Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes
Abstract
1. Introduction
2. Results and Discussion
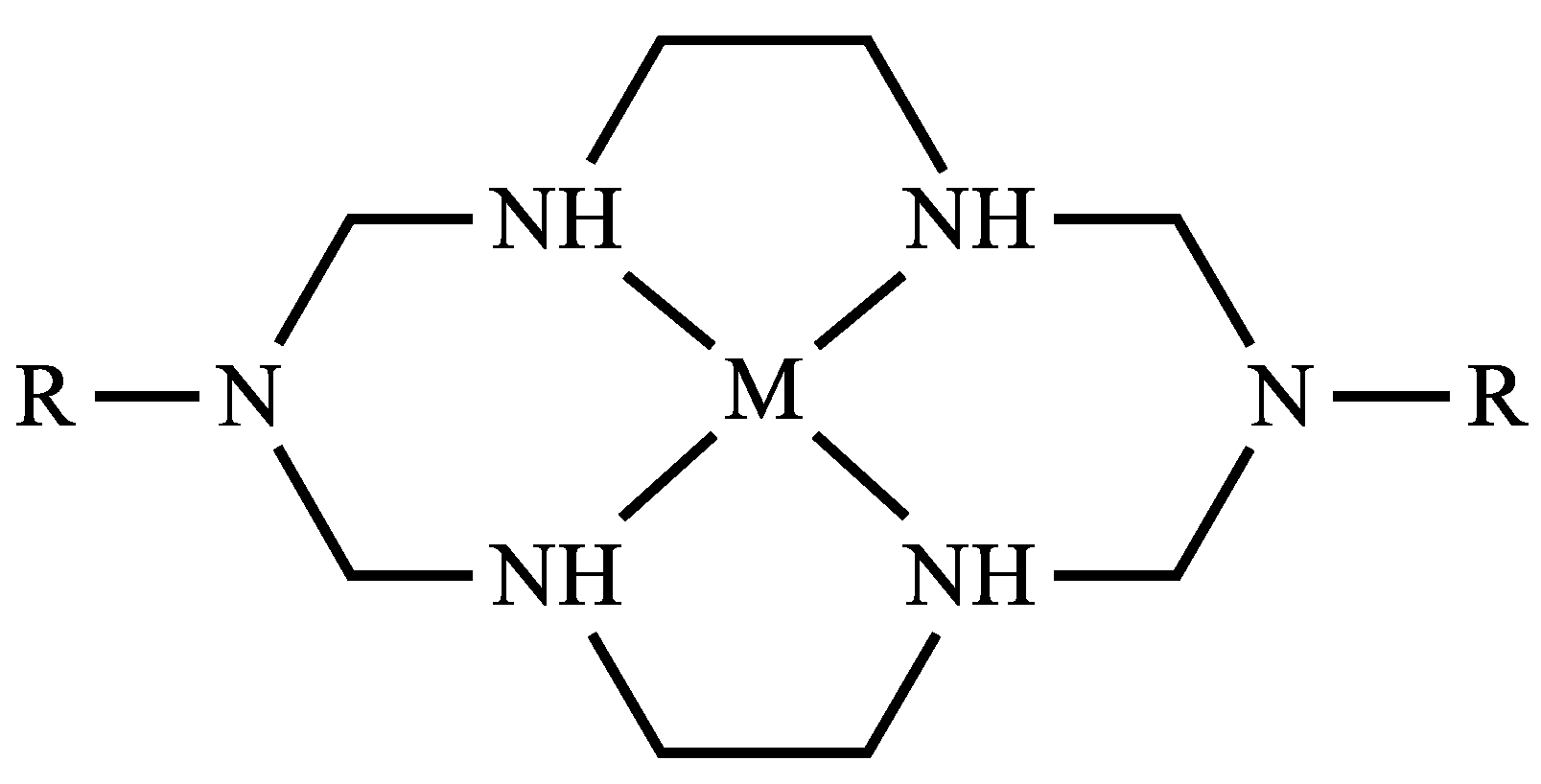
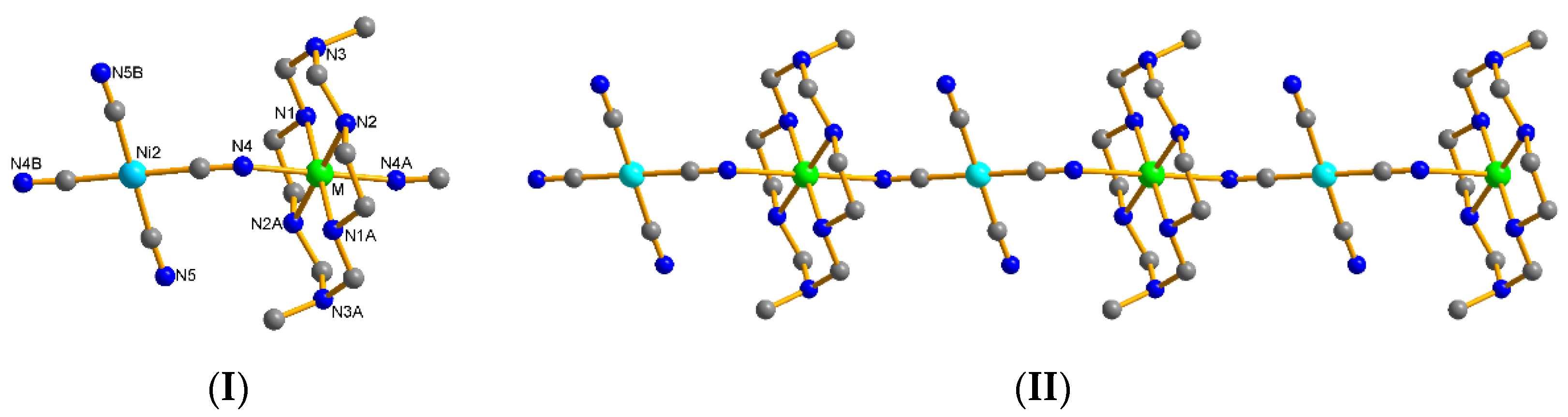
2.1. Description of Structures
2.2. IR Spectra
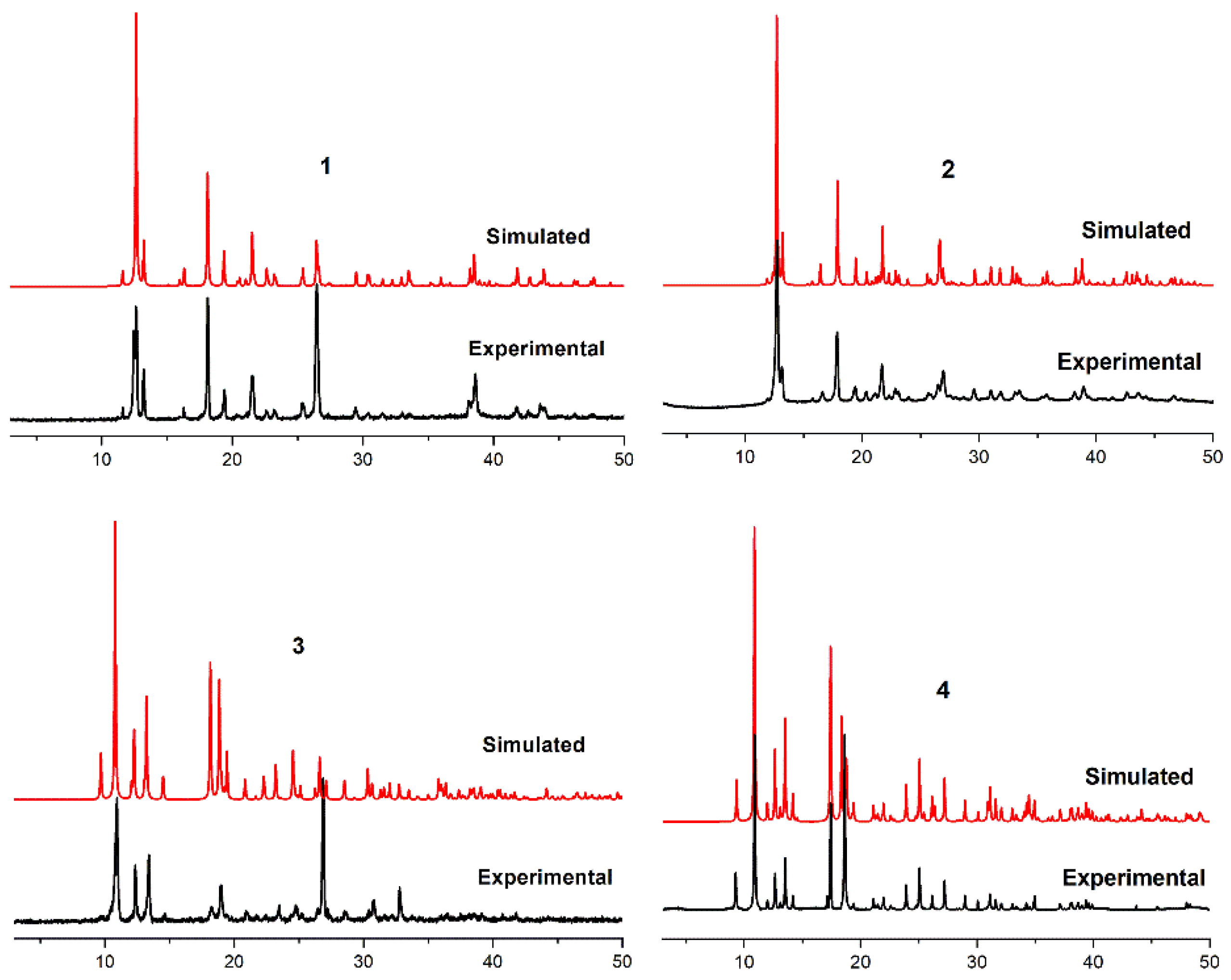
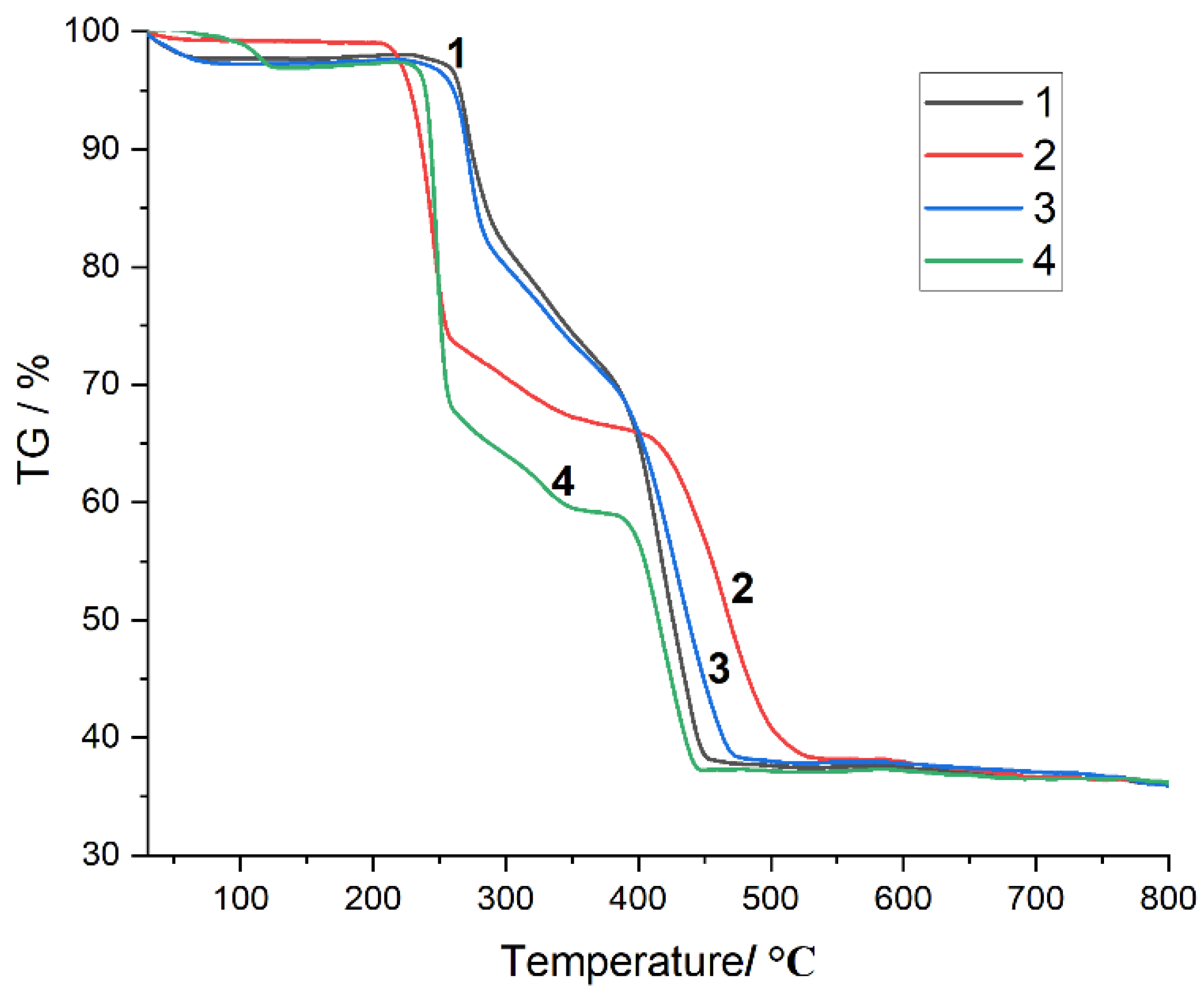
2.3. XRD and TG
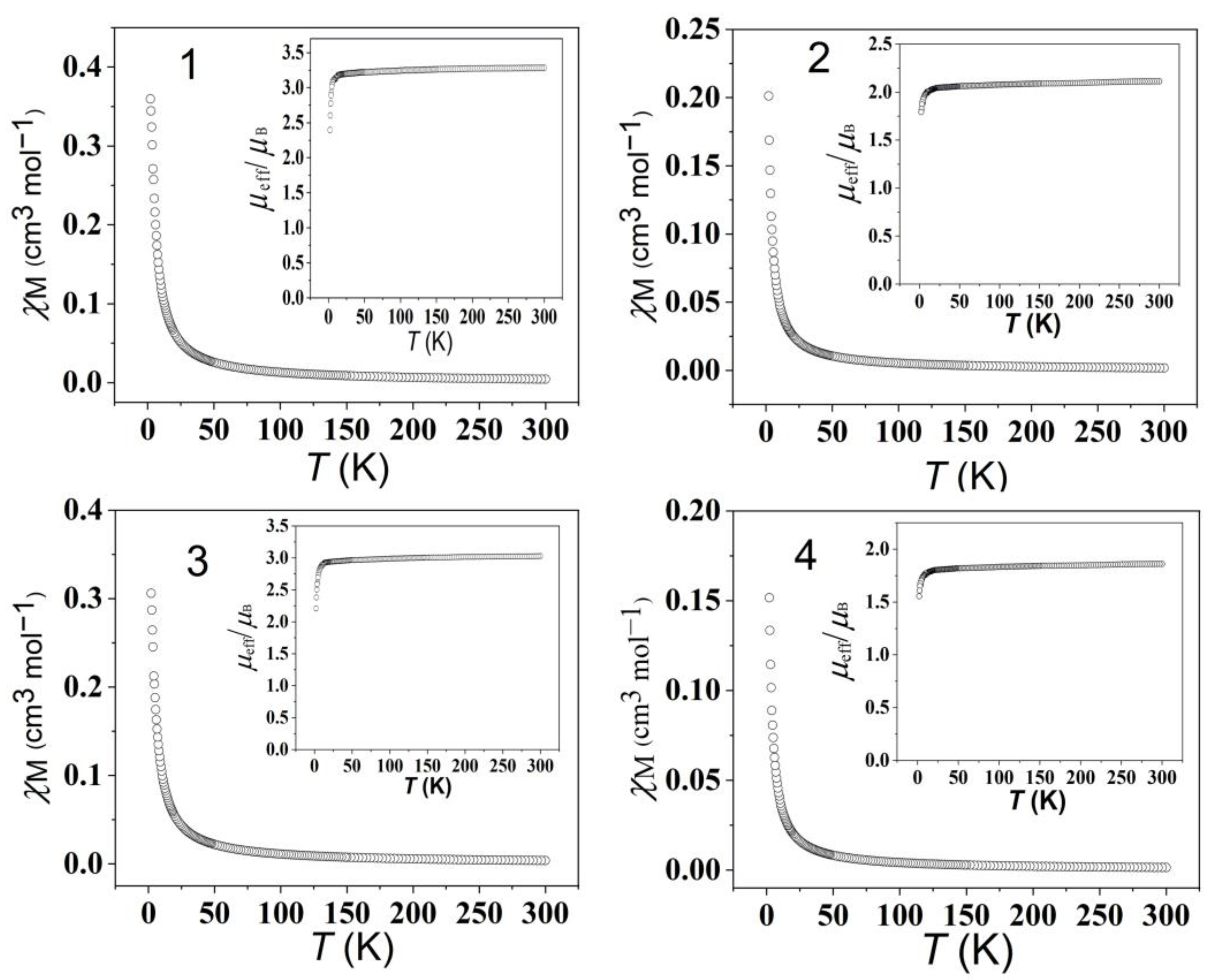
2.4. Magnetism
3. Materials and Methods
Preparation of the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Miras, H.N.; vilà-Nadal, L.; Cronin, L. Polyoxometalate based open-frameworks (POM-OFs). Chem. Soc. Rev. 2014, 43, 5679–5699. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Xiao, X.S.; Gong, S.F.; Yuan, L.; Tang, L.L. An electron-deficient supramolecular macrocyclic host for the selective separation of aromatics and cyclic aliphatics. Org. Chem. Front. 2022, 9, 4829–4833. [Google Scholar] [CrossRef]
- Ding, M.H.; Tang, L.L.; Liao, J.; Ou, G.C.; Zeng, F. High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene. Chin. Chem. Lett. 2021, 5, 1665–1668. [Google Scholar] [CrossRef]
- Mudoi, P.P.; Sarma, B.; Choudhury, A.; Gogoi, N. Evidence of protonation induced intra-molecular metal-to-metal charge transfer in a highly symmetric cyanido bridged {Fe2Ni2} molecular square. Dalton Trans. 2021, 50, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Young, C.; Kaneti, Y.V.; Yamauchi, Y.