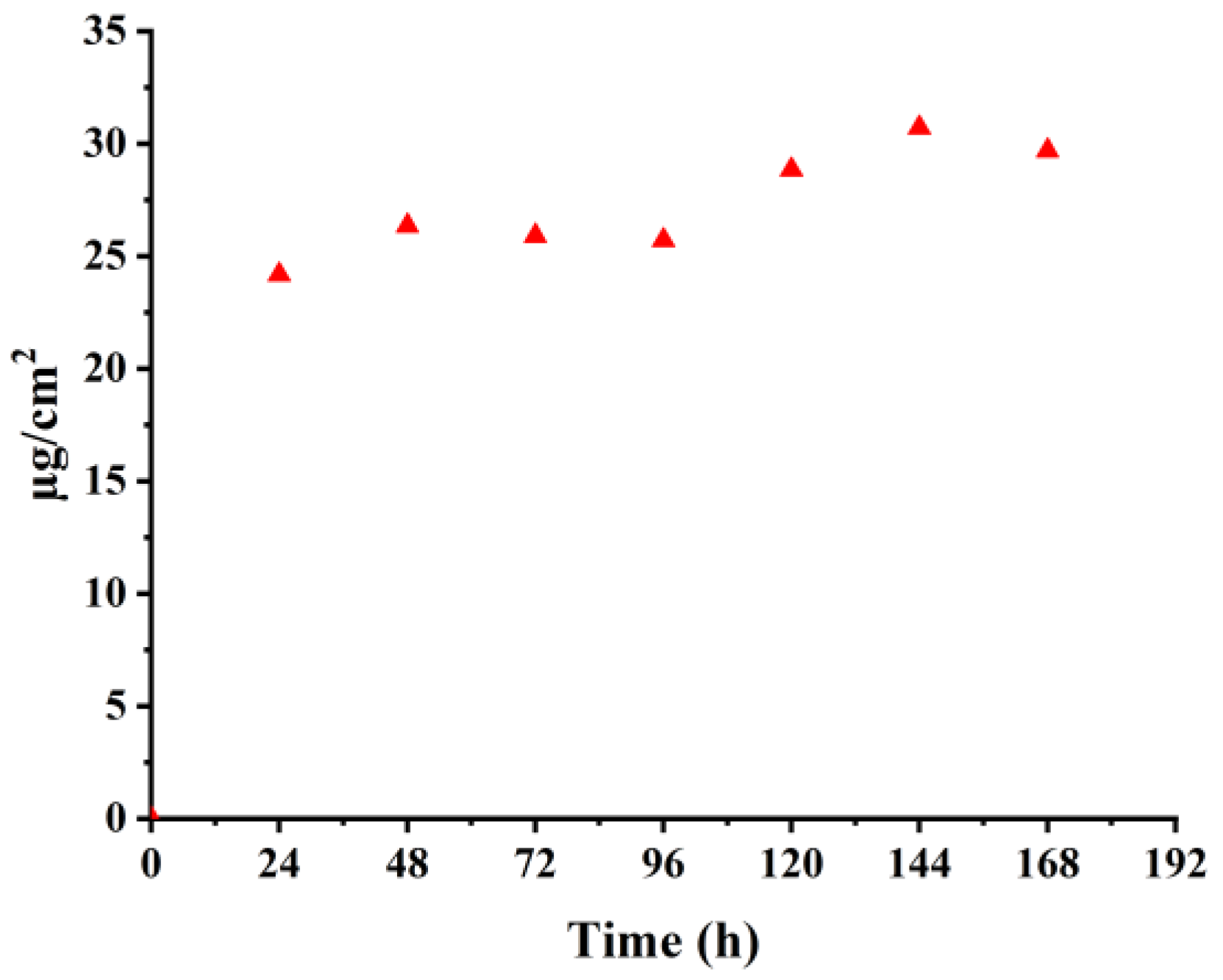
2.5.3. Release of OS-2 from the Treated Fabric
3. Materials and Methods
3.1. Textile Material
3.2. Chemicals
3.3. Bacterial Strains
4. Methods
4.1. Fabric Preparation
4.2. Cationization of Cotton Textile
4.3. OS Treatment of Textiles
4.3.1. OS Treatment of Bleached Textile
4.3.2. OS Treatment of Cationized Textile
4.4. CFU Antibacterial Assays
4.5. Color Measurements
4.6. Fixation of OS to the Textile
4.7. Release of OS from Treated Textile
4.8. Leaching of OS Due to Soaping
4.9. Durability of OS-Treatment
4.10. Fourier Transform Infrared Spectroscopy (FTIR)
4.11. X-ray Photoelectron Spectroscopy (XPS)
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Azam Ali, M.; Shavandi, A. Medical textiles testing and quality assurance. In Performance Testing of Textiles; Wang, L., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 129–153. [Google Scholar]
- Medical Textiles Market Size 2022 Global Growth, Trends, Industry Analysis, Key Players and Forecast to 2026. MarketWatch. Available online: https://www.grandviewresearch.com/industry-analysis/medical-textiles-market#:~:text=The%20global%20medical%20textiles%20market,for%20medical%2Dgrade%20textile%20products. (accessed on 23 August 2023).
