Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy
Abstract
1. Introduction
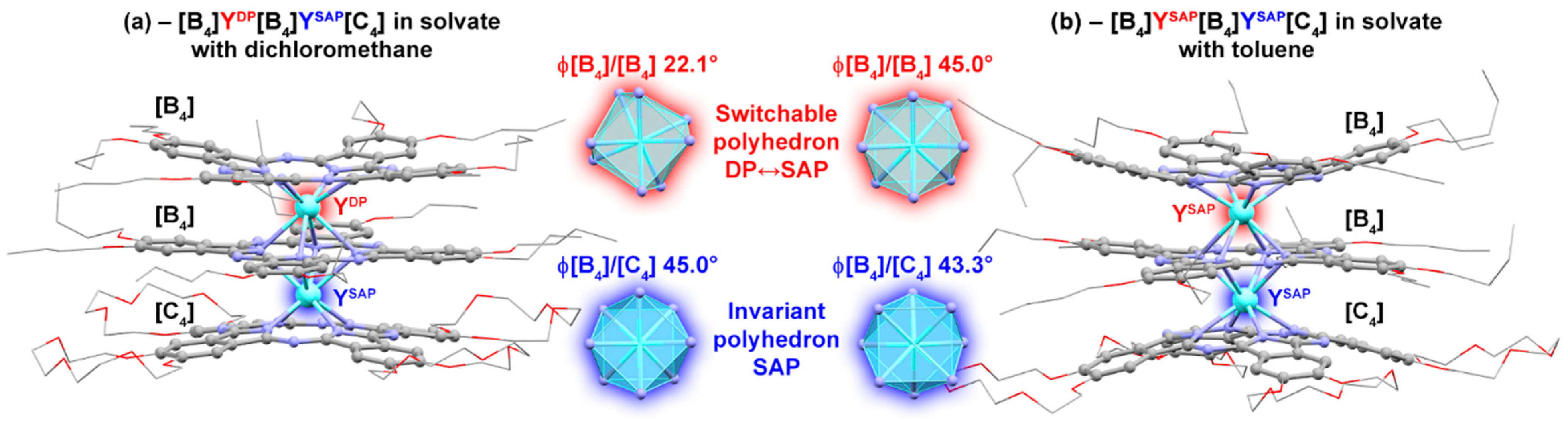
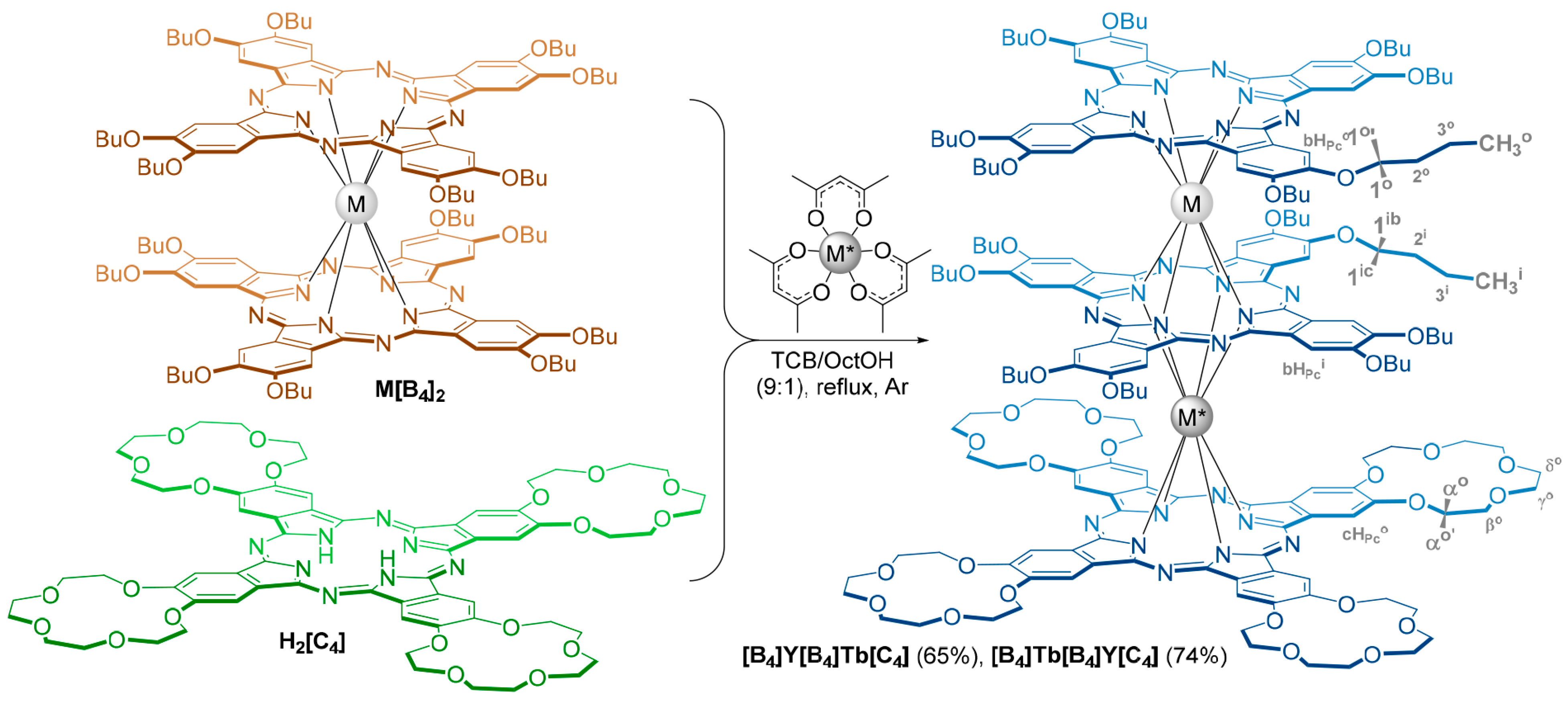
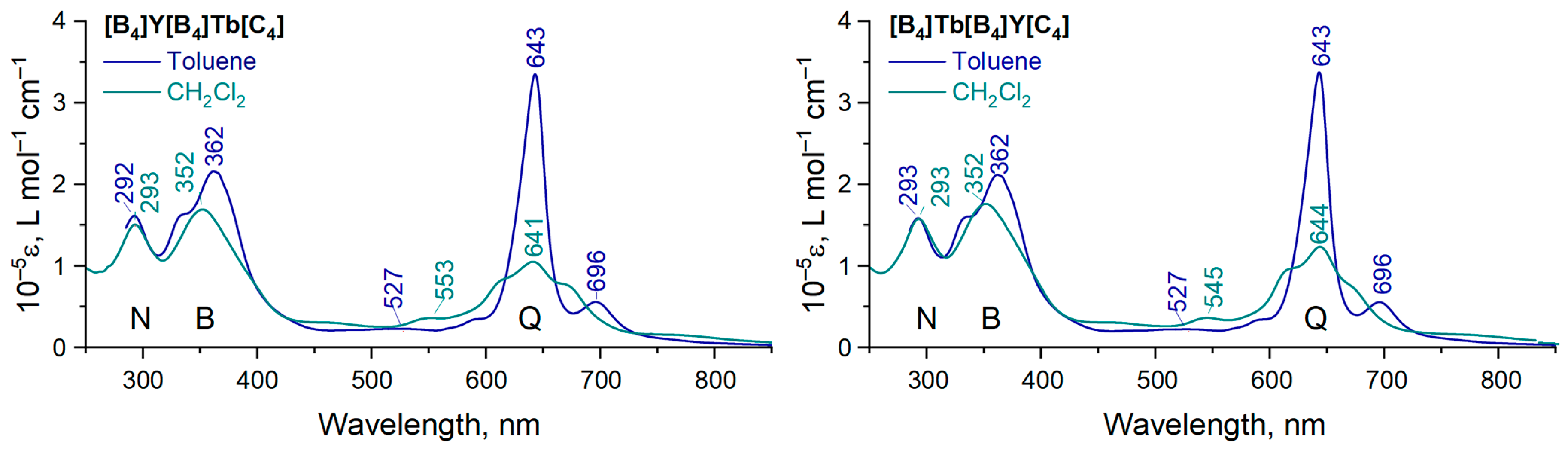
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Synthesis and Characterization of the Triple-Decker Complexes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Feng, J.; Zhang, H. Hybrid Materials Based on Lanthanide Organic Complexes: A Review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Martynov, A.G.; Horii, Y.; Katoh, K.; Bian, Y.; Jiang, J.; Yamashita, M.; Gorbunova, Y.G. Rare-Earth Based Tetrapyrrolic Sandwiches: Chemistry, Materials and Applications. Chem. Soc. Rev. 2022, 51, 9262–9339. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-Infrared (NIR) Lanthanide Molecular Probes for Bioimaging and Biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Lacerda, S.; Tóth, É. Lanthanide Complexes in Molecular Magnetic Resonance Imaging and Theranostics. ChemMedChem 2017, 12, 883–894. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N.; Pimenov, O.A.; Klochkov, V.V.; Khodov, I.A. La(III), Ce(III), Gd(III), and Eu(III) Complexation with Tris(Hydroxymethyl)Aminomethane in Aqueous Solution. Inorg. Chem. 2020, 59, 17783–17793. [Google Scholar] [CrossRef]
- Utochnikova, V.V. The Use of Luminescent Spectroscopy to Obtain Information about the Composition and the Structure of Lanthanide Coordination Compounds. Coord. Chem. Rev. 2019, 398, 113006. [Google Scholar] [CrossRef]
- Liddle, S.T.; Van Slageren, J. Improving F-Element Single Molecule Magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef]