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Alothman, Z.A.; Kim, J. Cyano-Bridged Cu-Ni Coordination Polymer Nanoflakes and Their Thermal Conversion to Mixed Cu-Ni Oxides. Nanomaterials 2018, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Nakabayashi, K.; Imoto, K.; Klimke, S.; Renz, F.; Ohkoshi, S.I. Second harmonic generation on chiral cyanido-bridged FeIINbIV spin-crossover complexes. Dalton Trans. 2021, 50, 8524–8532. [Google Scholar] [CrossRef]
- Kawabata, S.; Nakabayashi, K.; Imoto, K.; Ohkoshi, S. Spin crossover phenomenon in a three-dimensional cyanido-bridged FeIIMoIV assembly. J. Appl. Phys. 2021, 129, 105501. [Google Scholar] [CrossRef]
- Reczynski, M.; Pinkowicz, D.; Nakabayashi, K.; Nather, C.; Stanek, J.; Koziel, M.; Kalinowska-Tluscik, J.; Sieklucka, B.; Ohkoshi, S.I.; Nowicka, B. Room-temperature bistability in a Ni-Fe chain: Electron transfer controlled by temperature, pressure, light, and humidity. Angew. Chem. Int. Edit. 2021, 60, 2330–2338. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef]
- Jankowski, R.; Reczynski, M.; Chorazy, S.; Zychowicz, M.; Arczynski, M.; Koziel, M.; Ogorzaly, K.; Makowski, W.; Pinkowicz, D.; Sieklucka, B. Guest-dependent pressure-induced spin crossover in Fe4II[MIV(CN)8]2 (M = Mo, W) cluster-based material showing persistent solvent-driven structural transformations. Chem. Eur. J. 2020, 26, 11187–11198. [Google Scholar] [CrossRef] [PubMed]
- Pai, T.Y.; Stefanczyk, O.; Kumar, K.; Mathoniere, C.; Sieklucka, B.; Ohkoshi, S.I. Experimental and theoretical insights into the photomagnetic effects in trinuclear and ionic CuII-MoIV systems. Inorg. Chem. Front. 2022, 9, 771–783. [Google Scholar] [CrossRef]