- Murphy, F.; Tchetchik, A.; Furxhi, I. Reduction of health care-associated infections (HAIs) with antimicrobial inorganic nanoparticles incorporated in medical textiles: An economic assessment. Nanomaterials 2020, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.A.; Shakeri, M.; Bashari, A. Recent advances in application of chitosan and its derivatives in functional finishing of textiles. In The Impact and Prospects of Green Chemistry for Textile Technology; Shahid ul, I., Butola, B.S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 107–133. [Google Scholar]
- Fahmy, H.M.; Aly, A.A.; Abou-Okeil, A. A non-woven fabric wound dressing containing layer–by–layer deposited hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Ahmed, S.; Souqair, D.; Boateng, J. Antimicrobial dressings for improving wound healing. In Wound Healing—New Insights into Ancient Challenges; Alexandrescu, V.A., Ed.; InTechOpen: London, UK, 2016. [Google Scholar]
- Schneider, G.; Bim, F.L.; de Sousa, A.F.L.; Watanabe, E.; Andrade, D.; Fronteira, I. The use of antimicrobial-impregnated fabrics in health services: An integrative review. Rev. Lat. Am. De Enferm. 2021, 29, e3416. [Google Scholar] [CrossRef]
- Khan, A.M.; Islam, M.M.; Khan, M.M.R. Chitosan incorporation for antibacterial property improvement of jute-cotton blended denim fabric. J. Text. Inst. 2020, 111, 660–668. [Google Scholar] [CrossRef]
- Feng, Y. Nanomaterial antibacterial technology in the design of antibacterial fabrics for sports clothing. Adv. Mater. Sci. Eng. 2021, 2021, 1837729. [Google Scholar]
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Cotton; Baliño, S., Ed.; International Institute for Sustainable Development: Geneva, Switzerland, 2020. [Google Scholar]
- Tan, L.Y.; Bee, L.T.; Ratnam, C.T.; Woo, K.T.; Tee, T.T.; Rahmat, A.R. A review of antimicrobial fabric containing nanostructures metal-based compound. J. Vinyl Addit. Technol. 2019, 25, E3–E27. [Google Scholar] [CrossRef]
- Callewaert, C.; De Maeseneire, E.; Kerckhof, F.M.; de Verliefde Wiele, T.V.; Boon, N. Microbial odor profile of polyester and cotton clothes after a fitness session. Appl. Environ. Microbiol. 2014, 80, 6611–6619. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of novel antimicrobial agents for textiles-a review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 457–489. [Google Scholar]
- Asiedu-Gyekye, I.J.; Mahmood, A.S.; Awortwe, C.; Nyarko, A.K. Toxicological assessment of polyhexamethylene biguanide for water treatment. Interdiscip. Toxicol. 2015, 8, 193. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F.; Vineis, C.; Varesano, A.; Gozzelino, G. Novel Antimicrobial Agents and Processes for Textile Applications. In Antibacterial Agents; Intechopen: London, UK, 2017; Volume 17. [Google Scholar]
- Medici, S.; Peana, M.; Pelucelli, A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Ye, S.F.; Yang, Y.; Wu, L.; Ma, W.W.; Zeng, H. Ethaselen: A novel organoselenium anticancer agent targeting thioredoxin reductase 1 reverses cisplatin resistance in drug-resistant K562 cells by inducing apoptosis. Zhejiang Univ. Sci. B 2017, 18, 373–382. [Google Scholar] [CrossRef]