- Piguet, C.; Geraldes, C.F.G.C. Paramagnetic NMR Lanthanide Induced Shifts for Extracting Solution Structures. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; Volume 33, pp. 353–463. ISBN 9780444513236. [Google Scholar]
- Golding, R.; Halton, M. A Theoretical Study of the 14N and 17O N.M.R. Shifts in Lanthanide Complexes. Aust. J. Chem. 1972, 25, 2577. [Google Scholar] [CrossRef]
- Pinkerton, A.A.A.; Rossier, M.; Spiliadis, S.; Rower, M. Lanthanide-Induced Contact Shifts. the Average Electron Spin Polarization, Theory and Experiment. J. Magn. Reson. 1985, 64, 420–425. [Google Scholar] [CrossRef]
- Bleaney, B. Nuclear Magnetic Resonance Shifts in Solution Due to Lanthanide Ions. J. Magn. Reson. 1972, 8, 91–100. [Google Scholar] [CrossRef]
- Golding, R.M.; Pyykkö, P. On the Theory of Pseudocontact N.M.R. Shifts Due to Lanthanide Complexes. Mol. Phys. 1973, 26, 1389–1396. [Google Scholar] [CrossRef]
- Reilley, C.N.; Good, B.W.; Desreux, J.F. Structure-Independent Method for Dissecting Contact and Dipolar NMR Shifts in Lanthanide Complexes and Its Use in Structure Determination. Anal. Chem. 1975, 47, 2110–2116. [Google Scholar] [CrossRef]
- Reilley, C.N.; Good, B.W.; Allendoerfer, R.D. Separation of Contact and Dipolar Lanthanide Induced Nuclear Magnetic Resonance Shifts: Evaluation and Application of Some Structure Independent Methods. Anal. Chem. 1976, 48, 1446–1458. [Google Scholar] [CrossRef]
- Gorbunova, Y.G.; Martynov, A.G.; Birin, K.P.; Tsivadze, A.Y. NMR Spectroscopy—A Versatile Tool for Studying the Structure and Magnetic Properties of Paramagnetic Lanthanide Complexes in Solutions (Review). Russ. J. Inorg. Chem. 2021, 66, 202–216. [Google Scholar] [CrossRef]
