Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion
Abstract
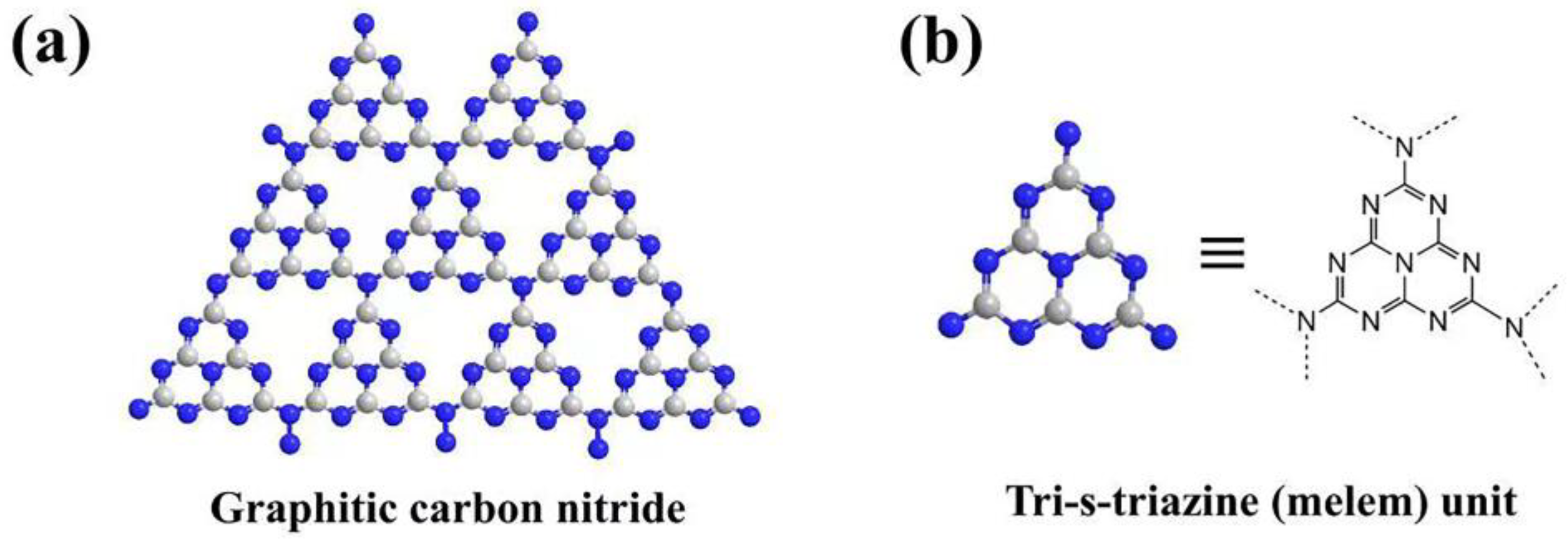
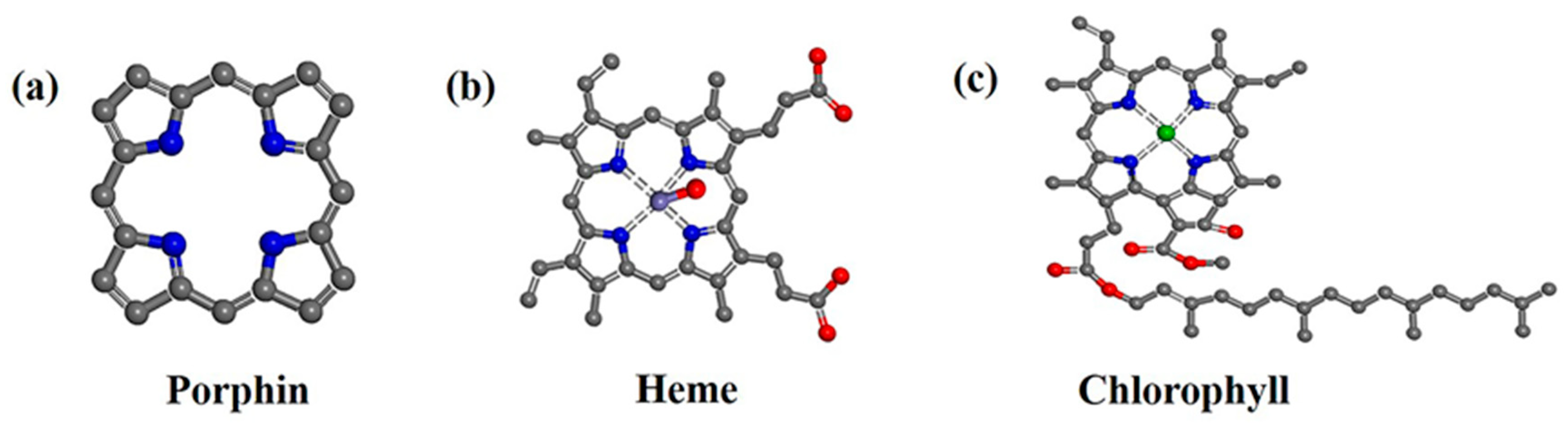
1. Introduction
2. Fabrication of Porphyrin Molecules/g-C3N4 Composite Photocatalysts
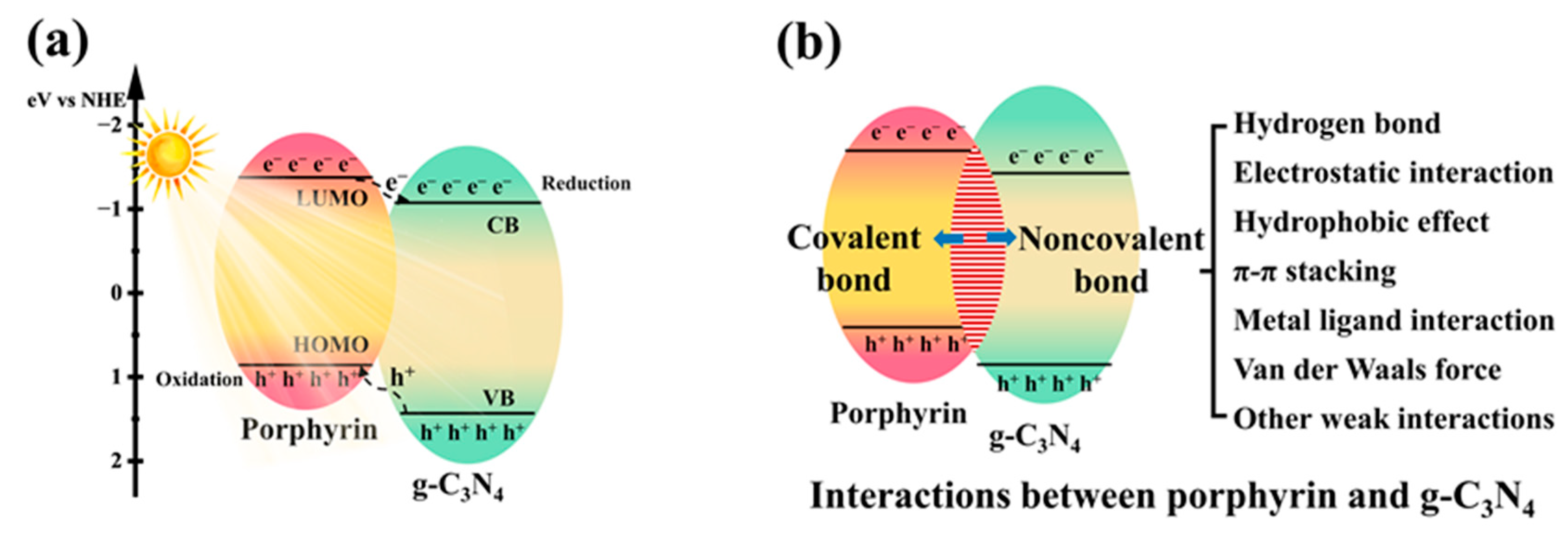
2.1. Noncovalent Bond Interactions between Porphyrin Molecules and g-C3N4
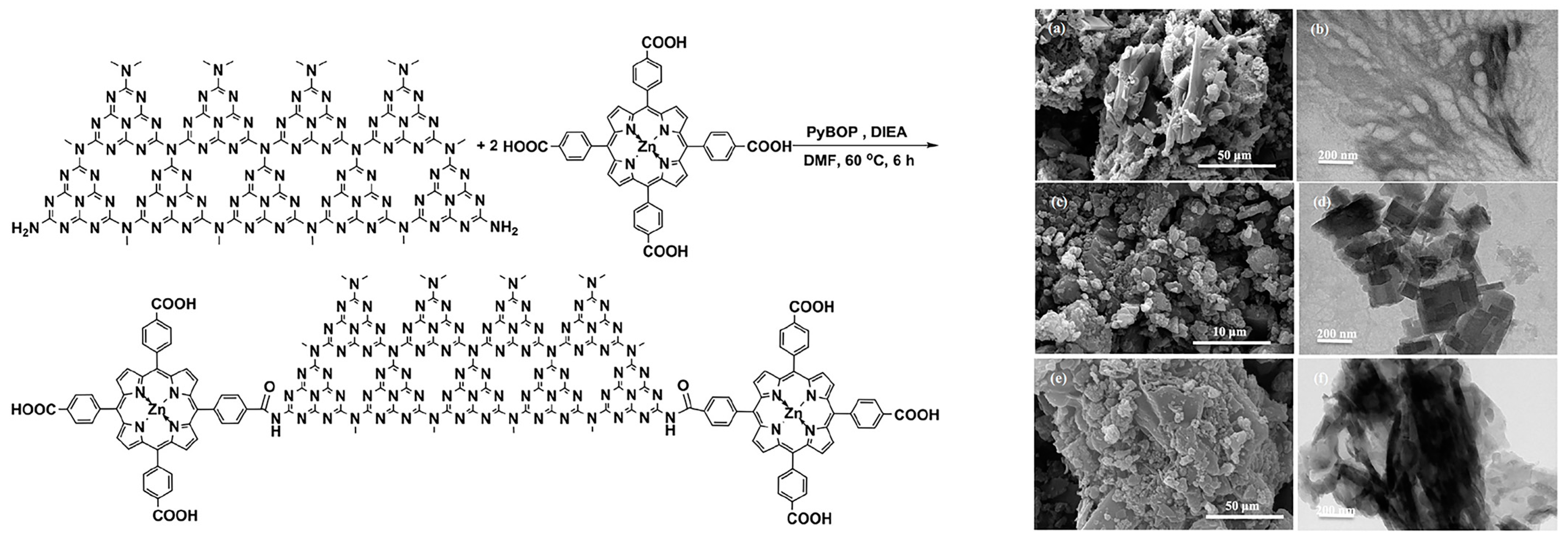
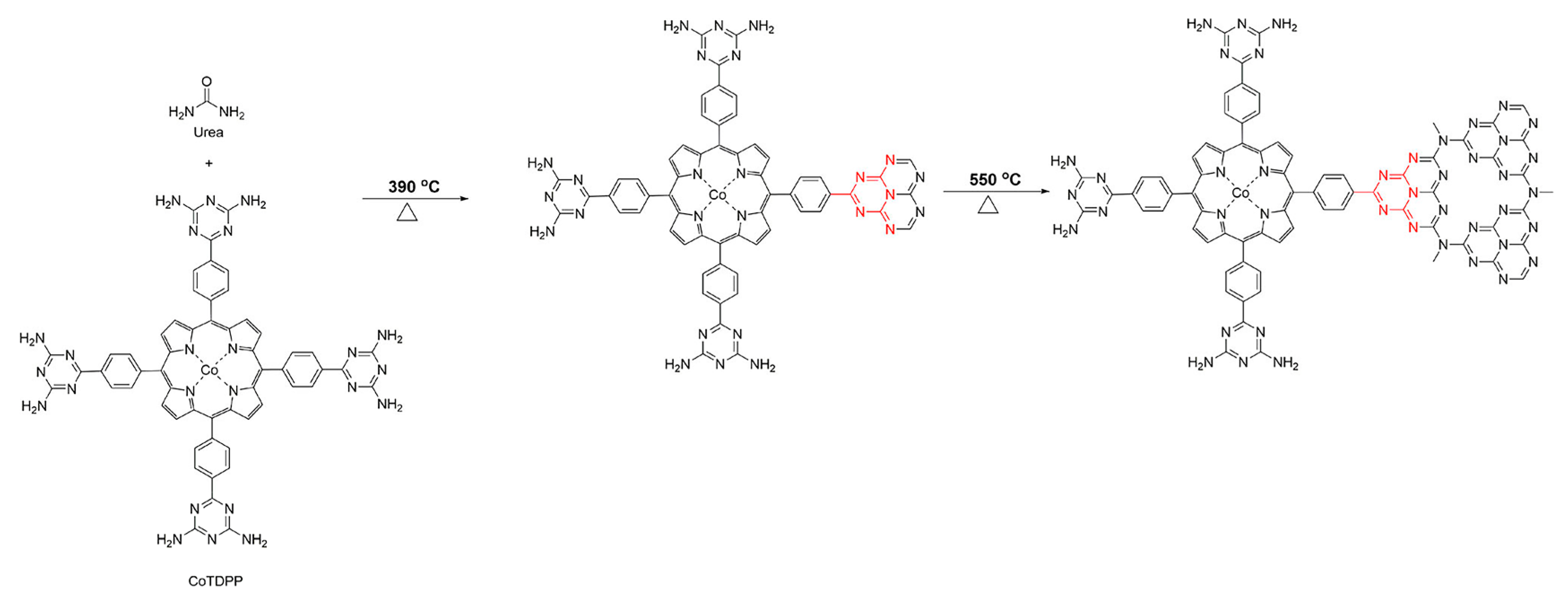
2.2. Covalent Bond Interactions between Porphyrin Molecules and g-C3N4
3. Fabrication of Porphyrin-Based Nanomaterials/g-C3N4 Composite Photocatalysts
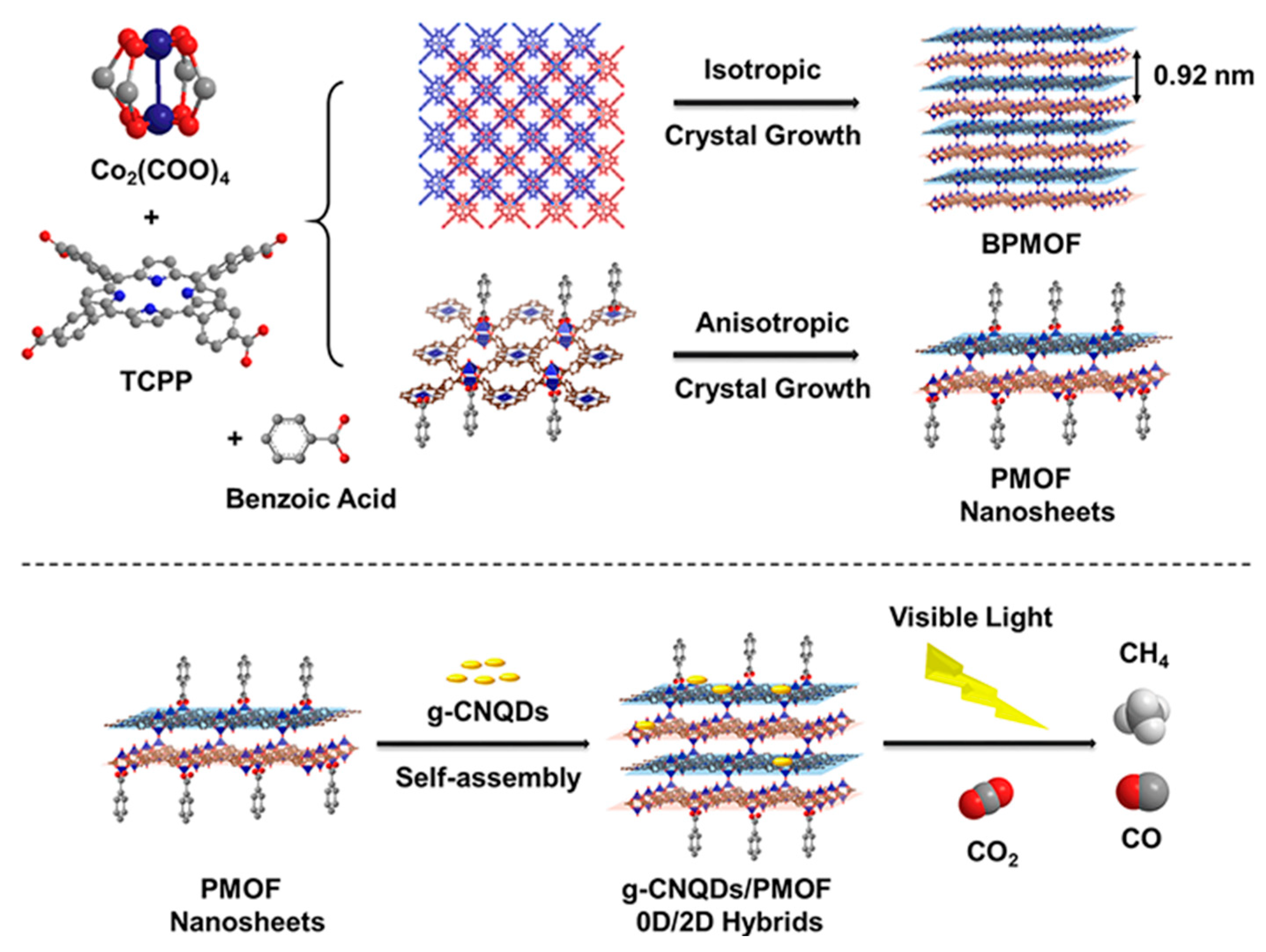
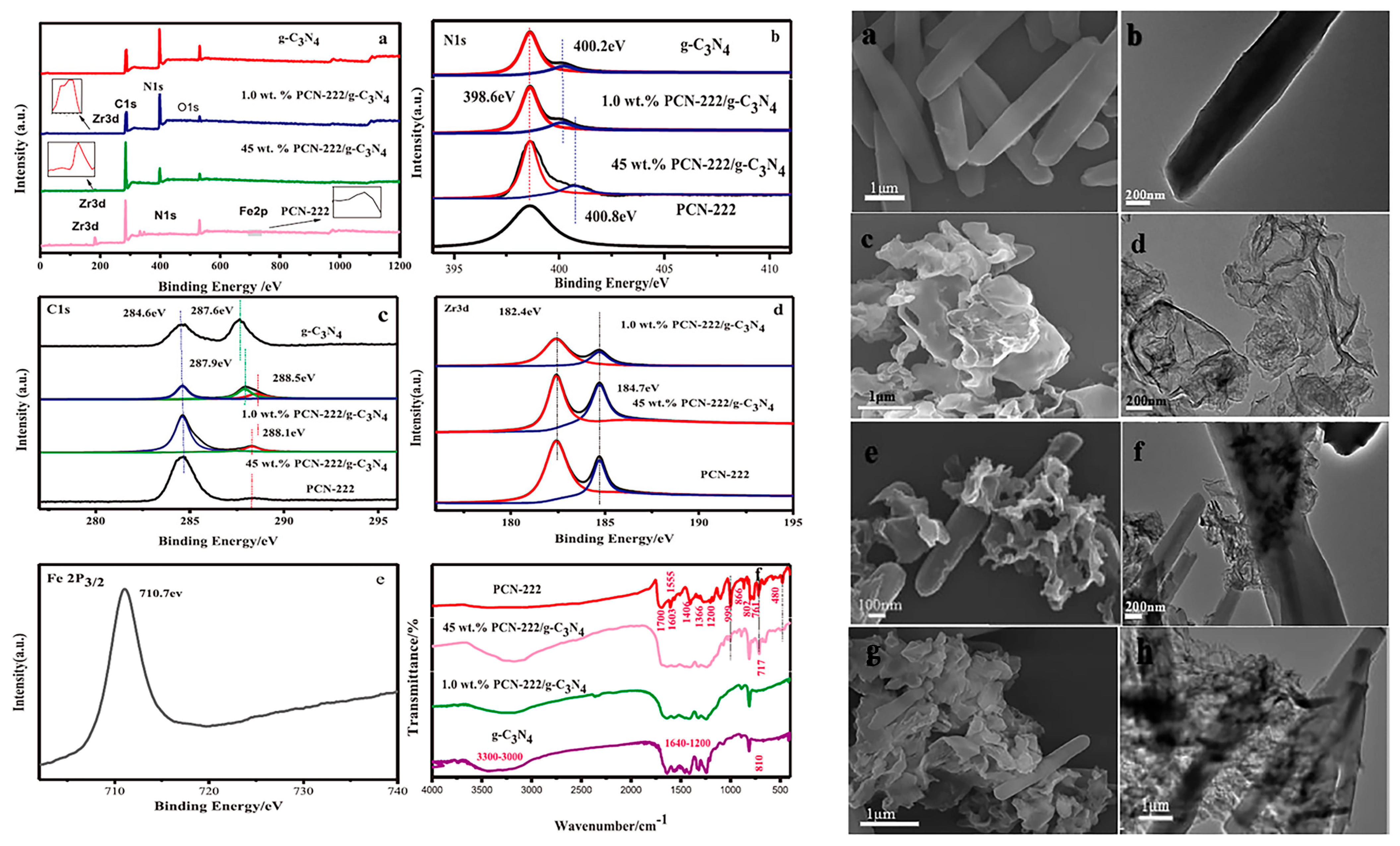
3.1. Porphyrin-Based MOF/g-C3N4 Hybrids for Solar Energy Utilization and Conversion