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: Glutathione peroxidase activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Ratushnaya, E.V.; Kirova, Y.I.; Suchkov, M.A.; Drevko, B.L.; Borodulin, V.B. Synthesis and antibacterial activity of organoselenium compounds. Pharm. Chem. J. 2002, 36, 652–653. [Google Scholar] [CrossRef]
- Tran, P.L.; Patel, S.; Hamood, A.N.; Enos, T.; Mosley, T.; Jarvis, C.; Desai, A.; Lin, P.; Reid, W.T. A novel organo-selenium bandage that inhibits biofilm development in a wound by gram-positive and gram-negative wound pathogens. Antibiotics 2014, 3, 435–449. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Kopel, J.; Fralick, J.A.; Reid, T.W. The use of an organo-selenium peptide to develop new antimicrobials that target a specific bacteria. Antibiotics 2021, 10, 611. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgreen, A.; Zou, L. Topical therapeutic efficacy of ebselen against multidrug-resistant staphylococcus aureus LT-1 targeting thioredoxin reductase. Front. Microbiol. 2020, 10, 3016. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Wang, Y.; Han, H.; Cui, B.; Hou, Y.; Wang, Y.; Wang, Q. A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp. ANT506: Cloning and heterologous expression of the gene and characterization of recombinant enzyme. Bioengineered 2017, 8, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Sheikhi-Mohammareh, S.; Shiri, A.; Maleki, E.H.; Matin, M.; Beyzaei, H.; Baranipour, P.; Oroojalian, F.; Memariania, T. Synthesis of various derivatives of [1,3]selenazolo[4,5-d]pyrimidine and exploitation of these heterocyclic systems as antibacterial, antifungal, and anticancer agents. ChemistrySelect 2020, 5, 10060–10066. [Google Scholar] [CrossRef]
- Piętka-Ottlik, M.; Wojtowicz-Mlochwska, H.; Kolodziejczyk, K.; Piasecki, E.; Mlochwski, J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem. Pharm. Bull. 2008, 56, 1423–1427. [Google Scholar] [CrossRef]
- Mukherjee, A.J.; Zade, S.S.; Singh, H.B.; Sunoj, R.B. Organoselenium chemistry: Role of intramolecular interactions. Chem. Rev. 2010, 110, 4357–4416. [Google Scholar] [CrossRef] [PubMed]
- Bouhafs, R.K.; Jarstrand, C. Effects of antioxidants on surfactant peroxidation by stimulated human polymorphonuclear leukocytes. Free Radic. Res. 2002, 36, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, R.; Yokota, T.; Fujimoto, T. Susceptibility of methicillin-resistant Staphylococcus aureus to the selenium-containing compound 2-phenyl-1,2-benzoisoselenazol-3(2H)-one (PZ51). Antimicrob. Agents Chemother. 1989, 33, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Soteropoulos, P.; Vaz, T.; Santangelo, R.; Paderu, P.; Huang, D.Y.; Tamas, M.J.; Perlin, D.S. Molecular characterization of the plasma membrane H+-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000, 44, 2349–2355. [Google Scholar] [CrossRef]
- Wójtowicz, H.; Kloc, K.; Maliszewska, I.; Mlochowski, J.; Pietka, M.; Piasecki, E. Azaanalogues of ebselen as antimicrobial and antiviral agents: Synthesis and properties. Il Farm. 2004, 59, 863–868. [Google Scholar] [CrossRef]