- Magott, M.; Pinkowicz, D. Chiral porous CN-bridged coordination polymer mimicking MOF-74 and showing magnetization photoswitching. Chem. Commun. 2021, 57, 9926–9929. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Guionneau, P.; Lafon, E.; Perot, S.; Kauffmann, B.; Mathoniere, C. New photomagnetic ionic salts based on [Mo(CN)8]4- and [W(CN)8]4- anions. Magnetochemistry 2021, 7, 97. [Google Scholar] [CrossRef]
- Sellin, M.; Rupf, S.M.; Abram, U.; Malischewski, M. Eightfold electrophilic methylation of octacyanotungstate [W(CN)8]4-/3-: Preparation of homoleptic, eight-coordinate methyl isocyanide complexes [W(CNMe)8]4+/5+. Inorg. Chem. 2021, 60, 5917–5924. [Google Scholar] [CrossRef]
- Ali, M.; Ray, A.; Sheldrick, W.S.; Mayer-Figge, H.; Gao, S.; Sahmes, A.I. Synthesis, crystal structure, EPR and magnetic properties of a cyano-bridged CuII–NiII heterobimetallic complex: An unusual structure with long-range ferromagnetic exchange through hydrogen bonding. New J. Chem. 2004, 28, 412–417. [Google Scholar] [CrossRef]
- Yeung, W.F.; Kwong, H.K.; Lau, T.C.; Gao, S.; Szeto, L.; Wong, W.T. Cyano-bridged molecular squares: Synthesis and structures of [Ni(cyclen)]2[Pt(CN)4]2·6H2O, [Ni(cyclen)]2[Ni(CN)4]2·6H2O and [Mn(cyclen)]2[Ni(CN)4]2·6H2O. Polyhedron 2006, 25, 1256–1262. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, T.B.; Feng, X.L. Two supramolecular isomers of molecular squares and 1D helical chains with alternating right- and left-handed chirality. Inorg. Chem. 2005, 44, 7056–7062. [Google Scholar] [CrossRef]