3.2. Porphyrin-Based COF/g-C3N4 Hybrids for Solar Energy Utilization and Conversion
3.3. Porphyrin-Based Assembly/g-C3N4 Hybrids for Solar Energy Utilization and Conversion
3.4. Other Porphyrin-Based Nanomaterial/g-C3N4 Hybrids for Solar Energy Utilization and Conversion
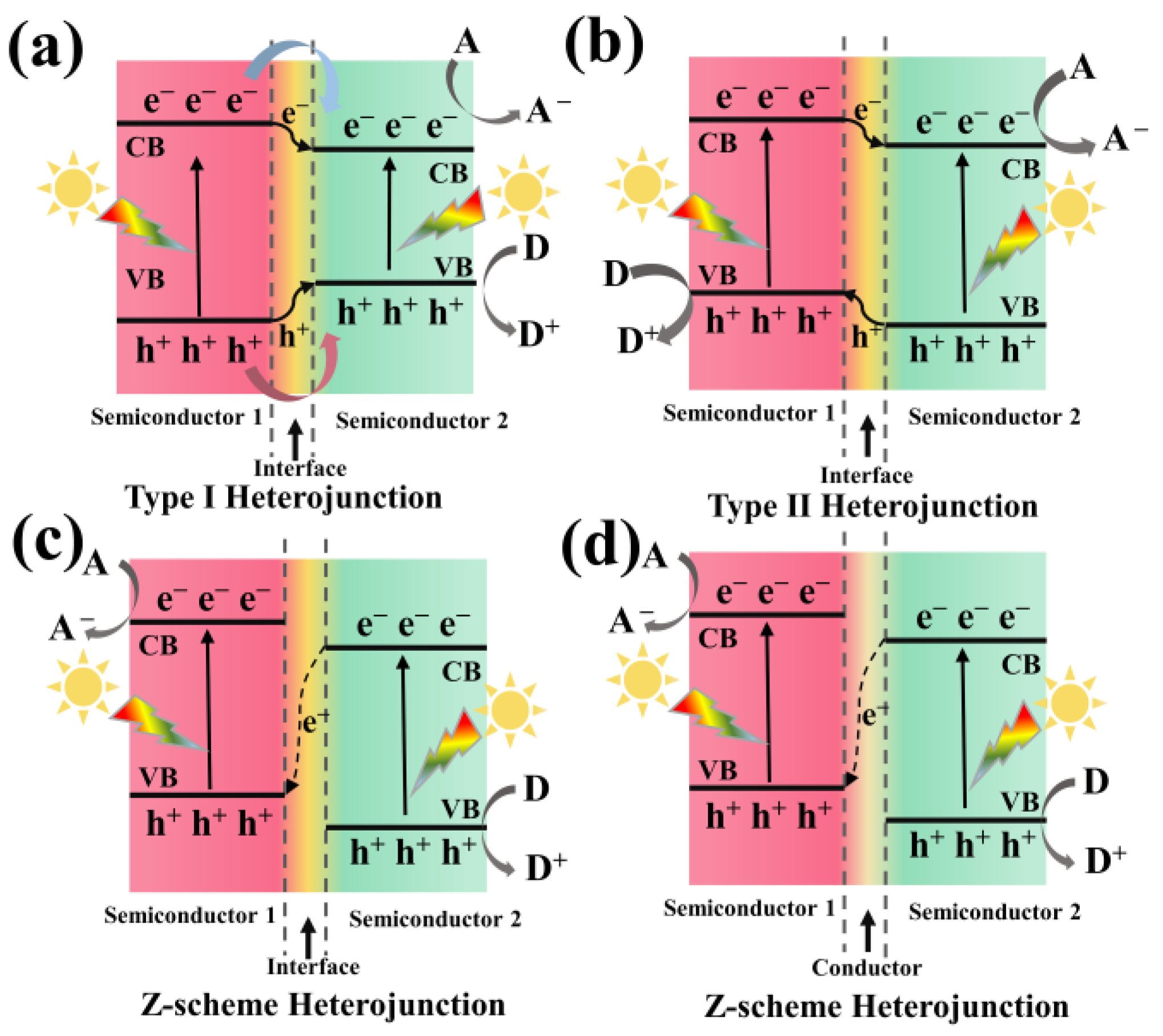
4. Photocatalytic Applications of Porphyrin/g-C3N4 Composite for Solar Energy Utilization and Conversion
4.1. Photocatalytic Water Splitting for Hydrogen Generation
4.2. Photocatalytic CO2 Reduction
(Eo = −1.90 V vs. NHE at pH 7)
(Eo = −0.61 V vs. NHE at pH 7)
(Eo = −0.53 V vs. NHE at pH 7)
(Eo = −0.48 V vs. NHE at pH 7)
(Eo = −0.38 V vs. NHE at pH 7)
(Eo = −0.24 V vs. NHE at pH 7)
4.3. Photocatalytic Degradation of Pollutants
5. Challenges and Perspectives
- (1)
- Although the porphyrin/g-C3N4 composites broaden the absorption range of visible light, there is a need to further increase their utilization of higher-wavelength solar energy (λ > 500 nm).
- (2)
- The efficiency of separating photo-induced charges should be substantially enhanced as the current efficiency of the porphyrin/g-C3N4 hybrid falls short of practical application requirements.
- (3)
- Fabrication of uniform single-layer or few-layer porphyrin/g-C3N4 photocatalysts is necessary to achieve more efficient solar-to-chemical conversion and energy storage.
- (4)
- Addressing the challenge of oxygen evolution is crucial for both water splitting and CO2 reduction. The scarcity of cocatalysts with high activity for oxygen evolution necessitates additional efforts.