- Babailov, S.P. Lanthanide Paramagnetic Probes for NMR Spectroscopic Studies of Molecular Conformational Dynamics in Solution: Applications to Macrocyclic Molecules. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 1–21. [Google Scholar] [CrossRef]
- Allegrozzi, M.; Bertini, I.; Janik, M.B.L.; Lee, Y.-M.; Liu, G.; Luchinat, C. Lanthanide-Induced Pseudocontact Shifts for Solution Structure Refinements of Macromolecules in Shells up to 40 Å from the Metal Ion. J. Am. Chem. Soc. 2000, 122, 4154–4161. [Google Scholar] [CrossRef]
- Müntener, T.; Joss, D.; Häussinger, D.; Hiller, S. Pseudocontact Shifts in Biomolecular NMR Spectroscopy. Chem. Rev. 2022, 122, 9422–9467. [Google Scholar] [CrossRef]
- Peters, J.A.; Huskens, J.; Raber, D.J. Lanthanide Induced Shifts and Relaxation Rate Enhancements. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 283–350. [Google Scholar] [CrossRef]
- Ishikawa, N.; Iino, T.; Kaizu, Y. Determination of Ligand-Field Parameters and f-Electronic Structures of Hetero-Dinuclear Phthalocyanine Complexes with a Diamagnetic Yttrium(III) and a Paramagnetic Trivalent Lanthanide Ion. J. Phys. Chem. A 2002, 106, 9543–9550. [Google Scholar] [CrossRef]
- Hiller, M.; Krieg, S.; Ishikawa, N.; Enders, M. Ligand-Field Energy Splitting in Lanthanide-Based Single-Molecule Magnets by NMR Spectroscopy. Inorg. Chem. 2017, 56, 15285–15294. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Okubo, T.; Tanaka, N.; Iino, T.; Kaizu, Y. Determination of Ligand-Field Parameters and f-Electronic Structures of Double-Decker Bis(Phthalocyaninato)Lanthanide Complexes. Inorg. Chem. 2003, 42, 2440–2446. [Google Scholar] [CrossRef]