- Melo, A.M.; Poester, V.R.; Trapaga, M.; Nogueira, C.W.; Zeni, G.; Martinez, M.; Sass, G.; Stevens, D.A. Diphenyl diselenide and its interaction with antifungals against Aspergillus spp. Med. Mycol. 2021, 59, 528–536. [Google Scholar] [CrossRef]
- Venturini, T.P.; Chassot, F.; Loreto, E.S.; Keller, J.T.; Azevedo, M.I.; Zeni, G.; Santurio, J.M.; Alves, S.H. Antifungal activities of diphenyl diselenide and ebselen alone and in combination with antifungal agents against Fusarium spp. Med. Mycol. 2016, 54, 550–555. [Google Scholar] [CrossRef]
- Rossato, L.; Loreto, E.; Venturini, T.P.; Azevedo, M.I.; Al-Hatmi, A.M.S.; Santurio, J.M.; Alves, S.H. In vitro combination between antifungals and diphenyl diselenide against Cryptococcus species. Mycoses 2019, 62, 508–512. [Google Scholar] [CrossRef]
- Denardi, L.B.; Benardi, L.B.; Mario, D.A.N.; Loerto, E.S.; Nogueira, C.W.; Santurio, J.M.; Alves, S.H. Antifungal activities of diphenyl diselenide alone and in combination with fluconazole or amphotericin B against Candida glabrata. Mycopathologia 2013, 176, 165–169. [Google Scholar] [CrossRef]
- Felli Kubiça, T.; Benardi, L.B.; Loreto, E.S.; Zeni, G.; Weiblen, C.; Oliviera, V.; Santurio, J.M.; Alves, S.H. In vitro activity of diphenyl diselenide and ebselen alone and in combination with antifungal agents against Trichosporon asahii. Mycoses 2019, 62, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Piccioni, M.; De Marco, S.; Pagiotti, R.; Nascimento, V.; Braga, L.A.; Santi, C.; Pietrella, D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection. BMC Microbiol. 2016, 16, 220. [Google Scholar] [CrossRef]
- Kuchar, J.; Rienhold, K.; Rosgen, V.; Nothling, N.; Lehman, C.W.; Mohr, F. Synthesis, reactivity and antimicrobial activity of a series of 2-arylamino-1,3-selenazoles. Molecules 2021, 26, 7695. [Google Scholar] [CrossRef] [PubMed]
- Karnik, A.V.; Kulkarni, A.M.; Malviya, N.J.; Mourya, B.R.; Jadhav, B.L. Synthesis and in vitro anti-bacterial evaluation of tetracyclic-ortho-fused 4H-naphtho[1′,2′–5,6]pyrano[3,4-d](1,2,3)selenadiazole and its derivatives. Eur. J. Med. Chem. 2008, 43, 2615–2617. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Sureshkumar, P.; Thanusu, J.; Kanagarajan, V. Design, synthesis, characterization, antibacterial and antifungal activities of a novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J. Enzym. Inhib. Med. Chem. 2008, 23, 347–351. [Google Scholar] [CrossRef]
- Al-Samadi, M.; Al-Momani, F. Synthesis, characterization an dantimicrobial activity of new 1,2,3-selenadiazoles. Molecules 2008, 13, 2740–2749. [Google Scholar] [CrossRef]
- Chitra, S.; Paul, N.; Muthusubramanian, S.; Manisankar, P.; Yogeeswari, P.; Sriram, D. A facile synthesis of carbocycle-fused mono and bis-1,2,3-selenadiazoles and their antimicrobial and antimycobacterial studies. Eur. J. Med. Chem. 2011, 46, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Mosolygó, T.; Kincses, A.; Csonka, A.; Tönki, Á.S.; Witek, K.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Kieć-Kononowicz, K.; Domínguez-Álvarez, E.; et al. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 2019, 24, 1487. [Google Scholar] [CrossRef] [PubMed]