- (5)
- The mechanism, cocatalysts, reaction pathways, and product selectivity involved in CO2 reduction catalyzed by porphyrin/g-C3N4 photocatalysts remain unclear and require further exploration and research.
- (6)
- The degradation of pollutants using porphyrin/g-C3N4 hybrid photocatalysts is still in its preliminary stages, and the photocatalytic degradation of gaseous pollutants has received less attention compared to organic pollutants in water.
- (7)
- The stability and recyclability of porphyrin/g-C3N4 photocatalysts are essential to meet industrial requirements and should be optimized for practical applications in the future.
Funding
Conflicts of Interest
Sample Availability
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, T.; Chang, X.; Gong, J. Effective Charge Carrier Utilization in Photocatalytic Conversions. Acc. Chem. Res. 2016, 49, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Long, R.; Huang, H.; Xiong, Y. Plasmonic nanostructures in solar energy conversion. J. Mater. Chem. C 2017, 5, 1008–1021. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Stone, D.; Banin, U. Rich Landscape of Colloidal Semiconductor-Metal Hybrid Nanostructures: Synthesis, Synergetic Characteristics, and Emerging Applications. Chem. Rev. 2023, 123, 3790–3851. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal electric field in carbon nitride-based heterojunctions for photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, M.; Lai, C.; Li, L.; Yang, X.; Du, L.; Zhang, G.; Wang, G.; Yang, L. Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis. Coord. Chem. Rev. 2023, 474, 214846. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, M.; Lai, C.; Wei, Z.; Zhang, G.; Li, L.; Tang, C.; Du, L.; Wang, G.; Liu, H. The Collision between g-C3N4 and QDs in the Fields of Energy and Environment: Synergistic Effects for Efficient Photocatalysis. Small 2023, 19, 2205902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In Situ Synthesis of Lead-Free Halide Perovskite-COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Yin, X.-L.; Liu, J.; Jiang, W.-J.; Zhang, X.; Hu, J.-S.; Wan, L.-J. Urchin-like Au@CdS/WO3 micro/nano heterostructure as a visible-light driven photocatalyst for efficient hydrogen generation. Chem. Commun. 2015, 51, 13842–13845. [Google Scholar] [CrossRef]
- Poulos, T.L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef]
- e Silva, R.C.; da Silva, L.O.; de Andrade Bartolomeu, A.; Brocksom, T.J.; de Oliveira, K.T. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow approaches. Beilstein J. Org. Chem. 2020, 16, 917–955. [Google Scholar] [CrossRef]
- Sheng, H.; Wang, J.; Huang, J.; Li, Z.; Ren, G.; Zhang, L.; Yu, L.; Zhao, M.; Li, X.; Li, G.; et al. Strong synergy between gold nanoparticles and cobalt porphyrin induces highly efficient photocatalytic hydrogen evolution. Nat. Commun. 2023, 14, 1528. [Google Scholar] [CrossRef]
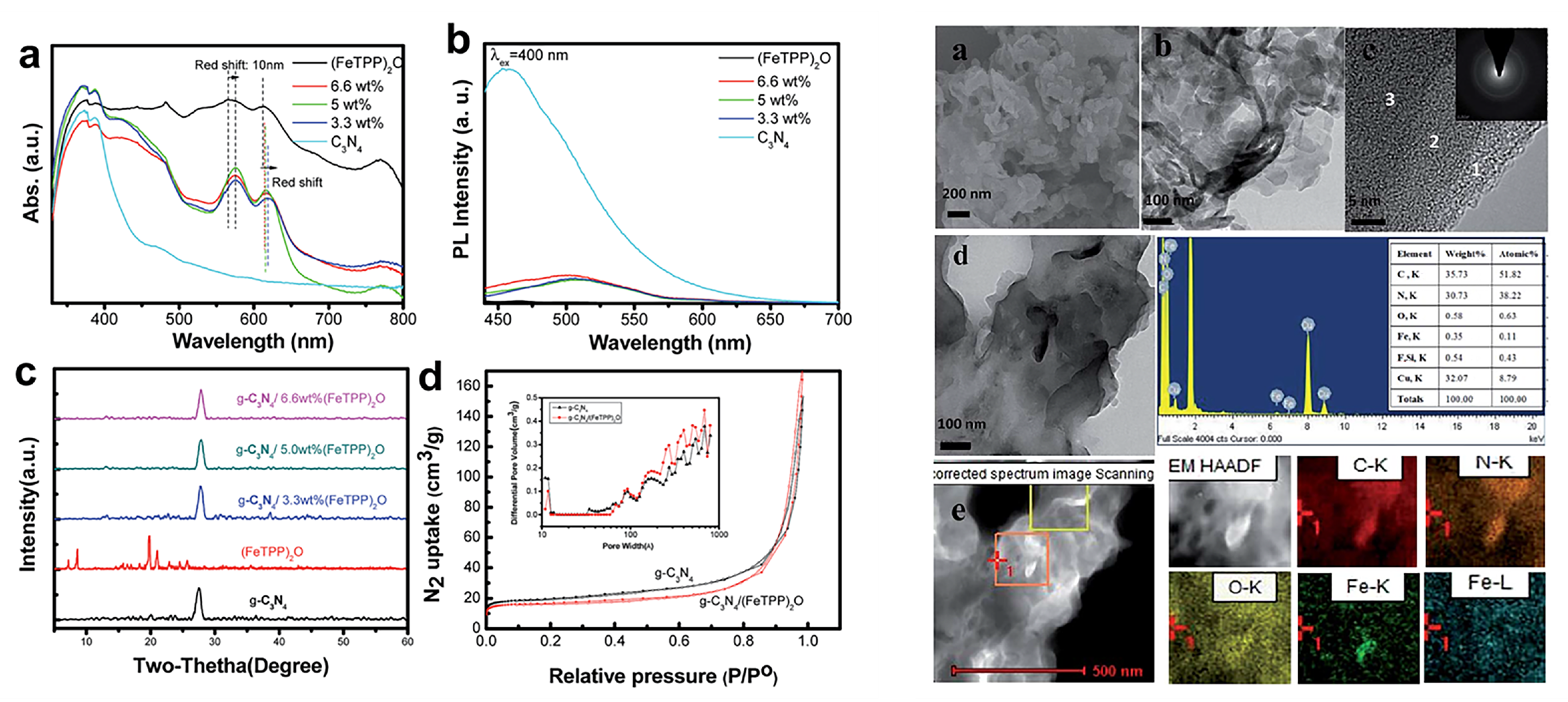
- Wang, D.H.; Pan, J.N.; Li, H.H.; Liu, J.J.; Wang, Y.B.; Kang, L.T.; Yao, J.N. Pure organic heterostructure of μ-oxo dimeric iron(III) porphyrin and graphitic-C3N4 for solar H2 reduction from water. J. Mater. Chem. A 2016, 4, 290–296. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Peng, T.; Zhang, J.; Li, R. Asymmetric Zinc Porphyrin Derivative-Sensitized Graphitic Carbon Nitride for Efficient Visible-Light-Driven H2 Production. ACS Sustain. Chem. Eng. 2017, 5, 7549–7556. [Google Scholar] [CrossRef]
- Mei, S.; Gao, J.; Zhang, Y.; Yang, J.; Wu, Y.; Wang, X.; Zhao, R.; Zhai, X.; Hao, C.; Li, R.; et al. Enhanced visible light photocatalytic hydrogen evolution over porphyrin hybridized graphitic carbon nitride. J. Colloid Interface Sci. 2017, 506, 58–65. [Google Scholar] [CrossRef]
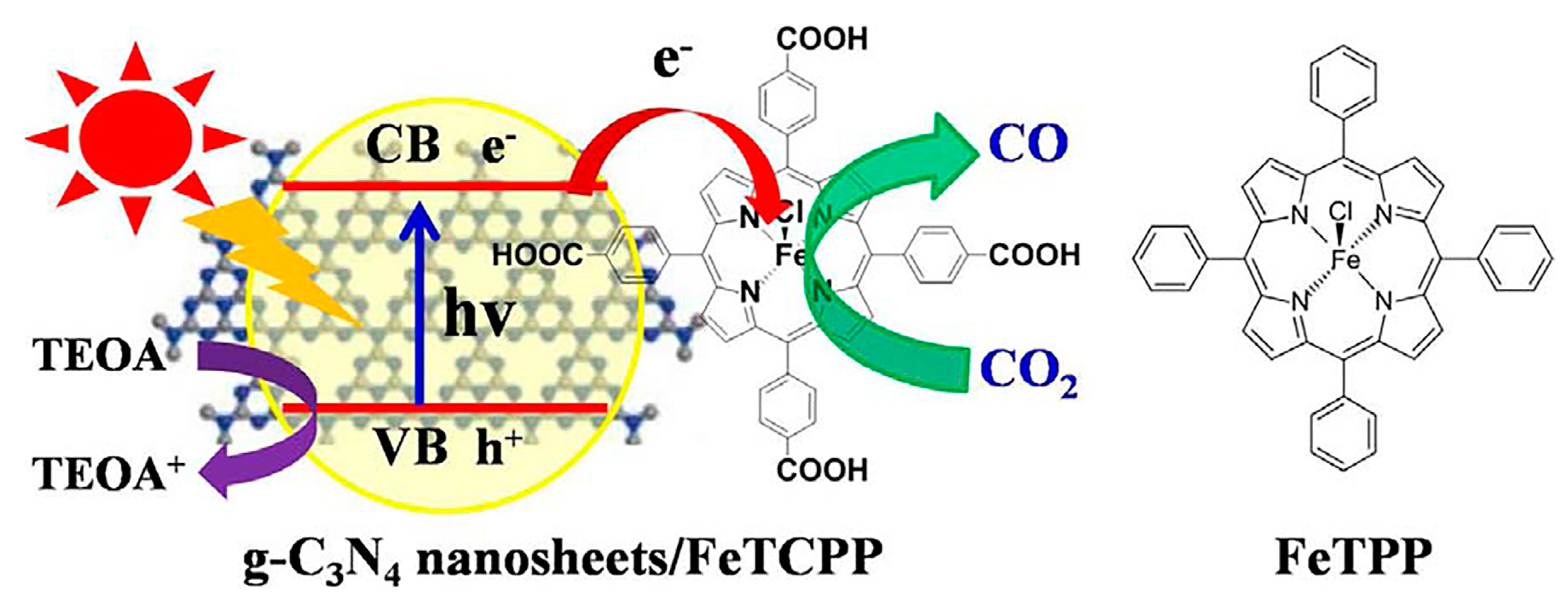
- Lin, L.; Hou, C.; Zhang, X.; Wang, Y.; Chen, Y.; He, T. Highly efficient visible-light driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra(4-carboxyphenyl)porphyrin iron(III) chloride heterogeneous catalysts. Appl. Catal. B 2018, 221, 312–319. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Shen, Q.; Guo, C.; Zhao, G. A biomimetic photoelectrocatalyst of Co-porphyrin combined with a g-C3N4 nanosheet based on π-π supramolecular interaction for high-efficiency CO2 reduction in water medium. Green Chem. 2017, 19, 5900–5910. [Google Scholar] [CrossRef]