- Ishikawa, N. Simultaneous Determination of Ligand-Field Parameters of Isostructural Lanthanide Complexes by Multidimensional Optimization. J. Phys. Chem. A 2003, 107, 5831–5835. [Google Scholar] [CrossRef]
- Martynov, A.G.; Sinelshchikova, A.A.; Dorovatovskii, P.V.; Polovkova, M.A.; Ovchenkova, A.E.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Solvation-Induced Conformational Switching of Trisphthalocyanates for Control of Their Magnetic Properties. Inorg. Chem. 2023, Submitted. [Google Scholar]
- Martynov, A.G.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Redox-Triggered Switching of Conformational State in Triple-Decker Lanthanide Phthalocyaninates. Molecules 2022, 27, 6498. [Google Scholar] [CrossRef]
- Martynov, A.G.; Yagodin, A.V.; Birin, K.P.; Gorbunova, Y.G.; Tsivadze, A.Y. Solvation-Induced Switching of the Conformational State of Alkoxy- and Crown-Substituted Trisphthalocyaninates Studied by UV-Vis and 1 H-NMR Spectroscopy. J. Porphyr. Phthalocyanines 2023, 27, 414–422. [Google Scholar] [CrossRef]
- Babailov, S.P.; Polovkova, M.A.; Kirakosyan, G.A.; Martynov, A.G.; Zapolotsky, E.N.; Gorbunova, Y.G. NMR Thermosensing Properties on Binuclear Triple-Decker Complexes of Terbium(III) and Dysprosium(III) with 15-Crown-5-Phthalocyanine. Sens. Actuators A Phys. 2021, 331, 112933. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Kirakosyan, G.A.; Zapolotsky, E.N.; Babailov, S.P.; Gorbunova, Y.G. 1H NMR Spectral Analysis of Structural Features in a Series of Paramagnetic Homoleptic Binuclear Triple-Decker Phthalocyaninato Lanthanide Complexes. Polyhedron 2022, 219, 115792. [Google Scholar] [CrossRef]