- Spengler, G.; Kincses, A.; Csonka, A.; Tonki, A.S.; Witek, K.; Sanmartin, C.; Marc, M.A.; Handzilik, J.; Kiec-Kononowicz, K.; Dominguez-Alvarez, E.; et al. Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules 2019, 24, 4264. [Google Scholar] [CrossRef]
- Nové, M.; Kincses, A.; Szalontai, B.; Racz, B.; Blair, J.M.A.; Gonzalez-Pradena, A.; Benito-Lama, M.; Domingez-Alvarez, E.; Spengler, G. Biofilm eradication by symmetrical selenoesters for food-borne pathogens. Microorganisms 2020, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Enos, T.; Luth, K.; Hamood, A.; Ray, C.; Mitchell, K.; Reid, T.W. Organo-Selenium-Containing Polyester Bandage Inhibits Bacterial Biofilm Growth on the Bandage and in the Wound. Biomedicines 2020, 8, 62. [Google Scholar] [CrossRef]
- Tran, P.; Arnett, A.; Jarvis, C.; Moeley, T.; Tran, K.; Hanes, R.; Webster, D.; Mitchell, K.; Dominguez, L.; Hamood, A.; et al. Organo-selenium coatings inhibit gram-negative and gram-positive bacterial attachment to ophthalmic scleral buckle material. Transl. Vis. Sci. Technol. 2017, 6, 1. [Google Scholar] [CrossRef][Green Version]
- Tran, P.L.; Huynh; Pham, P.; Lacky, B.; Jarvis, C.; Moeley, T.; Hamood, A.N.; Hanes, R.; Reid, T. Organoselenium polymer inhibits biofilm formation in polypropylene contact lens case material. Eye Contact Lens 2017, 43, 110–115. [Google Scholar] [CrossRef]
- Jacobo, U.; Vopni, R.; Tran, P.; Patel, S.; Jain, S.; de Riese, C.; Reid, T.W.; de Reise, W. Efficacy of Organo-selenium-incorporated urinary catheter tugging for in-vitro growth inhibition of E. coli, K. pneunoniae, P. aeruginosa, and H. influenzae. Int. Urol. Nephrol. 2023, 3, 503–510. [Google Scholar]
- Liu, M.; Zhang, X.; Chu, S.; Ge, Y.; Huang, T.; Liu, Y.; Yu, L. Selenization of cotton products with NaHSe endowing the antibacterial activities. Chin. Chem. Lett. 2022, 33, 205–208. [Google Scholar] [CrossRef]
- Arivithamani, N.; Dev, V.R.G. Cationization of cotton for industrial scale salt-free reactive dyeing of garments. Clean Technol. Environ. Policy 2017, 19, 2317–2326. [Google Scholar] [CrossRef]
- Gavade, S.N.; Markad, V.L.; Kodam, K.M.; Shingare, M.S.; Mane, D.V. Synthesis and biological evaluation of novel 2,4,6-triazine derivatives as antimicrobial agents. Bioorganic Med. Chem. Lett. 2012, 22, 5075–5077. [Google Scholar] [CrossRef] [PubMed]
- Solankee, A.; Kapadia, K.; Ćirić, A.; Soković, M.; Irini Doytchinova, I.; Geronikaki, A. Synthesis of some new S-triazine based chalcones and their derivatives as potent antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 510–518. [Google Scholar] [CrossRef]
- Singh, P.K. Synthesis and fungicidal activity of novel 3-(substituted/unsubstituted phenylselenonyl)-1-ribosyl/deoxyribosyl-1H-1,2,4-triazole. J. Agric. Food Chem. 2012, 60, 5813–5858. [Google Scholar] [CrossRef]
- Farrell, M.J. Cationic cotton, reservations to reality. In Proceedings of the AATCC International Conference, Charlotte, NC, USA, 21–23 March 2012. [Google Scholar]
- Acharya, S.; Abidi, N.; Rajbhandari, R.; Meulewater, F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 2014, 21, 4693–4706. [Google Scholar] [CrossRef]