- Da Silva, E.S.; Moura, N.M.; Neves, M.G.P.; Coutinho, A.; Prieto, M.; Silva, C.G.; Faria, J.L. Novel hybrids of graphitic carbon nitride sensitized with free-base meso-tetrakis(carboxyphenyl) porphyrins for efficient visible light photocatalytic hydrogen production. Appl. Catal. B 2018, 221, 56–69. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Zhao, W.; Li, C.; Chen, X.; Wang, Y.; Zhu, W.; Zhong, Q. Influence of metal-porphyrins on the photocatalysis of graphitic carbon nitride. Dyes Pigm. 2018, 153, 241–247. [Google Scholar] [CrossRef]
- Li, L.; Bodedla, G.B.; Liu, Z.; Zhu, X. Naphthalimide-porphyrin hybridized graphitic carbon nitride for enhanced photocatalytic hydrogen production. Appl. Surf. Sci. 2020, 499, 143755. [Google Scholar] [CrossRef]
- Mistry, L.; Le-Quang, L.; Masdeu, G.; Bjorkman, W.; Harelind, H.; Abrahamsson, M. Selective Photocatalytic Reduction of CO2-to-CO in Water using a Polymeric Carbon Nitride Quantum Dot/Fe-Porphyrin Hybrid Assembly. ChemCatChem 2022, 14, e202200897. [Google Scholar] [CrossRef]
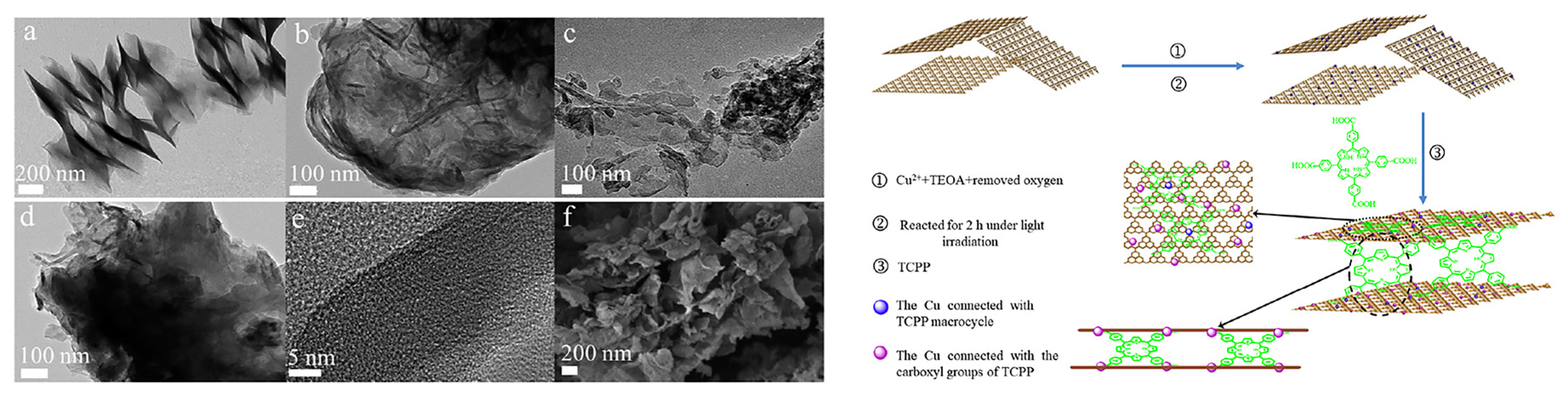
- Zhu, K.; Zhang, M.; Feng, X.; Qin, L.; Kang, S.-Z.; Li, X. A novel copper-bridged graphitic carbon nitride/porphyrin nanocomposite with dramatically enhanced photocatalytic hydrogen generation. Appl. Catal. B 2020, 268, 118434. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, K.; Qin, L.; Kang, S.-Z.; Li, X. Enhanced electron transfer and photocatalytic hydrogen production over the carbon nitride/porphyrin nanohybrid finely bridged by special copper. Catal. Sci. Technol. 2020, 10, 1640–1649. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Qu, D.; Yang, J.; Weng, Y.; Wang, Z.; Sun, Z.; Chen, Y.; He, T. Boosting visible-light driven solar-fuel production over g-C3N4/tetra(4-carboxyphenyl)porphyrin iron(III) chloride hybrid photocatalyst via incorporation with carbon dots. Appl. Catal. B 2020, 265, 118595. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cheng, Z.-E.; Tahir, A.A.; Luo, W.; Wang, C. Au surface plasmon resonance promoted charge transfer in Z-scheme system enables exceptional photocatalytic hydrogen evolution. Appl. Catal. B 2022, 310, 121322. [Google Scholar] [CrossRef]
- Yang, Y.J.; Sun, B.W.; Qian, D.J.; Chen, M. Fabrication of multiporphyrin@g-C3N4 nanocomposites via Pd(II)-directed layer-by-layer assembly for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 478, 1027–1036. [Google Scholar] [CrossRef]
- Zhao, G.; Pang, H.; Liu, G.; Li, P.; Liu, H.; Zhang, H.; Shi, L.; Ye, J. Co-porphyrin/carbon nitride hybrids for improved photocatalytic CO2 reduction under visible light. Appl. Catal. B 2017, 200, 141–149. [Google Scholar] [CrossRef]
- Ma, Z.; Zeng, C.; Hu, L.; Zhao, Q.; Yang, Q.; Niu, J.; Yao, B.; He, Y. A high-performance photocatalyst of ZnTCPP sensitized porous graphitic carbon nitride for antibiotic degradation under visible light irradiation. Appl. Surf. Sci. 2019, 484, 489–500. [Google Scholar] [CrossRef]
- Tian, S.; Chen, S.; Ren, X.; Cao, R.; Hu, H.; Bai, F. Bottom-up fabrication of graphitic carbon nitride nanosheets modified with porphyrin via covalent bonding for photocatalytic H2 evolution. Nano Res. 2019, 12, 3109–3115. [Google Scholar] [CrossRef]
- Tian, S.; Chen, S.; Ren, X.; Hu, Y.; Hu, H.; Sun, J.; Bai, F. An efficient visible-light photocatalyst for CO2 reduction fabricated by cobalt porphyrin and graphitic carbon nitride via covalent bonding. Nano Res. 2020, 13, 2665–2672. [Google Scholar] [CrossRef]