- Babailov, S.P.; Polovkova, M.A.; Zapolotsky, E.N.; Kirakosyan, G.A.; Martynov, A.G.; Gorbunova, Y.G. Nuclear Magnetic Resonance Thermosensing Properties of Holmium(III) and Thulium(III) Tris(Tetra-15-Crown-5-Phthalocyaninato) Complexes. J. Porphyr. Phthalocyanines 2022, 26, 334–339. [Google Scholar] [CrossRef]
- Polovkova, M.A.; Martynov, A.G.; Birin, K.P.; Nefedov, S.E.; Gorbunova, Y.G.; Tsivadze, A.Y. Determination of the Structural Parameters of Heteronuclear (Phthalocyaninato)Bis(Crownphthalocyaninato)Lanthanide(III) Triple-Deckers in Solution by Simultaneous Analysis of NMR and Single-Crystal X-Ray Data. Inorg. Chem. 2016, 55, 9258–9269. [Google Scholar] [CrossRef]
- Ishikawa, N.; Iino, T.; Kaizu, Y. Study of 1 H NMR Spectra of Dinuclear Complexes of Heavy Lanthanides with Phthalocyanines Based on Separation of the Effects of Two Paramagnetic Centers. J. Phys. Chem. A 2003, 107, 7879–7884. [Google Scholar] [CrossRef]
- Mironov, V.S.; Galyametdinov, Y.G.; Ceulemans, A.; Görller-Walrand, C.; Binnemans, K. Room-Temperature Magnetic Anisotropy of Lanthanide Complexes: A Model Study for Various Coordination Polyhedra. J. Chem. Phys. 2002, 116, 4673–4685. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Berezhnoy, G.S.; Sinelshchikova, A.A.; Khrustalev, V.N.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Heteroleptic Crown-Substituted Tris(Phthalocyaninates) as Dynamic Supramolecular Scaffolds with Switchable Rotational States and Tunable Magnetic Properties. Inorg. Chem. 2021, 60, 9110–9121. [Google Scholar] [CrossRef]