- Jiang, L.; Feng, X.L.; Su, C.Y.; Chen, X.M.; Lu, T.B. Interchain-solvent-induced chirality change of 1D helical chains: From achiral to chiral crystallization. Inorg. Chem. 2007, 46, 2637–2644. [Google Scholar] [CrossRef]
- Curtis, N.F.; Flood, K.; Robinson, W.T. N-rac-(5,7,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,14(1)-diene)copper(II): Structures of the perchlorate salt and a trinuclear tetracyanonickelato-bridged compound. Polyhedron 2006, 25, 1579–1584. [Google Scholar] [CrossRef]
- Černák, J.; Kuchár, J.; Stolárová, M.; Kajnaková, M.; Vavra, M.; Potocǹák, I.; Falvello, L.R.; Tomás, M. Preparation, spectroscopic and magnetic characterization of Cu(cyclam)M(CN)4 complexes exhibiting one-dimensional crystal structures (cyclam = 1,4,8,11-tetraazacyclotetradecane, M = Ni, Pd, Pt). Transit. Met. Chem. 2010, 35, 737–744. [Google Scholar] [CrossRef]
- Kartal, Z.; Sahin, O.; Yavuz, A. Synthesis of Hofmann-type Zn(H2O)2Ni(CN)4·nG (G = water and 1,4-dioxane) clathrates and the determination of their structural properties by various spectroscopic methods. Turk. J. Chem. 2019, 43, 1608–1621. [Google Scholar] [CrossRef]
- Zhang, D.P.; Si, W.J.; Wang, P.; Chen, X.; Jiang, J.Z. 1D to 3D Heterobimetallic complexes tuned by cyanide precursors: Synthesis, crystal structures, and magnetic properties. Inorg. Chem. 2014, 53, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.C.; Zhang, M.; Li, Z.Z.; Yuan, X.Y. Construction of one-dimensional homochiral helical supramolecular stereoismers using chiral macrocyclic nickel(II) complexes as building blocks. Inorg. Chim. Acta 2014, 411, 11–16. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nowicka, B.; Chorazy, S.; Reczynski, M.; Sieklucka, B. Magnetic clusters based on octacyanidometallates. Inorg. Chem. Front. 2015, 2, 10–27. [Google Scholar] [CrossRef]
- Jiang, X.; Tao, B.L.; Yu, X.; Wang, Y.H.; Xia, H. Syntheses, crystal structures and properties of three cyano-bridged one-dimensional coordination polymers based on macrocyclic metallic tectons. RSC Adv. 2015, 5, 19034. [Google Scholar] [CrossRef]
- Vafazadeh, R.; Dehghani-Firouzabadi, A.; Willis, A.C. Synthesis of hetero- and homo-multinuclear complexes with a tetracyanonickelate anion: Structural characterization [Cu(bcen)Ni(CN)4]2. Acta Chim Slov. 2017, 64, 686–691. [Google Scholar] [CrossRef]
- Azhar, A.; Zakaria, M.B.; Kim, J.; Na, J.; Kaneti, Y.V.; Fatehmulla, A.; Aldhafiri, A.M.; Farooq, W.A.; Bando, Y.; Yamauchi, Y. Single crystal growth of two-dimensional cyano-bridged coordination polymer of Co(H2O)2Ni(CN)4·4H2O using trisodium citrate dihydrate. Bull. Chem. Soc. Jpn. 2019, 92, 1263–1267. [Google Scholar] [CrossRef]
- Suh, M.P.; Kang, S.G. Synthesis and properties of nickel(II) and copper(II) complexes of 14-membered hexaaza macrocycles, 1,8-dimethyl- and 1,8-diethyl-1,3,6,8,10,13-hexaazacyclotetradecane. Inorg. Chem. 1988, 27, 2544–2546. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]