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Liu, B.; Kuang, Y.; Gulzar, A.; He, F.; Gai, S.; Yang, P.; Lin, J. Recent advances in porphyrin-based MOFs for cancer therapy and diagnosis therapy. Coord. Chem. Rev. 2021, 439, 213945. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.-P.; Jiang, H.-L. Boosting Photocatalytic Hydrogen Production of Porphyrinic MOFs: The Metal Location in Metalloporphyrin Matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Lan, Y.-Q.; Liu, M.; Chen, Y.-J.; Huang, X.; Dong, L.-Z.; Lu, M.; Guo, C.; Yuan, D.; Chen, Y.; Xu, G.; et al. Porphyrin-Based COF 2D Materials: Variable Modification of Sensing Performances by Post-Metallization. Angew. Chem. Int. Ed. 2022, 61, e202115308. [Google Scholar]
- Chen, M.; Li, H.; Liu, C.; Liu, J.; Feng, Y.; Wee, A.G.H.; Zhang, B. Porphyrin- and porphyrinoid-based covalent organic frameworks (COFs): From design, synthesis to applications. Coord. Chem. Rev. 2021, 435, 213778. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Xu, Y.-S.; Qiao, Z.-Y.; Wang, H. Covalent organic frameworks (COFs) for photothermal therapy. RSC Nanosci. Nanotechnol. 2022, 54, 286–304. [Google Scholar]
- Cao, R.; Wang, G.; Ren, X.; Duan, P.-C.; Wang, L.; Li, Y.; Chen, X.; Zhu, R.; Jia, Y.; Bai, F. Self-Assembled Porphyrin Nanoleaves with Unique Crossed Transportation of Photogenerated Carriers to Enhance Photocatalytic Hydrogen Production. Nano Lett. 2022, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Feng, H.; Ren, X.; Ji, J.; Bai, F.; Fan, H. Microemulsion-assisted self-assembly and synthesis of size-controlled porphyrin nanocrystals with enhanced photocatalytic hydrogen evolution. Nano Lett. 2019, 19, 2614–2619. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Wang, X.; Yang, W.; Bai, F.; Zhang, B.; Alarid, L.; Bian, K.; Fan, H. pH-dependent assembly of porphyrin-silica nanocomposites and their application in targeted photodynamic therapy. Nano Lett. 2017, 17, 6916–6921. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Zhang, R.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Interfacial Self-Assembly Driven Formation of Hierarchically Structured Nanocrystals with Photocatalytic Activity. ACS Nano 2014, 8, 827–833. [Google Scholar] [CrossRef]
- Jing, J.; Li, J.; Su, Y.; Zhu, Y. Non-covalently linked donor-acceptor interaction enhancing photocatalytic hydrogen evolution from porphyrin assembly. Appl. Catal. B 2023, 324, 122284. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Li, W.; Zhu, Y. Electron Donor-Acceptor Interface of TPPS/PDI Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Sci. 2022, 9, 2201134. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C.; Bian, G.; Xu, J.; Dong, Y.; Zhang, Y.; Lou, Y.; Liu, W.; Zhu, Y. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 2023, 8, 361–371. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhang, H.; Chang, K.; Zhao, G.; Kako, T.; Ye, J. Implantation of Iron(III) in porphyrinic metal organic frameworks for highly improved photocatalytic performance. Appl. Catal. B 2018, 224, 60–68. [Google Scholar] [CrossRef]
- Son, H.-J.; Pac, C.; Kang, S.O. Inorganometallic Photocatalyst for CO2 Reduction. Acc. Chem. Res. 2021, 54, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yang, J.; Zhang, S.; Zhang, J.; Li, Z.; Wang, J.; Chen, X. Bodipy-Based Metal-Organic Frameworks Transformed in Solid States from 1D Chains to 2D Layer Structures as Efficient Visible Light Heterogeneous Photocatalysts for Forging C-B and C-C Bonds. J. Am. Chem. Soc. 2023, 145, 6123–6134. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, X.; Han, J.; Wu, Y.; Liu, S. Zero-Dimensional-g-CNQD-Coordinated Two-Dimensional Porphyrin MOF Hybrids for Boosting Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 42243–42249. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Deng, C.; Huang, Q.; Zhang, C.; Chen, C.; Zhao, J.; Sheng, H. Facilitated Photocatalytic CO2 Reduction in Aerobic Environment on a Copper-Porphyrin Metal-Organic Framework. Angew. Chem. Int. Ed. 2023, 62, e202216717. [Google Scholar]
- Jia, H.; Ma, D.; Zhong, S.; Li, L.; Li, L.; Xu, L.; Li, B. Boosting photocatalytic activity under visible-light by creation of PCN-222/g-C3N4 heterojunctions. Chem. Eng. J. 2019, 368, 165–174. [Google Scholar] [CrossRef]
