Inhibition of Mitochondrial Antioxidant Defense and CDK4/6 in Mesothelioma
Abstract
1. Introduction
1.1. Mitochondrial Antioxidant Defense
1.2. Cell Cycle-Related Proteins
1.3. Interaction between Mitochondrial Antioxidant Defense and the Cell Cycle
2. Results
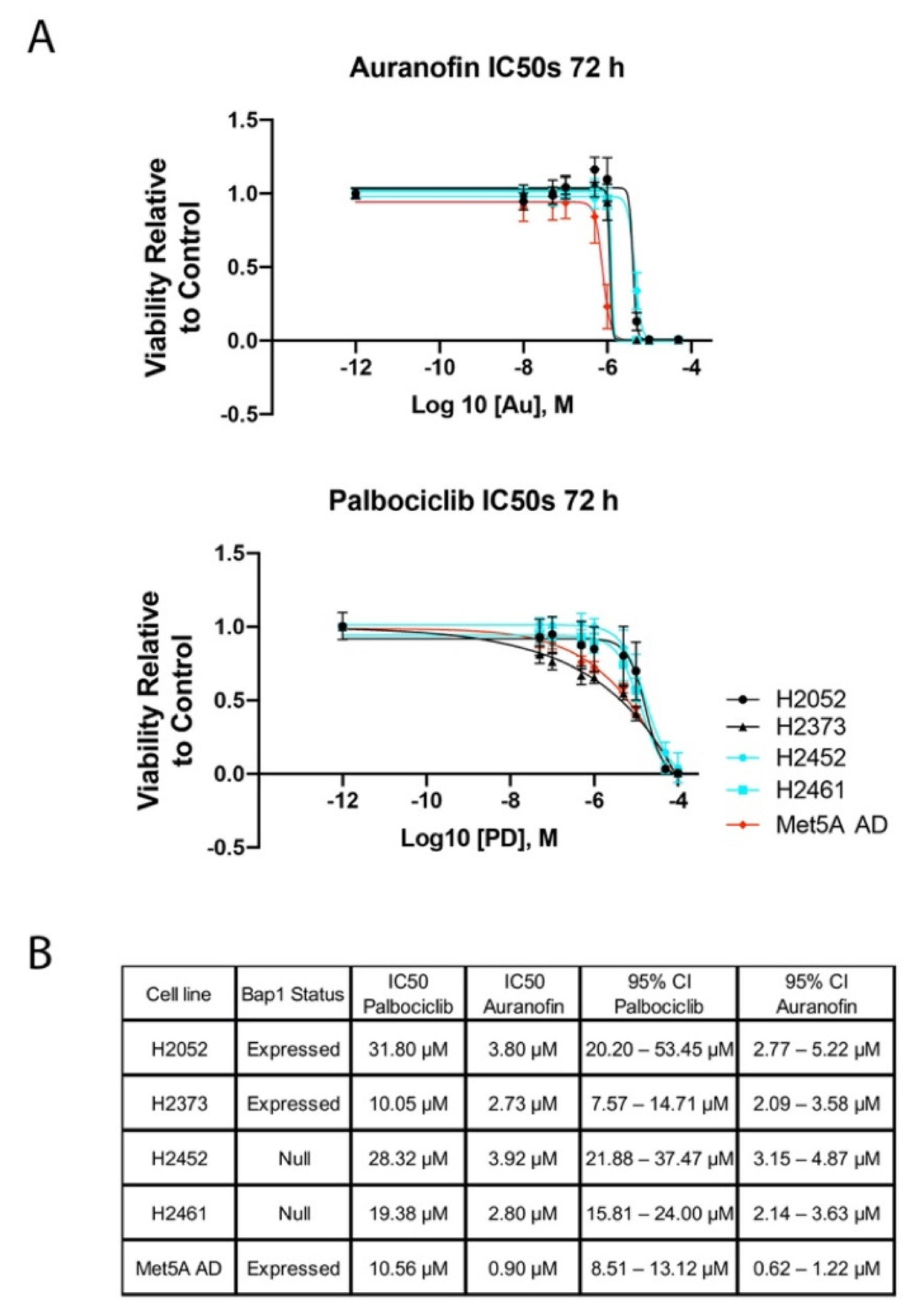
2.1. Inhibition of Cell Proliferation by Auranofin and Palbociclib
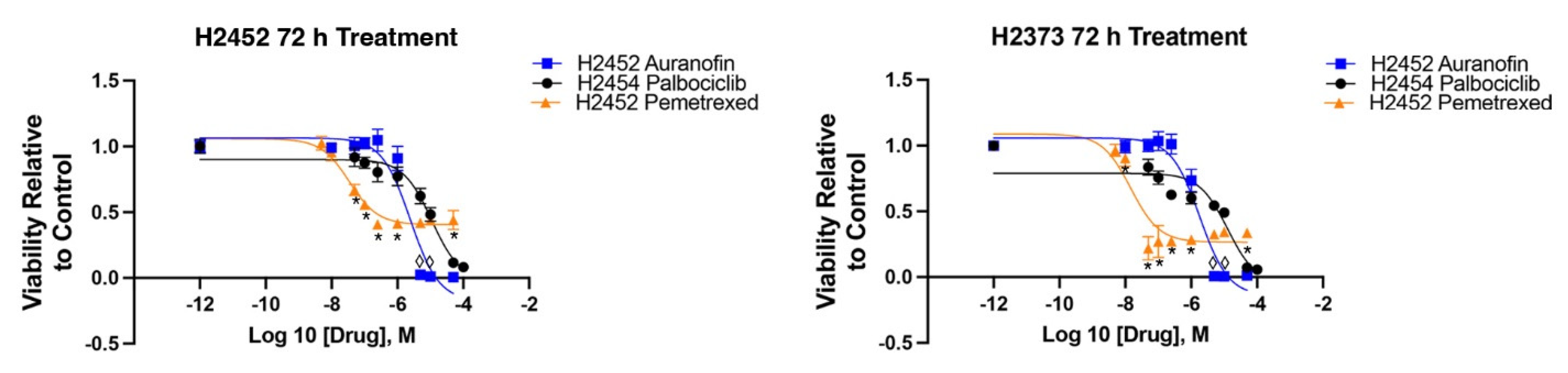
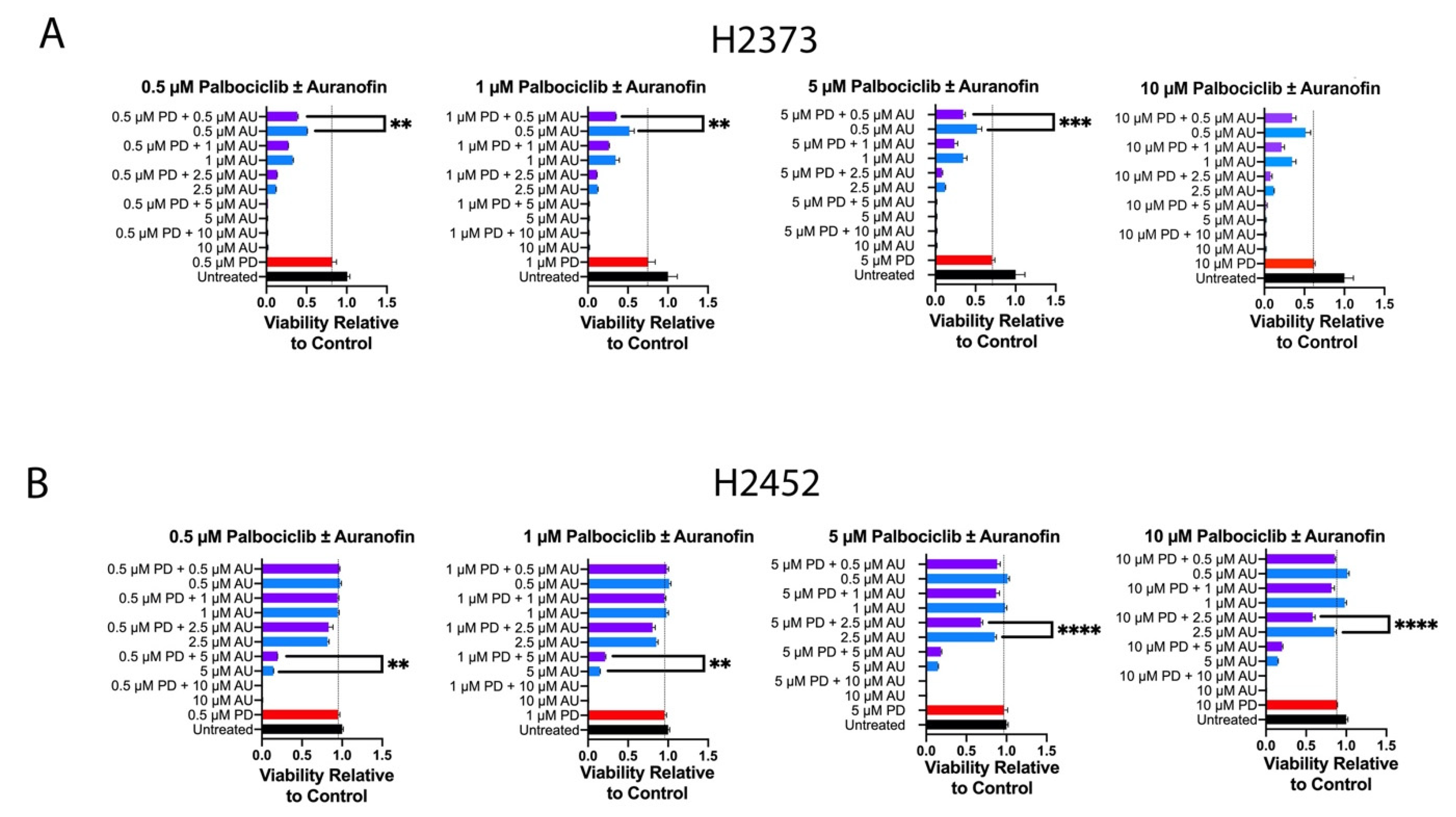
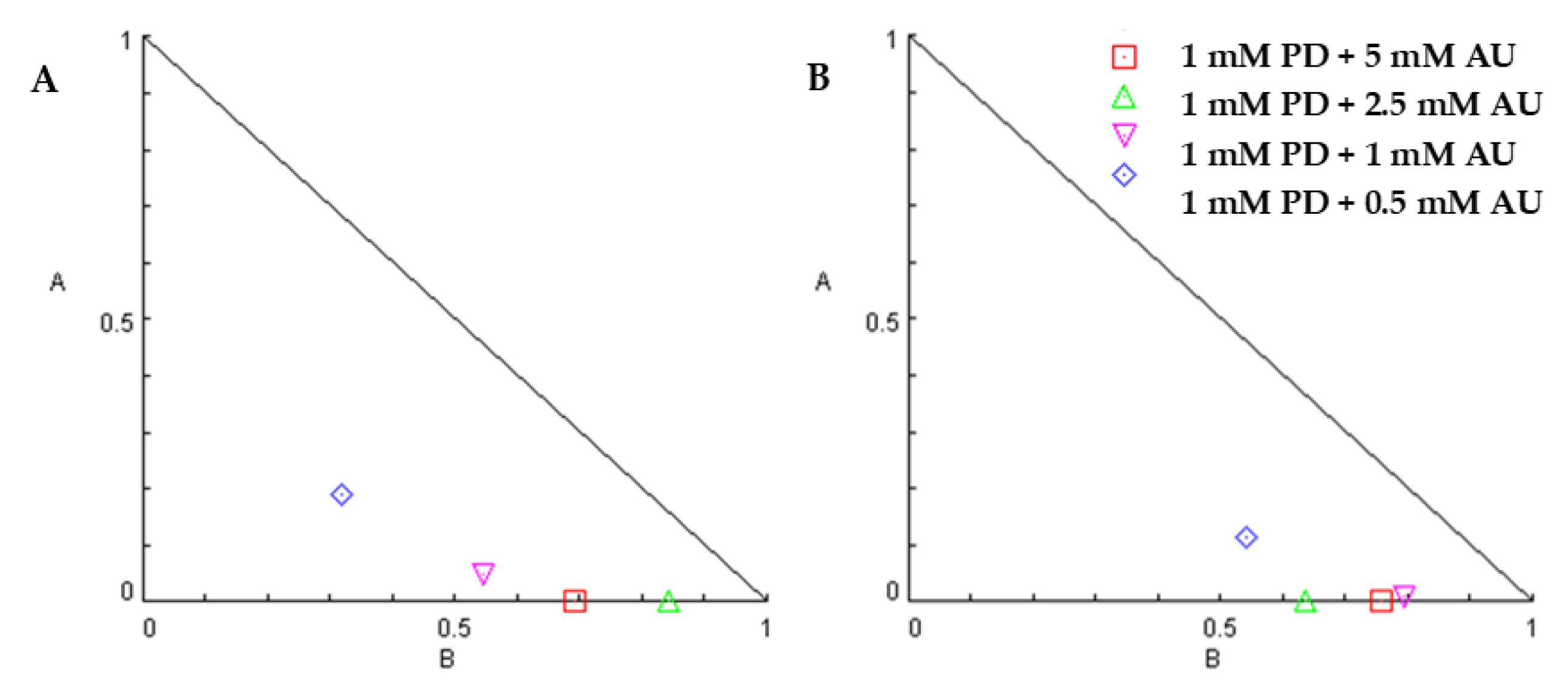
2.2. Inhibition of Cell Proliferation by Combination of Auranofin and Palbociclib
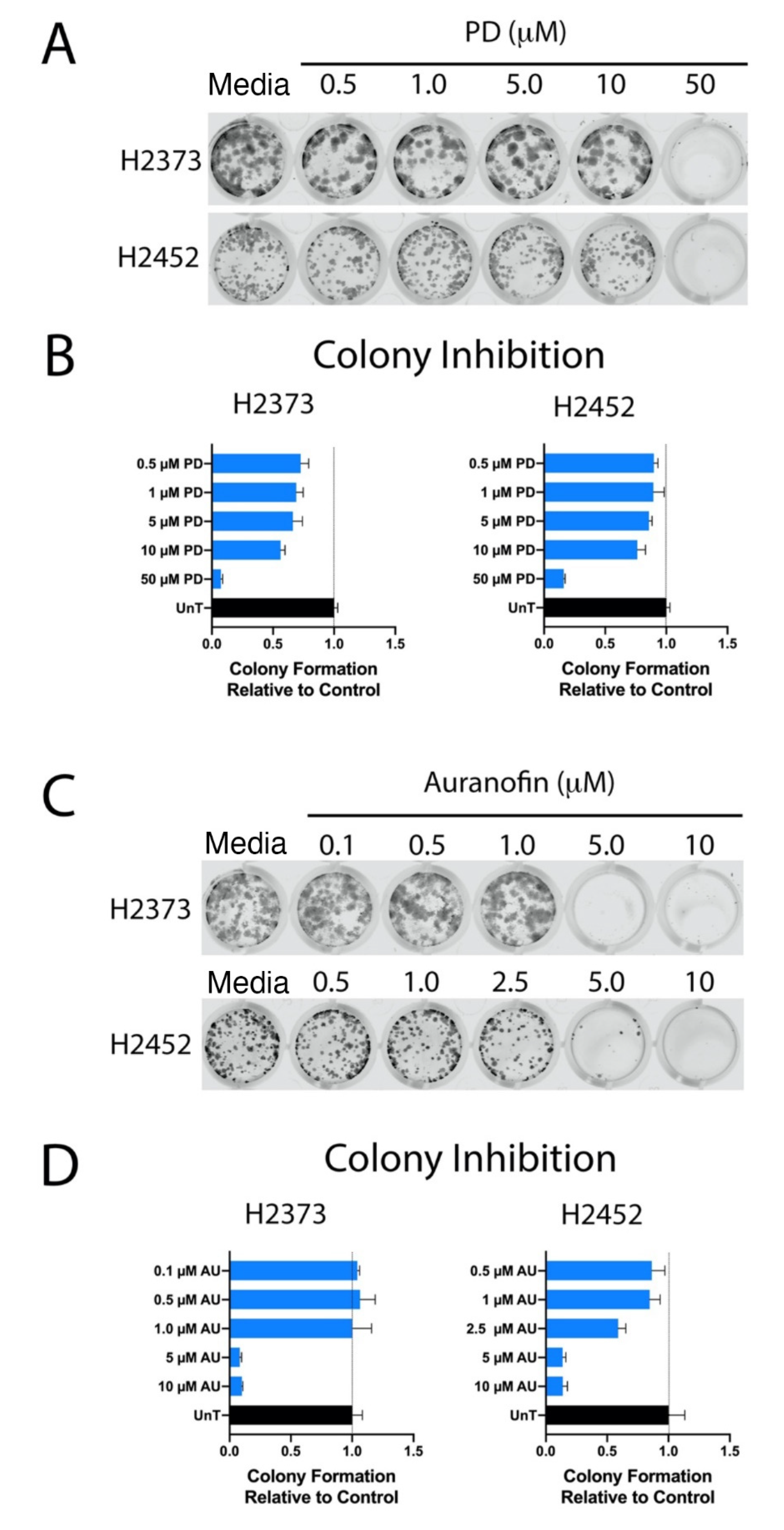
2.3. Colony-Forming Assays
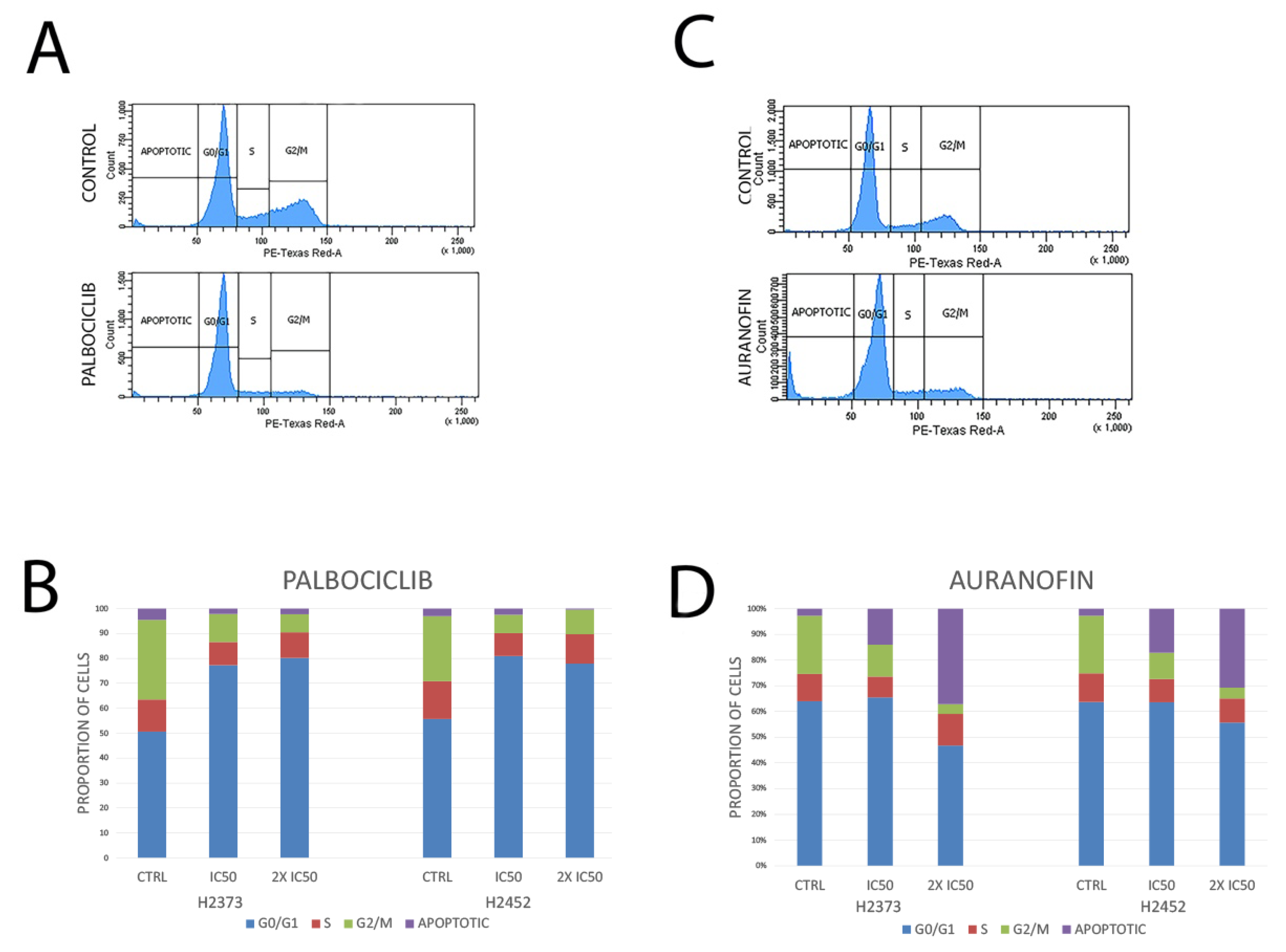
2.4. Cell Cycle Analysis
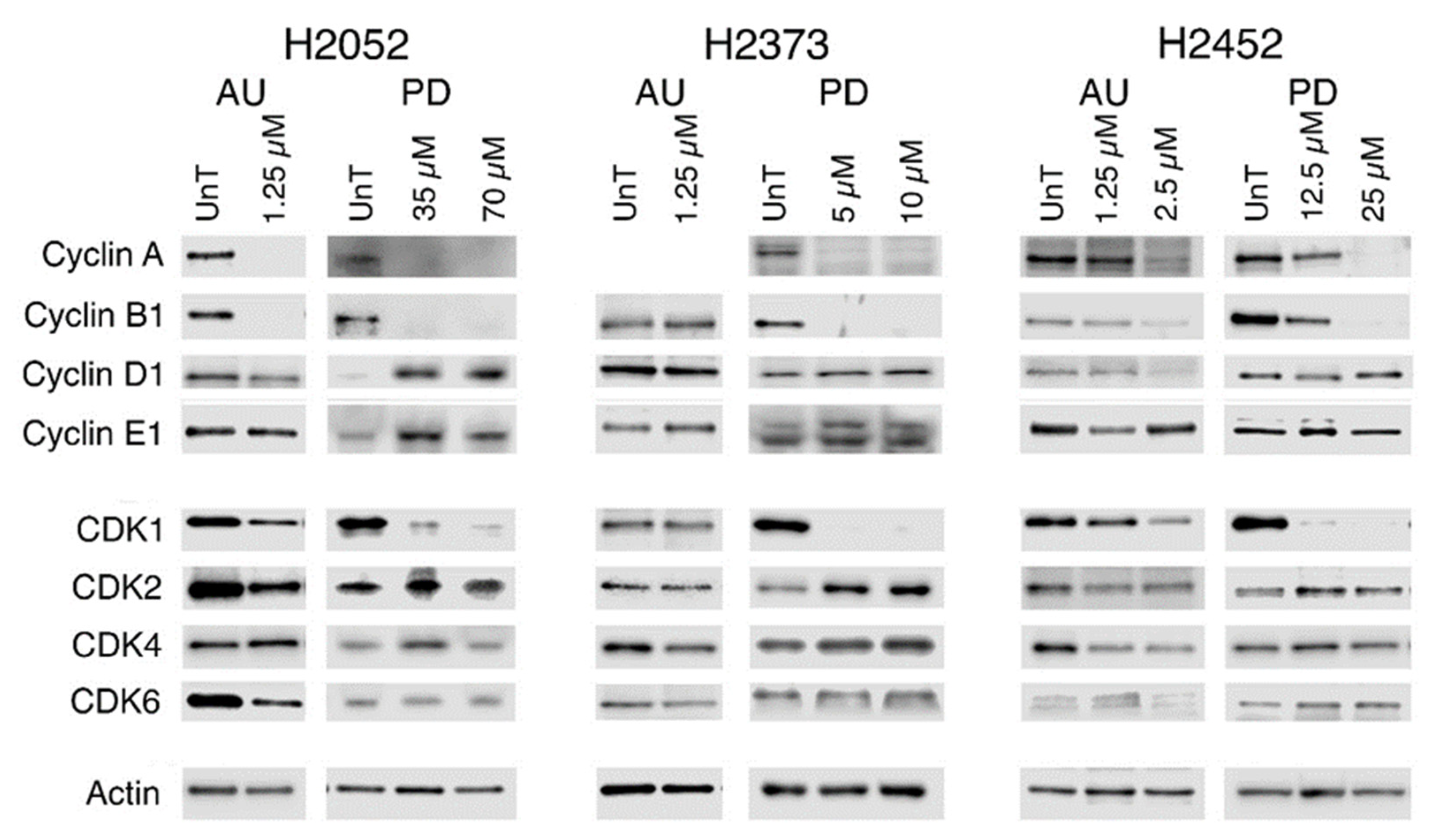
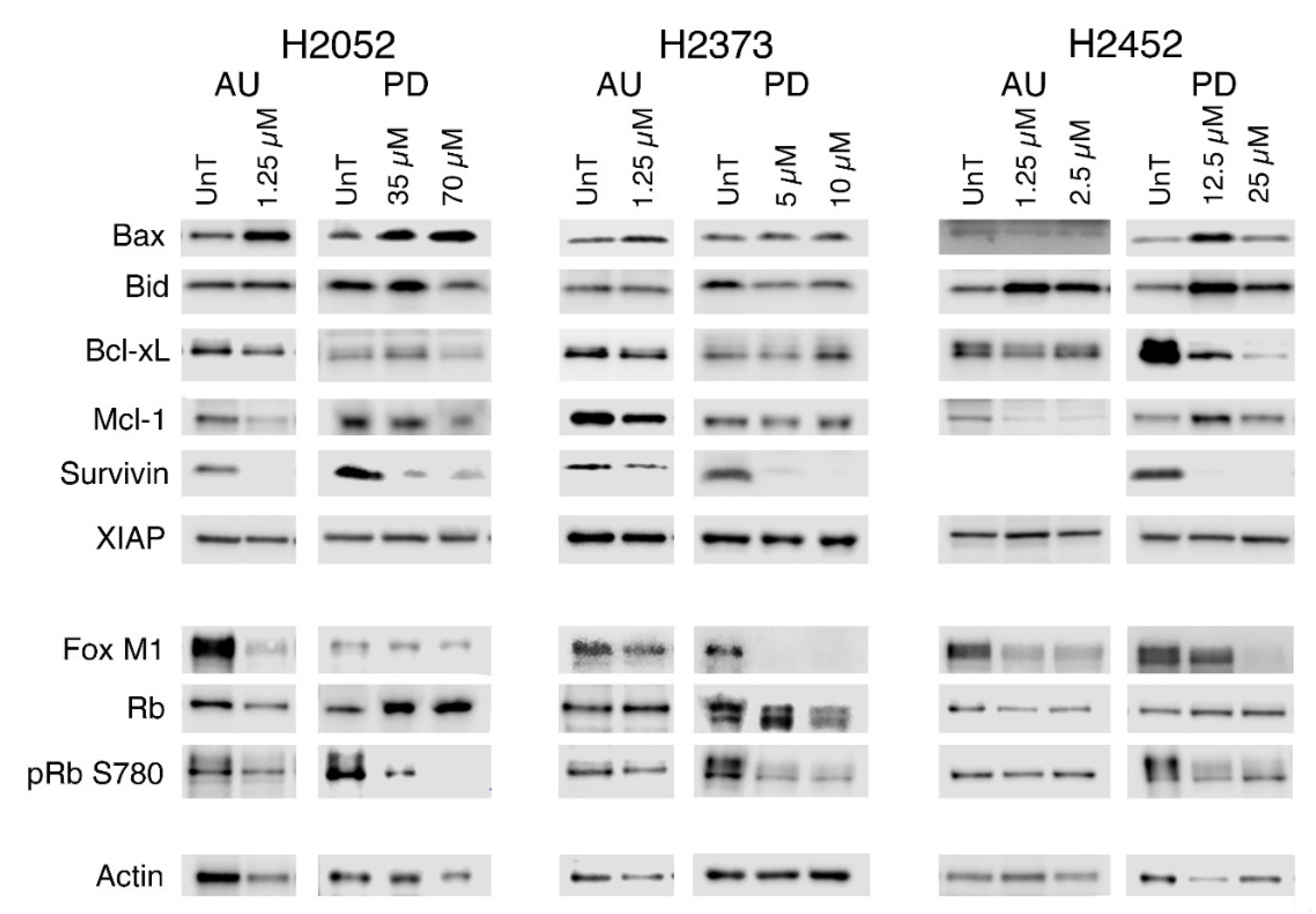
2.5. Modulation of Gene Expression in Response to Treatment with Palbociclib or Auranofin
3. Materials and Methods
3.1. Cell Lines and Reagents
3.2. Cell Proliferation Assays
3.3. Colony-Forming Assays
3.4. Cell Cycle Analysis