| 1 | |||||
| Bond | Length | Bond | Length | Bond | Length |
| Ni(1)-N(1) | 2.0498(16) | Ni(1)-N(2) | 2.0712(16) | Ni(1)-N(4) | 2.1489(17) |
| Ni(2)-C(6) | 1.866(2) | Ni(2)-C(7) | 1.858(2) | ||
| Bond | Angle | Bond | Angle | Bond | Angle |
| N(1)-Ni(1)-N(2) | 94.15(7) | N(1)-Ni(1)-N(2) #1 | 85.85(7) | N(1)-Ni(1)-N(4) #1 | 90.27(6) |
| N(2)-Ni(1)-N(4) #1 | 88.33(6) | N(1)-Ni(1)-N(4) | 89.73(6) | N(2)-Ni(1)-N(4) | 91.67(6) |
| N(5)-C(7)-Ni(2) | 176.0(2) | N(4)-C(6)-Ni(2) | 171.6(7) | C(6)-N(6)-Ni(1) | 149.7(5) |
| 2 | |||||
| Bond | Length | Bond | Length | Bond | Length |
| Cu(1)-N(1) | 2.0009(15) | Cu(1)-N(2) | 2.0240(16) | Cu(1)-N(5) | 2.563(2) |
| Ni(1)-C(7) | 1.865(2) | Ni(1)-C(6) | 1.861(2) | ||
| Bond | Angle | Bond | Angle | Bond | Angle |
| N(1)-Cu(1)-N(2) | 93.72(7) | N(1)-Cu(1)-N(2) #1 | 86.28(7) | C(6)-Ni(1)-C(7) | 88.08(9) |
| C(6)-Ni(1)-C(7) #2 | 91.92(9) | N(5)-C(7)-Ni(1) | 175.7(9) | N(4)-C(6)-Ni(1) | 177.3(2) |
| C(7)-N(5)-Cu(1) | 127.8(8) | ||||
| 3 | |||||
| Bond | Length | Bond | Length | Bond | Length |
| Ni(1)-N(1) | 2.061(2) | Ni(1)-N(2) | 2.068(2) | Ni(1)-N(4) | 2.118(2) |
| Ni(2)-C(8) | 1.864(3) | Ni(2)-C(9) | 1.862(3) | ||
| Bond | Angle | Bond | Angle | Bond | Angle |
| N(1)-Ni(1)-N(2) #1 | 85.66(10) | N(1)-Ni(1)-N(2) | 94.34(10) | N(1)-Ni(1)-N(4) | 92.09(10) |
| N(2)-Ni(1)-N(4) | 91.21(9) | N(1)-Ni(1)-N(4) #1 | 87.91(10) | N(2)-Ni(1)-N(4) #1 | 88.79(9) |
| C(9)-Ni(2)-C(8) | 91.14(11) | C(9)-Ni(2)-C(8) #3 | 88.86(11) | N(4)-C(8)-Ni(2) | 177.1(2) |