- Morita, T.; Damjanović, M.; Katoh, K.; Kitagawa, Y.; Yasuda, N.; Lan, Y.; Wernsdorfer, W.; Breedlove, B.K.; Enders, M.; Yamashita, M. Comparison of the Magnetic Anisotropy and Spin Relaxation Phenomenon of Dinuclear Terbium(III) Phthalocyaninato Single-Molecule Magnets Using the Geometric Spin Arrangement. J. Am. Chem. Soc. 2018, 140, 2995–3007. [Google Scholar] [CrossRef]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.-E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A Trigonal Prismatic Mononuclear Cobalt(II) Complex Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Nelyubina, Y.V.; Kats, S.V.; Penkova, L.V.; Efimov, N.N.; Dmitrienko, A.O.; Vologzhanina, A.V.; Belov, A.S.; Voloshin, Y.Z.; Novikov, V.V. Polymorphism in a Cobalt-Based Single-Ion Magnet Tuning Its Barrier to Magnetization Relaxation. J. Phys. Chem. Lett. 2016, 7, 4111–4116. [Google Scholar] [CrossRef]
- Horii, Y.; Damjanovic, M.; Ajayakumar, M.R.; Katoh, K.; Kitagawa, Y.; Chibotaru, L.; Ungur, L.; Mas-Torrent, M.; Wernsdorfer, W.; Breedlove, B.K.; et al. Highly Oxidized States of Phthalocyaninato Terbium(III) Multiple-Decker Complexes Showing Structural Deformations, Biradical Properties and Decreases in Magnetic Anisotropy. Chem. A Eur. J. 2020, 26, 8621–8630. [Google Scholar] [CrossRef]
- Katoh, K.; Kajiwara, T.; Nakano, M.; Nakazawa, Y.; Wernsdorfer, W.; Ishikawa, N.; Breedlove, B.K.; Yamashita, M. Magnetic Relaxation of Single-Molecule Magnets in an External Magnetic Field: An Ising Dimer of a Terbium(III)-Phthalocyaninate Triple-Decker Complex. Chem. A Eur. J. 2011, 17, 117–122. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Berezhnoy, G.S.; Sinelshchikova, A.A.; Dolgushin, F.M.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Cation-Induced Dimerization of Heteroleptic Crown-Substituted Trisphthalocyaninates as Revealed by X-Ray Diffraction and NMR Spectroscopy. Inorg. Chem. 2020, 59, 9424–9433. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kishiue, S.; Damjanović, M.; Katoh, K.; Breedlove, B.K.; Enders, M.; Yamashita, M. Supramolecular Approach for Enhancing Single-Molecule Magnet Properties of Terbium(III)-Phthalocyaninato Double-Decker Complexes with Crown Moieties. Chem. A Eur. J. 2018, 24, 4320–4327. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tomita, Y.; Hada, Y.; Tsubota, K.; Handa, M.; Kasuga, K.; Sogabe, K.; Tokii, T. Preparation and Electrochemical Properties of the Green Ytterbium(III) and Lutetium(III) Sandwich Complexes of Octabutoxy-Substituted Phthalocyanine. Chem. Lett. 1992, 21, 759–762. [Google Scholar] [CrossRef]
- Martynov, A.G.; Berezhnoy, G.S.; Safonova, E.A.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Aromatic Nucleophilic Substitution as a Side Process in the Synthesis of Alkoxy- and Crown-Substituted (Na)Phthalocyanines. Macroheterocycles 2019, 12, 75–81. [Google Scholar] [CrossRef]
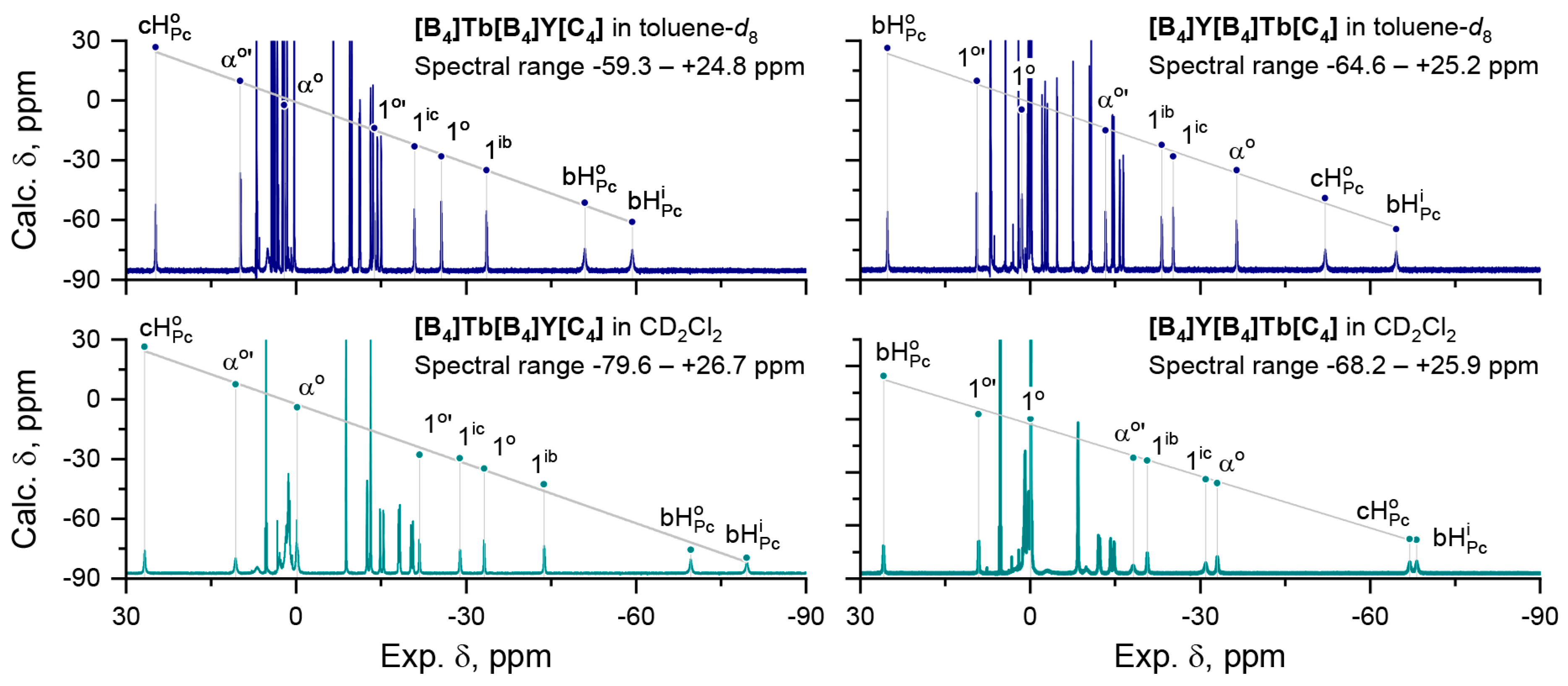
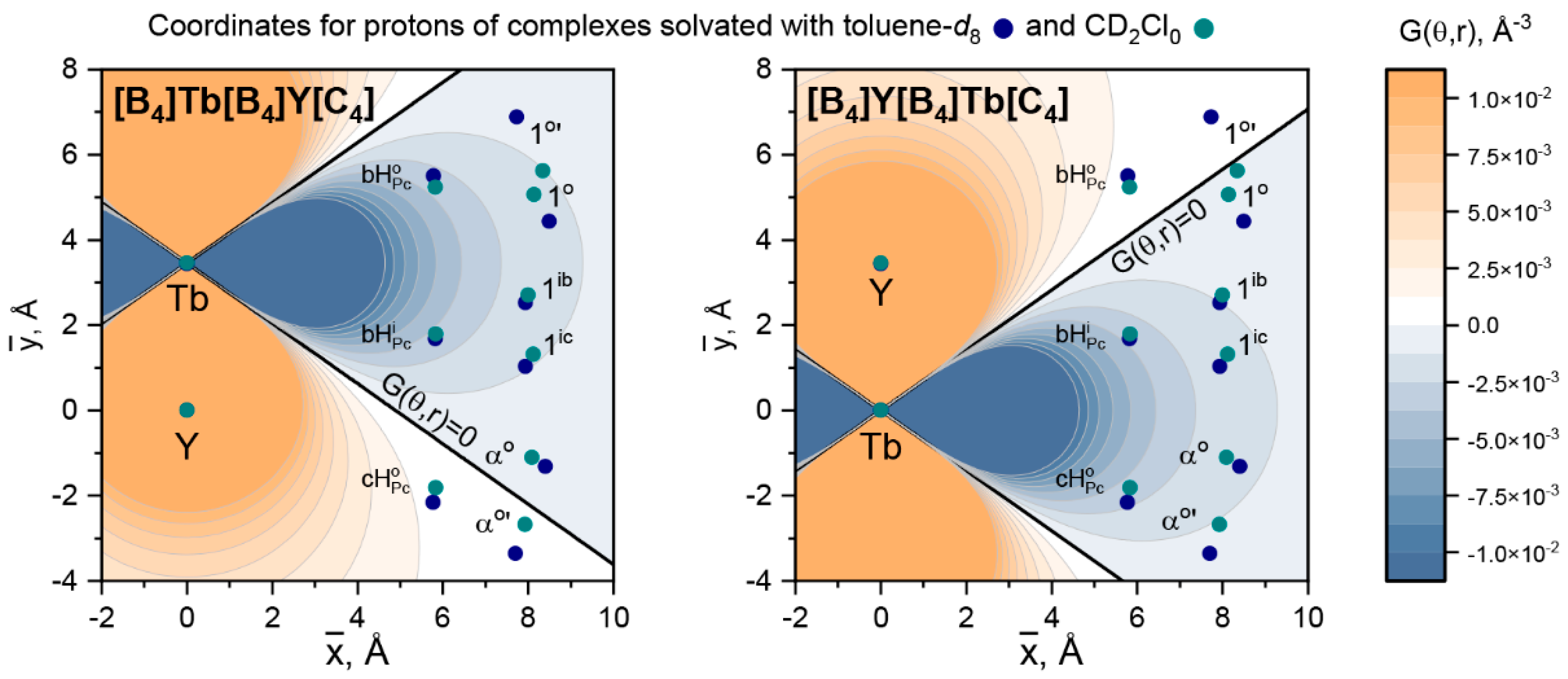

| [B4]Tb[B4]Y[C4], | [B4]Y[B4]Tb[C4], | |||||||
|---|---|---|---|---|---|---|---|---|
| Toluene-d8 | CD2Cl2 | Toluene-d8 | CD2Cl2 | |||||
| Proton | , Å−3 | δ, ppm | , Å−3 | δ, ppm | , Å−3 | δ, ppm | , Å−3 | δ, ppm |
| bHPco | −2.85 × 10−3 | −51.0 | −3.28 × 10−3 | −69.7 | 8.36 × 10−4 | 25.2 | 7.10 × 10−4 | 25.9 |