3.5. Immunoblotting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brautigam, L.; Schutte, L.D.; Godoy, J.R.; Prozorovski, T.; Gellert, M.; Hauptmann, G.; Holmgren, A.; Lillig, C.H.; Berndt, C. Vertebrate-specific glutaredoxin is essential for brain development. Proc. Natl. Acad. Sci. USA 2011, 108, 20532–20537. [Google Scholar] [CrossRef]
- Nonn, L.; Williams, R.R.; Erickson, R.P.; Powis, G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003, 23, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.I.; Lew, C.M.; Cortez, L.A.; Webb, C.R.; Rodriguez, M.; Liu, Y.; Qi, W.; Li, Y.; Chaudhuri, A.; Van Remmen, H.; et al. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 2008, 44, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Zhang, H.; Jones, D.P. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol. Sci. 2006, 91, 643–650. [Google Scholar] [CrossRef]
- Benhar, M.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 2008, 320, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hosoi, F.; Yamaguchi-Iwai, Y.; Nakamura, H.; Masutani, H.; Ueda, S.; Nishiyama, A.; Takeda, S.; Wada, H.; Spyrou, G.; et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002, 21, 1695–1703. [Google Scholar] [CrossRef]
- Damdimopoulos, A.E.; Miranda-Vizuete, A.; Pelto-Huikko, M.; Gustafsson, J.A.; Spyrou, G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J. Biol. Chem. 2002, 277, 33249–33257. [Google Scholar] [CrossRef]
- Cunniff, B.; Benson, K.; Stumpff, J.; Newick, K.; Held, P.; Taatjes, D.; Joseph, J.; Kalyanaraman, B.; Heintz, N.H. Mitochondrial-targeted nitroxides disrupt mitochondrial architecture and inhibit expression of peroxiredoxin 3 and FOXM1 in malignant mesothelioma cells. J. Cell. Physiol. 2013, 228, 835–845. [Google Scholar] [CrossRef]
- Cunniff, B.; Newick, K.; Nelson, K.J.; Wozniak, A.N.; Beuschel, S.; Leavitt, B.; Bhave, A.; Butnor, K.; Koenig, A.; Chouchani, E.T.; et al. Disabling Mitochondrial Peroxide Metabolism via Combinatorial Targeting of Peroxiredoxin 3 as an Effective Therapeutic Approach for Malignant Mesothelioma. PLoS ONE 2015, 10, e0127310. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, B.; Wozniak, A.N.; Sweeney, P.; DeCosta, K.; Heintz, N.H. Peroxiredoxin 3 levels regulate a mitochondrial redox setpoint in malignant mesothelioma cells. Redox Biol. 2014, 3, 79–87. [Google Scholar] [CrossRef]