| N(5)-C(9)-Ni(2) | 178.9(3) | C(8)-N(4)-Ni(1) | 148.4(2) | ||
| 4 | |||||
| Bond | Length | Bond | Length | Bond | Length |
| Cu(1)-N(1) | 2.016(2) | Cu(1)-N(2) | 2.0159(19) | Cu(1)-N(4) | 2.410(2) |
| Ni(1)-C(8) | 1.864(3) | Ni(1)-C(9) | 1.865(3) | ||
| Bond | Angle | Bond | Angle | Bond | Angle |
| N(1)-Cu(1)-N(2) #4 | 85.93(8) | N(1)-Cu(1)-N(2) | 94.07(8) | N(1)-Cu(1)-N(4) #4 | 91.93(8) |
| N(2)-Cu(1)-N(4) #4 | 91.37(9) | N(1)-Cu(1)-N(4) | 88.07(8) | N(2)-Cu(1)-N(4) | 88.63(9) |
| C(8)-Ni(1)-C(9) | 90.18(11) | C(8)-Ni(1)-C(9) #5 | 89.82(11) | N(4)-C(8)-Ni(1) | 178.0(2) |
| N(5)-C(9)-Ni(1) | 178.8(3) | C(8)-N(4)-Cu(1) | 160.8(2) | ||
| Compound | [NiL1][Ni(CN)4] | [CuL1][Ni(CN)4] | [NiL2][Ni(CN)4]·2H2O | [CuL2][Ni(CN)4]·2H2O |
|---|---|---|---|---|
| Empirical formula | C14H26Ni2N10 | C14H26CuNiN10 | C18H38Ni2O2N10 | C18H38CuNiN10O2 |
| Formula weight | 451.86 | 456.70 | 544.00 | 548.83 |
| Temperature (K) | 296(2) | 296(2) | 296(2) | 296(2) |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic |
| Space group | P2(1)/n | P2(1)/n | P-1 | P-1 |
| a/Å | 9.834(3) | 9.906(4) | 8.588(8) | 8.400(4) |
| b/Å | 9.167(3) | 9.110(4) | 8.873(8) | 8.771(4) |
| c/Å | 10.910(4) | 10.785(5) | 10.001(9) | 10.098(4) |
| α/° | 90 | 90 | 80.930(11) | 81.180(4) |
| β/° | 95.177(4) | 92.268(4) | 65.954(10) | 69.590(4) |
| γ/° | 90 | 90 | 67.544(10) | 67.991(4) |
| V/Å3 | 979.5(6) | 972.6(7) | 643.1(10) | 646.2(5) |
| Z | 4 | 2 | 2 | 1 |
| Dc/Mg cm−3 | 1.532 | 1.559 | 1.405 | 1.410 |