- Nallathambi, A.; Rengaswami, G.D.V. Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr. Polym. 2016, 152, 1–11. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, D.; Xu, Q.; Zhang, Y.; Fu, F.; Diago, H.; Liu, X. Excellent binding effect of l-methionine for immobilizing silver nanoparticles onto cotton fabrics to improve the antibacterial durability against washing. RSC Adv. 2018, 8, 24458–24463. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Gao, C.; Li, S.; Chung, C.T.W.; Xin, J.H. Non-leaching and durable antibacterial textiles finished with reactive zwitterionic sulfobetaine. J. Ind. Eng. Chem. 2017, 46, 373–378. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Eswayah, A.; Romero-Gonzalez, M.; Gardiner, P.H.; Solari, P.L.; Merroun, M.L. Chemical and structural characterization of SeIV biotransformations by Stenotrophomonas bentonitica into Se0 nanostructures and volatiles Se species. Environ. Sci. Nano 2020, 7, 2140–2155. [Google Scholar] [CrossRef]
- Felix, L.; Mylonakis, E.; Fuchs, B.B. Thioredoxin Reductase is a valid target for antimicrobial therapeutic development against gram-positive bacteria. Front. Microbiol. 2021, 12, 663481. [Google Scholar] [CrossRef]
- Vido, K.; Diemer, H.; Doresselaer, A.D.; Leize, E.; Juillard, V.; Gruss, A.; Gaudu, P. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. J. Bacteriol. 2005, 187, 601–610. [Google Scholar] [CrossRef]
- Binder, J.; Shadkchan, Y.; Oshrov, N.; Krappman, S. The essential thioredoxin reductase of the human pathogenic mold Aspergillus fumigatus Is a promising antifungal target. Front. Microbiol. 2020, 11, 1383. [Google Scholar] [CrossRef]
- Thangamani, S.; Eldesouky, H.E.; Mohammad, H.; Pascuzzi, P.E.; Avramova, L.; Hazbun, T.R.; Seleem, M.N. Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3002–3010. [Google Scholar] [CrossRef]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS ONE 2015, 10, e0133877. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K. Ebselen bearing polar functionality: Identification of potent antibacterial agents against multidrug-resistant Gram-negative bacteria. Bioorganic Chem. 2019, 93, 103286. [Google Scholar] [CrossRef] [PubMed]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Lu, J.; Vlamis-Gardikas, A.; Kandasamy, K.; Zhao, R.; Gustafsson, T.N.; Engstrand, L.; Hoffner, S.; Engman, L.; Holmgen, A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 2013, 27, 1394–1403. [Google Scholar] [CrossRef]
- Zhou, C.E.; Kan, C.; Yuen, C.M.; Lo, K.C.; Ho, C.; Lau, K. Regenerable Antimicrobial Finishing of Cotton with Nitrogen Plasma Treatment. BioResources 2016, 11, 1554–1570. [Google Scholar] [CrossRef]
- Srikulkit, K.; Santifuengkul, P. Salt-free dyeing of cotton cellulose with a model cationic reactive dye. Color. Technol. 2000, 116, 398–402. [Google Scholar] [CrossRef]
- Aķ⋅it, A.C.; Onar, N. Leaching and fastness behavior of cotton fabrics dyed with different type of dyes using sol-gel process. J. Appl. Polym. Sci. 2008, 109, 97–105. [Google Scholar] [CrossRef]

| Bleached Cotton | ||||
| Sample Name | a* | b* | L* | ΔE* |
| Control (BC) | −0.31 | 2.61 | 95.50 | 0 |
| Control (CC) | −0.26 | 2.56 | 95.58 | 0 |
| Bleached Cotton/OS-1 | ||||
| 1% OS-1@BC | −0.05 | 5.30 | 94.81 | 3.40 |