- Kahlos, K.; Anttila, S.; Asikainen, T.; Kinnula, K.; Raivio, K.O.; Mattson, K.; Linnainmaa, K.; Kinnula, V.L. Manganese superoxide dismutase in healthy human pleural mesothelium and in malignant pleural mesothelioma. Am. J. Respir. Cell Mol. Biol. 1998, 18, 570–580. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Lehtonen, S.; Sormunen, R.; Kaarteenaho-Wiik, R.; Kang, S.W.; Rhee, S.G.; Soini, Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J. Pathol. 2002, 196, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Newick, K.; Cunniff, B.; Preston, K.; Held, P.; Arbiser, J.; Pass, H.; Mossman, B.; Shukla, A.; Heintz, N. Peroxiredoxin 3 is a redox-dependent target of thiostrepton in malignant mesothelioma cells. PLoS ONE 2012, 7, e39404. [Google Scholar] [CrossRef] [PubMed]
- Anders, L.; Ke, N.; Hydbring, P.; Choi, Y.J.; Widlund, H.R.; Chick, J.M.; Zhai, H.; Vidal, M.; Gygi, S.P.; Braun, P.; et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 2011, 20, 620–634. [Google Scholar] [CrossRef]
- Jin, C.; Qin, L.; Shi, Y.; Candas, D.; Fan, M.; Lu, C.L.; Vaughan, A.T.; Shen, R.; Wu, L.S.; Liu, R.; et al. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic. Biol. Med. 2015, 81, 77–87. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.J.; Huang, Q.; Lu, L.; Min, W. Novel action and mechanism of auranofin in inhibition of vascular endothelial growth factor receptor-3-dependent lymphangiogenesis. Anticancer Agents Med. Chem. 2014, 14, 946–954. [Google Scholar] [CrossRef]
- Choi, Y.J.; Anders, L. Signaling through cyclin D-dependent kinases. Oncogene 2014, 33, 1890–1903. [Google Scholar] [CrossRef]
- Frizelle, S.P.; Grim, J.; Zhou, J.; Gupta, P.; Curiel, D.T.; Geradts, J.; Kratzke, R.A. Re-expression of p16INK4a in mesothelioma cells results in cell cycle arrest, cell death, tumor suppression and tumor regression. Oncogene 1998, 16, 3087–3095. [Google Scholar] [CrossRef]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C. Cell-cycle targeted therapies. Lancet Oncol. 2004, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Diehl, J.A. Nuclear cyclin D1: An oncogenic driver in human cancer. J. Cell. Physiol. 2009, 220, 292–296. [Google Scholar] [CrossRef]
- de Assis, L.V.; Locatelli, J.; Isoldi, M.C. The role of key genes and pathways involved in the tumorigenesis of Malignant Mesothelioma. Biochim. Biophys. Acta. 2014, 1845, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Cadoo, K.A.; Gucalp, A.; Traina, T.A. Palbociclib: An evidence-based review of its potential in the treatment of breast cancer. Breast Cancer 2014, 6, 123–133. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Bonelli, M.; Terenziani, R.; Zoppi, S.; Fumarola, C.; La Monica, S.; Cretella, D.; Alfieri, R.; Cavazzoni, A.; Digiacomo, G.; Galetti, M.; et al. Dual Inhibition of CDK4/6 and PI3K/AKT/mTOR Signaling Impairs Energy Metabolism in MPM Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5165. [Google Scholar] [CrossRef]
- Bonelli, M.A.; Digiacomo, G.; Fumarola, C.; Alfieri, R.; Quaini, F.; Falco, A.; Madeddu, D.; La Monica, S.; Cretella, D.; Ravelli, A.; et al. Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR Pathways Induces a Synergistic Anti-Tumor Effect in Malignant Pleural Mesothelioma Cells. Neoplasia 2017, 19, 637–648. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Li, Z.; Zou, W.; Zhang, J.; Zhang, Y.; Xu, Q.; Li, S.; Chen, C. Mechanisms of CDK4/6 Inhibitor Resistance in Luminal Breast Cancer. Front. Pharmacol. 2020, 11, 580251. [Google Scholar] [CrossRef]