- Pu, Z.; Xiao, B.; Mao, S.; Sun, Y.; Ma, D.; Wang, H.; Zhou, J.; Cheng, Y.; Shi, J.-W. An electron-hole separation mechanism caused by the pseudo-gap formed at the interfacial Co-N bond between cobalt porphyrin metal organic framework and boron-doped g-C3N4 for boosting photocatalytic H2 production. J. Colloid Interface Sci. 2022, 628, 477–487. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Zhu, W.; Qin, L.; Kang, S.-Z.; Li, X. Enhanced light absorption and electron transfer in dimensionally matched carbon nitride/porphyrin nanohybrids for photocatalytic hydrogen production. Fuel 2023, 338, 127394. [Google Scholar] [CrossRef]
- Hou, Y.; Cui, C.-X.; Zhang, E.; Wang, J.-C.; Li, Y.; Zhang, Y.; Zhang, Y.; Wang, Q.; Jiang, J. A hybrid of g-C3N4 and porphyrin-based covalent organic frameworks via liquid-assisted grinding for enhanced visible-light-driven photoactivity. Dalton Trans. 2019, 48, 14989–14995. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Wang, Z.; Zhu, Y. An all-organic 0D/2D supramolecular porphyrin/g-C3N4 heterojunction assembled via π-π interaction for efficient visible photocatalytic oxidation. Appl. Catal. B 2021, 291, 120059. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; You, E.; Jiang, Q.; Chen, X.; Wang, Y.; Song, Z.; Chang, K.; Xie, Z.; Kuang, Q. Accelerated water oxidation kinetics triggered by supramolecular porphyrin nanosheet for robust visible-light-driven carbon dioxide reduction. Small 2022, 18, 2204924. [Google Scholar] [CrossRef]
- Lai, H.T.; Nguyen, G.T.; Tran, N.T.; Nguyen, T.T.; Van Tran, C.; Nguyen, D.K.; Chang, S.W.; Chung, W.J.; Nguyen, D.D.; Thi, H.P.N.; et al. Assembled Porphyrin Nanofiber on the Surface of g-C3N4 Nanomaterials for Enhanced Photocatalytic Degradation of Organic Dyes. Catalysts 2022, 12, 1630. [Google Scholar] [CrossRef]
- Zou, W.; Liu, X.-H.; Xue, C.; Zhou, X.-T.; Yu, H.-Y.; Fan, P.; Ji, H.-B. Enhancement of the visible-light absorption and charge mobility in a zinc porphyrin polymer/g-C3N4 heterojunction for promoting the oxidative coupling of amines. Appl. Catal. B 2021, 285, 119863. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, J.-F.; Li, X.-A.; Lv, C.-Y.; Hou, C.-P.; Liu, J.-M. Direct Z-scheme photochemical hybrid systems: Loading porphyrin-based metal-organic cages on graphitic-C3N4 to dramatically enhance photocatalytic hydrogen evolution. Chin. J. Catal. 2022, 43, 2249–2258. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/Visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- John, A.T.; George, D.; Hariharan, M. Charge Transport through Discrete Crystalline Architectures. J. Phys. Chem. C 2023, 127, 3389–3397. [Google Scholar] [CrossRef]
- Kamat, P.V. Manipulation of Charge Transfer Across Semiconductor Interface. A Criterion That Cannot Be Ignored in Photocatalyst Design. J. Phys. Chem. Lett. 2012, 3, 663–672. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Zhang, J.; Peng, T.; Li, R. Syntheses of asymmetric zinc porphyrins bearing different pseudo-pyridine substituents and their photosensitization for visible-light-driven H2 production activity. Dalton Trans. 2017, 46, 8219–8228. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; Garcia, H. Photocatalytic CO2 reduction to C2+ products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Chen, X.; Ma, B.; Zhu, M. Metal halide perovskites for photocatalytic CO2 reduction: An overview and prospects. Coord. Chem. Rev. 2023, 482, 215076. [Google Scholar] [CrossRef]
- Shao, F.; Mi, L.; Tian, Z.; Zheng, C.; Zhang, Y.; Li, Q.; Liu, S. Promoting Photodegradation Efficiency via a Heterojunction Photocatalyst Combining with Oxygen Direct and Fast Diffusion from the Gas Phase to Active Catalytic Sites. ACS Appl. Mater. Interfaces 2019, 11, 44922–44930. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, S.; Hu, C.; Duan, M.; Song, M.; Huang, F.; Cai, J. Coupling graphitic carbon nitrides with tetracarboxyphenyl porphyrin molecules through π-π stacking for efficient photocatalysis. J. Mater. Sci. Mater. Electron. 2020, 31, 10677–10688. [Google Scholar] [CrossRef]





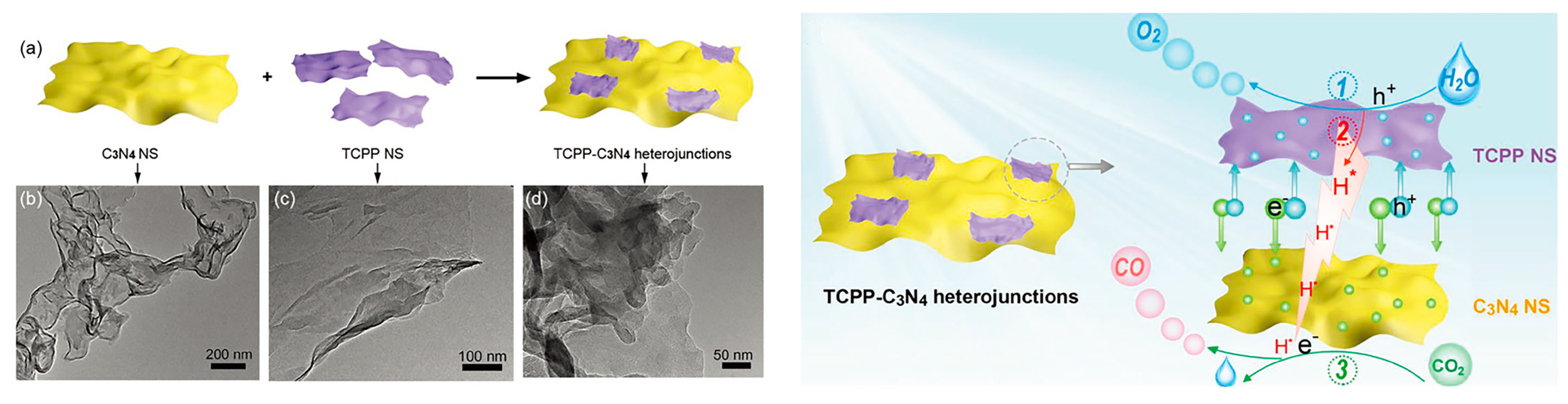
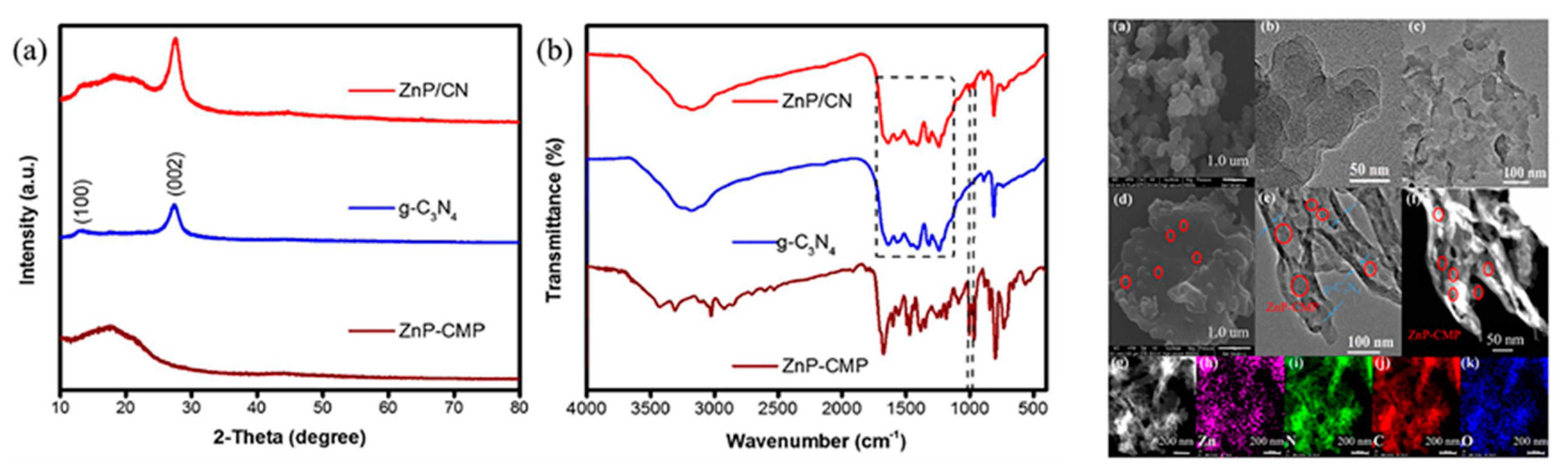

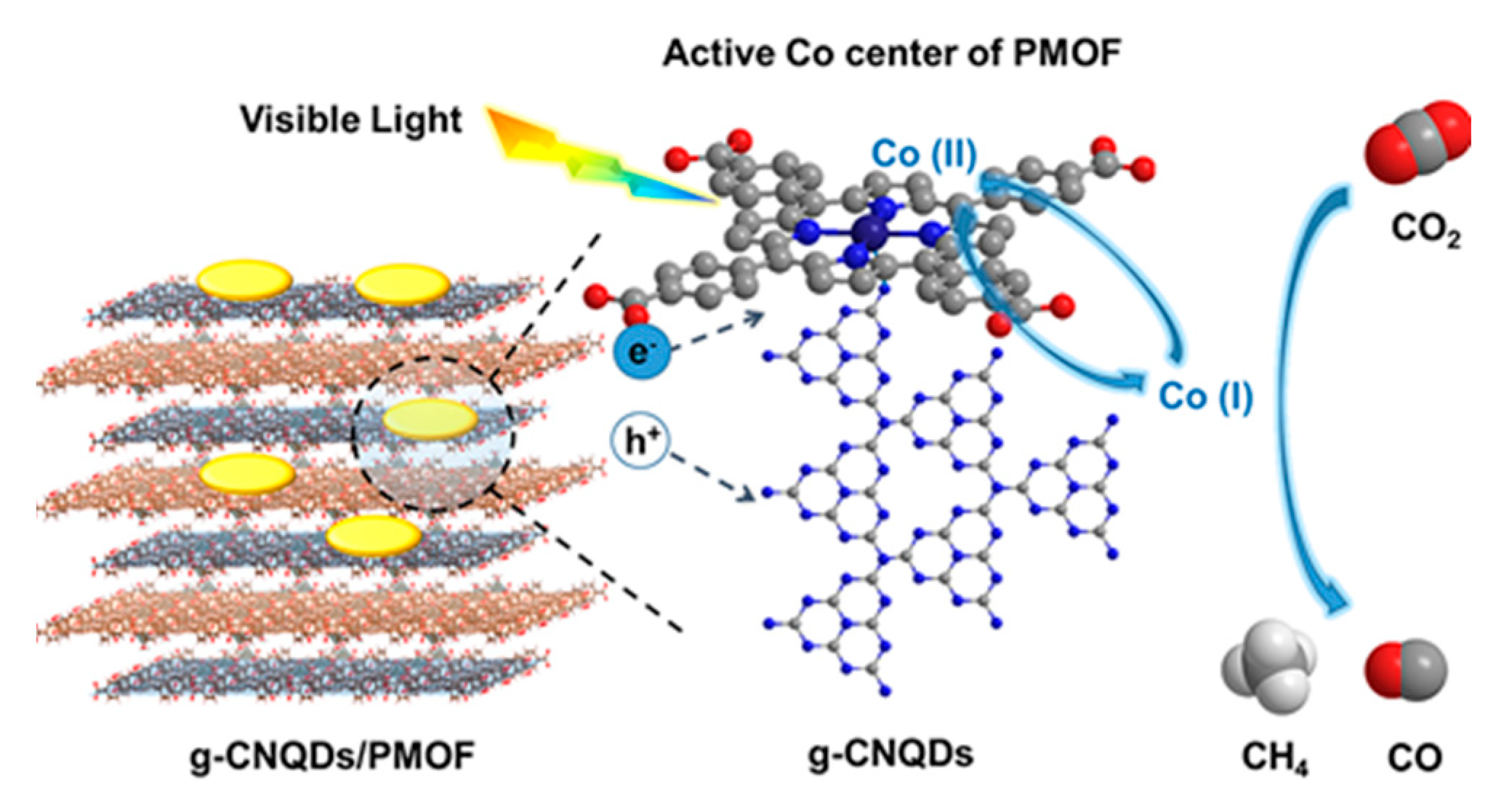
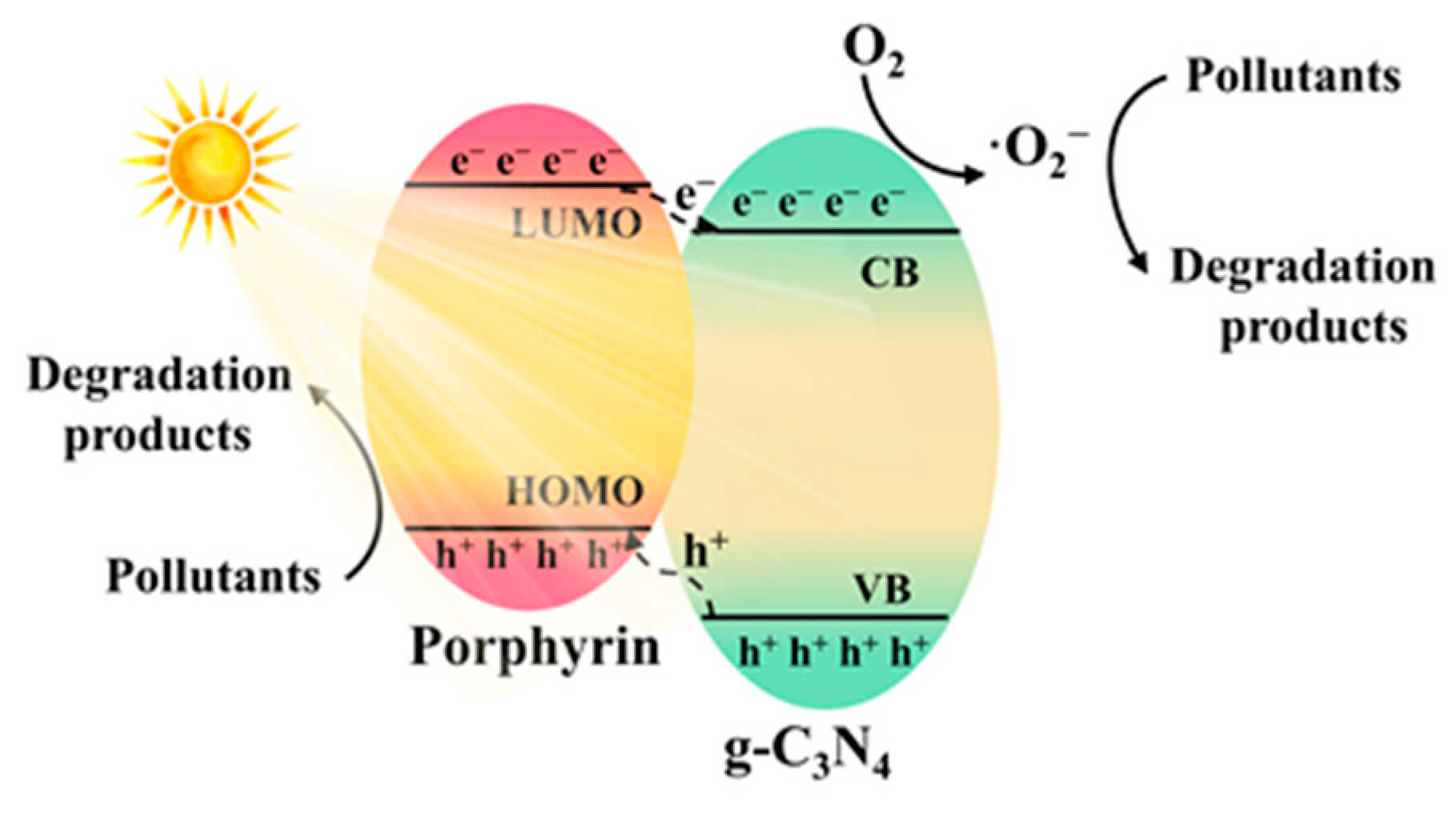