| 3% OS-1@BC | 1.85 | 13.64 | 92.12 | 14.12 |
| 5% OS-1@BC | 0.70 | 12.42 | 93.02 | 12.38 |
| Cationized Cotton/OS-1 | ||||
| 1% OS-1@CC | 2.29 | 14.16 | 91.66 | 14.98 |
| 3% OS-1@CC | 4.12 | 22.86 | 89.23 | 26.16 |
| 5% OS-1@CC | 12.31 | 28.23 | 82.69 | 36.19 |
| Bleached Cotton/OS-2 | ||||
| 1% OS-2@BC | −1.69 | 10.67 | 94.40 | 10.81 |
| 3% OS-2@BC | 0.08 | 27.77 | 90.57 | 32.55 |
| 5% OS-2@BC | 1.79 | 28.04 | 89.26 | 33.03 |
| Cationized Cotton/OS-2 | ||||
| 1% OS-2@CC | −1.09 | 22.57 | 89.17 | 25.06 |
| 3% OS-2@CC | 5.14 | 44.73 | 85.07 | 53.24 |
| 5% OS-2@CC | 6.25 | 48.14 | 82.97 | 57.69 |
| Sample Name | S. aureus | Escherichia coli | Candida albicans | S. aureus | Escherichia coli | Candida albicans |
|---|---|---|---|---|---|---|
| Percent Reduction (%) | Log Reduction a | |||||
| Control (BC) | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Control (CC) | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| OS-1 | ||||||
| Bleached Cotton, BC | ||||||
| 1% OS-1 | 97.24 | 6.06 | 79.35 | 1.56 | 0.03 | 0.69 |
| 3% OS-1 | 97.63 | 30.30 | 85.00 | 1.63 | 0.16 | 0.82 |
| 5% OS-1 | 100.00 | 54.55 | 95.60 | 8.00 | 0.34 | 1.36 |
| Cationized Cotton, CC | ||||||
| 1% OS-1 | 100.00 | 43.55 | 51.72 | 8.00 | 0.25 | 0.32 |
| 3% OS-1 | 100.00 | 67.74 | 72.41 | 8.00 | 0.49 | 0.56 |
| 5% OS-1 | 100.00 | 74.19 | 100.00 | 8.00 | 0.59 | 8.00 |
| OS-2 | ||||||
| Bleached Cotton, BC | ||||||
| 1% OS-2 | 100.00 | 39.39 | 92.99 | 8.00 | 0.22 | 1.15 |
| 3% OS-2 | 100.00 | 60.61 | 99.15 | 8.00 | 0.40 | 2.07 |
| 5% OS-2 | 100.00 | 72.73 | 99.98 | 8.00 | 0.56 | 3.70 |
| Cationized Cotton, CC | ||||||
| 1% OS-2 | 100.00 | 43.55 | 100.00 | 8.00 | 0.25 | 8.00 |
| 3% OS-2 | 100.00 | 67.74 | 100.00 | 8.00 | 0.49 | 8.00 |
| 5% OS-2 | 100.00 | 74.19 | 100.00 | 8.00 | 0.59 | 8.00 |
| Abbreviation | Description |
|---|---|
| BC | Bleached cotton fabric |
| CC | Cationized cotton fabric |
| 5%OS-1@BC | 5% o.w.f. OS-1 applied to a bleached cotton fabric |
| 5%OS-2@BC | 5% o.w.f. OS-2 applied to a bleached cotton fabric |
| 5%OS-1@CC | 5% o.w.f. OS-1 applied to a cationized cotton fabric |
| 5%OS-2@CC | 5% o.w.f. OS-2 applied to a cationized cotton fabric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, E.; Tran, P.; Jacobo, U.; Bergfeld, N.; Acharya, S.; Shamshina, J.L.; Reid, T.W.; Abidi, N. Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds. Molecules 2023, 28, 6381. https://doi.org/10.3390/molecules28176381
Hoque E, Tran P, Jacobo U, Bergfeld N, Acharya S, Shamshina JL, Reid TW, Abidi N. Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds. Molecules. 2023; 28(17):6381. https://doi.org/10.3390/molecules28176381
Chicago/Turabian StyleHoque, Ejajul, Phat Tran, Unique Jacobo, Nicholas Bergfeld, Sanjit Acharya, Julia L. Shamshina, Ted W. Reid, and Noureddine Abidi. 2023. "Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds" Molecules 28, no. 17: 6381. https://doi.org/10.3390/molecules28176381
APA StyleHoque, E., Tran, P., Jacobo, U., Bergfeld, N., Acharya, S., Shamshina, J. L., Reid, T. W., & Abidi, N. (2023). Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds. Molecules, 28(17), 6381. https://doi.org/10.3390/molecules28176381