| bHPci | −3.34 × 10−3 | −59.3 | −3.46 × 10−3 | −79.6 | −3.46 × 10−3 | −64.6 | −3.27 × 10−3 | −68.2 |
| cHPco | 8.72 × 10−4 | 24.8 | 7.19 × 10−4 | 26.7 | −2.70 × 10−3 | −52.1 | −3.23 × 10−3 | −67.0 |
| 1o | −8.28 × 10−4 | −13.8 | −1.26 × 10−3 | −21.8 | 2.94 × 10−4 | 9.4 | −6.11 × 10−5 | 9.1 |
| 1o’ | −1.53 × 10−3 | −25.7 | −1.55 × 10−3 | −33.2 | −4.08 × 10−4 | 1.5 | −1.86 × 10−4 | 0.0 |
| 1ib | −1.88 × 10−3 | −33.6 | −1.88 × 10−3 | −43.7 | −1.25 × 10−3 | −23.2 | −1.15 × 10−3 | −18.2 |
| 1ic | −1.31 × 10−3 | −20.9 | −1.36 × 10−3 | −28.9 | −1.85 × 10−3 | −36.5 | −1.66 × 10−3 | −31.0 |
| αo | −3.03 × 10−4 | 2.1 | −3.45 × 10−4 | −0.2 | −1.51 × 10−3 | −25.2 | −1.74 × 10−3 | −33.0 |
| αo’ | 2.89 × 10−4 | 9.9 | 1.23 × 10−4 | 10.7 | −8.77 × 10−4 | −13.3 | −1.18 × 10−3 | −20.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynov, A.G.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy. Molecules 2023, 28, 4474. https://doi.org/10.3390/molecules28114474
Martynov AG, Birin KP, Kirakosyan GA, Gorbunova YG, Tsivadze AY. Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy. Molecules. 2023; 28(11):4474. https://doi.org/10.3390/molecules28114474
Chicago/Turabian StyleMartynov, Alexander G., Kirill P. Birin, Gayane A. Kirakosyan, Yulia G. Gorbunova, and Aslan Yu. Tsivadze. 2023. "Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy" Molecules 28, no. 11: 4474. https://doi.org/10.3390/molecules28114474
APA StyleMartynov, A. G., Birin, K. P., Kirakosyan, G. A., Gorbunova, Y. G., & Tsivadze, A. Y. (2023). Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy. Molecules, 28(11), 4474. https://doi.org/10.3390/molecules28114474