| Composite | Cocatalyst | Sacrificial Agent | Light Source | Activity | AQY | Ref. |
|---|---|---|---|---|---|---|
| C3N4/[(FeTPP)2O] | N/A | 10 vol% TEOA | 300 W Xe Lamp | 187.5 μmol g−1 h−1 | 0.0415% (420 nm) | [19] |
| ZnMT3PyP-Pt/C3N4 | Pt | 50 mM AA | 300 W Xe Lamp | 2.28 mmol g−1 h−1 | 25.1% (420 nm) | [20] |
| TCPP/Pt/g-C3N4 | Pt | 10 vol% TEOA | 450 W Hg Lamp (λ > 380 nm) | 1.21 mmol g−1 h−1 | N/A | [21] |
| mTCPP/CN | Pt | 20 mM EDTA | Mercury vapour lamp | 2.28 mmol g−1 h−1 | N/A | [24] |
| NP/g-C3N4 | Pt | 10 vol% TEOA | 300 W Xe Lamp | 2.29 mmol g−1 h−1 | N/A | [26] |
| g-C3N4-Cu-TCPP | N/A | 10 vol% TEOA | 300 W Xe Lamp | 1.67 mmol g−1 h−1 | N/A | [28] |
| g-C3N4-Cu-THPP | N/A | 10 vol% TEOA | 300 W Xe Lamp | 7.5 μmol h−1 | N/A | [29] |
| Au-P/Fe-CN | N/A | 20 vol% methanol | 300 W Xe Lamp | 3.17 mmol g−1 h−1 | 3.26% (420 nm) | [31] |
| g-CNU-TDPP | Pt | 10 vol% TEOA | 300 W Xe Lamp | 7.6 mmol g−1 h−1 | 13.3% (450 nm) | [35] |
| HBCNN/CoPMOF | Pt | AA | 225 W Xe Lamp | 33.5 mmol g−1 h−1 | N/A | [56] |
| C3N4/Co/PMOF | N/A | 17 vol% TEOA | 300 W Xe Lamp | 1.81 mmol g−1 h−1 | N/A | [57] |
| MOC-Py-Zn/g-C3N4 | N/A | 10 vol% TEOA | 300 W Xe Lamp | 10.3 mmol g−1 h−1 | N/A | [63] |
| ZnPy-4-Pt/C3N4 | Pt | 50 mM AA | 300 W Xe Lamp | 3.49 mmol g−1 h−1 | 32.3% (420 nm) | [68] |
| Composite | Reaction Solvent | Sacrificial Agent | Light Source | Activity | Ref. |
|---|---|---|---|---|---|
| g-C3N4/0.75% FeTCPP | CH3CN:H2O = 3:1 | 20 vol% TEOA | 300 W Xe Lamp | CO: 1.09 mmol g−1 h−1 | [22] |
| CNQD·[Fe-p-TMA] | H2O | TEOA | Hg-Xe Lamp (55 mW cm−2) | CO: 279 μmol h−1 TON: >105 Selectivity: 96% | [27] |
| g-C3N4-CDs/FeTCPP | CH3CN:H2O = 3:1 | 20 vol% TEOA | 300 W Xe Lamp | CO: 11.85 mmol g−1 h−1 | [30] |
| Co-porphyrin/g-C3N4 | CH3CN | 20 vol% TEOA | 300 W Xe Lamp | CO: 17 μmol g−1 h−1 CH4: 0.7 μmol g−1 h−1 Selectivity: 80% | [33] |
| g-CNU-CoTDPP | CH3CN | 20 vol% TEOA | 300 W Xe Lamp | CO: 57 μmol g−1 h−1 H2: 14.2 μmol g−1 h−1 Selectivity: 79% | [36] |
| g-CNQDs/PMOF | CH3CN:H2O = 3:1 | 20 vol% TEOA | 300 W Hg Lamp | CO: 16.1 μmol g−1 h−1 CH4: 6.86 μmol g−1 h−1 Selectivity: 70% | [53] |
| Cu-Zn-TCPP/g-C3N4 | N/A | TEA | 300 W Xe Lamp | CO: 43.75 μmol g−1 h−1 CH4: 323.75 μmol g−1 h−1 CH4 selectivity: 88% | [54] |
| 3% TCPP-C3N4 | N/A | H2O | 300 W Xe Lamp | CO: 16.8 μmol g−1 h−1 O2: 10 μmol g−1 h−1 | [60] |
| Composite | Light Source | Decomposition Rate | Initial Concentration | Ref. |
|---|---|---|---|---|
| CoTPP/g-C3N4 | 350 W Xe Lamp | RhB: 99.79% in 90 min | 25 μmol L−1 | [25] |
| O-C3N4@(Pd-TPyP)3 | 320W Xe Lamp | RhB: about 90% in 40 min | 10 mg L−1 | [32] |
| ZnTCPP/g-C3N4 | 300 W Xe Lamp | RhB: 96% in 30 min TC: 80.3% in 120 min | RhB: 10 mg L−1 TC: 30 mg L−1 | [34] |
| Co-porphyrin/g-C3N4 | 300 W Xe Lamp | RhB: 97.9% in 120 min Ofloxacin: 95.9% in 200 min | 20 mg L−1 | [55] |
| CuPor-Ph-COF/g-C3N4 | 300 W Xe Lamp | RhB: 86% in 90 min | 10 mg L−1 | [58] |
| SA-TCPP/O-CN | 500 W Xe Lamp | BPA: 0.150 h−1 | 10 ppm | [59] |
| C3N4/TCPP | 350 W Xe Lamp | RhB: 0.033 min−1 | 10 ppm | [61] |
| C3N4-TCPP/CP | 300 W Xe Lamp | RhB: 95% in 150 min | 50 mg L−1 | [72] |
| 0.1% TCPP/g-C3N4 | Xe Lamp | RhB: 0.033 min−1 | 22 ppm | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wei, J.; Ren, X.; Song, K.; Sun, J.; Bai, F.; Tian, S. Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion. Molecules 2023, 28, 4283. https://doi.org/10.3390/molecules28114283
Chen S, Wei J, Ren X, Song K, Sun J, Bai F, Tian S. Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion. Molecules. 2023; 28(11):4283. https://doi.org/10.3390/molecules28114283
Chicago/Turabian StyleChen, Sudi, Jiajia Wei, Xitong Ren, Keke Song, Jiajie Sun, Feng Bai, and Shufang Tian. 2023. "Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion" Molecules 28, no. 11: 4283. https://doi.org/10.3390/molecules28114283
APA StyleChen, S., Wei, J., Ren, X., Song, K., Sun, J., Bai, F., & Tian, S. (2023). Recent Progress in Porphyrin/g-C3N4 Composite Photocatalysts for Solar Energy Utilization and Conversion. Molecules, 28(11), 4283. https://doi.org/10.3390/molecules28114283