| μ/mm−1 | 1.943 | 2.082 | 1.498 | 1.585 |
| F (000) | 472 | 474 | 288 | 289 |
| Crystal size (mm) | 0.36 × 0.28 × 0.23 | 0.46 × 0.36 × 0.28 | 0.28 × 0.23 × 0.15 | 0.38 × 0.26 × 0.20 |
| θ range | 2.67–27.46 | 2.74–27.47 | 2.23–27.44 | 2.15–27.53 |
| Collected/unique | 10687/2221(0.022) | 10594/2206(0.021) | 7227/2838(0.0295) | 6873/2872(0.0306) |
| Completeness to θ | 99.3% | 99.1% | 97.0% | 96.3% |
| Goodness-of-fit on F2 | 1.041 | 1.012 | 0.979 | 0.991 |
| Final R indices [I > 2σ (I)] | 0.0279, 0.0721 | 0.0268, 0.0716 | 0.0340, 0.0828 | 0.0356, 0.0893 |
| R indices (all data) | 0.0411, 0.0799 | 0.0417, 0.0792 | 0.0554, 0.0910 | 0.0551, 0.0981 |
| Max. peak/hole (e. Å−3) | 0.290/−0.173 | 0.196/−0.178 | 0.460/−0.464 | 0.605/−0.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, G.; Wang, Q.; Tan, Y.; Zhou, Q. Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes. Molecules 2023, 28, 4529. https://doi.org/10.3390/molecules28114529
Ou G, Wang Q, Tan Y, Zhou Q. Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes. Molecules. 2023; 28(11):4529. https://doi.org/10.3390/molecules28114529
Chicago/Turabian StyleOu, Guangchuan, Qiong Wang, Yingzhi Tan, and Qiang Zhou. 2023. "Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes" Molecules 28, no. 11: 4529. https://doi.org/10.3390/molecules28114529
APA StyleOu, G., Wang, Q., Tan, Y., & Zhou, Q. (2023). Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes. Molecules, 28(11), 4529. https://doi.org/10.3390/molecules28114529