- Broaddus, V.C.; Dansen, T.B.; Abayasiriwardana, K.S.; Wilson, S.M.; Finch, A.J.; Swigart, L.B.; Hunt, A.E.; Evan, G.I. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J. Biol. Chem. 2005, 280, 12486–12493. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Kinnula, V.; Kaarteenaho-Wiik, R.; Kurttila, E.; Linnainmaa, K.; Paakko, P. Apoptosis and expression of apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in malignant mesothelioma. Clin. Cancer Res. 1999, 5, 3508–3515. [Google Scholar] [PubMed]
- Wu, M.; Sun, Y.; Li, G.; Desman, G.; Wang, B.; Gil, J.; Burstein, D.E. Immunohistochemical detection of XIAP in mesothelium and mesothelial lesions. Am. J. Clin. Pathol. 2007, 128, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Survival of Adherent Cells with the Colony-Forming Assay. Cold Spring Harb. Protoc. 2016, 2016, 087171. [Google Scholar] [CrossRef]
- Scaria, G.S.; Kren, B.T.; Klein, M.A. Cyclin-Dependent Kinase and Antioxidant Gene Expression in Cancers with Poor Therapeutic Response. Pharmaceuticals 2020, 13, 26. [Google Scholar] [CrossRef]
- Dean, J.L.; Thangavel, C.; McClendon, A.K.; Reed, C.A.; Knudsen, E.S. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene 2010, 29, 4018–4032. [Google Scholar] [CrossRef]
- Min, A.; Kim, J.E.; Kim, Y.J.; Lim, J.M.; Kim, S.; Kim, J.W.; Lee, K.H.; Kim, T.Y.; Oh, D.Y.; Bang, Y.J.; et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018, 430, 123–132. [Google Scholar] [CrossRef]
- Burch, P.M.; Heintz, N.H. Redox regulation of cell-cycle re-entry: Cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid. Redox Signal. 2005, 7, 741–751. [Google Scholar] [CrossRef]
- Burhans, W.C.; Heintz, N.H. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radic. Biol. Med. 2009, 47, 1282–1293. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zhao, J.; Shi, J.; Wang, M.; Qiu, S.; Hu, Y.; Xu, Y.; Cui, Y.; Liu, C.; et al. The Specific Inhibition of SOD1 Selectively Promotes Apoptosis of Cancer Cells via Regulation of the ROS Signaling Network. Oxid. Med. Cell. Longev. 2019, 2019, 9706792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Li, M.; Wang, H.; Dong, X. Metal Complexes or Chelators with ROS Regulation Capacity: Promising Candidates for Cancer Treatment. Molecules 2021, 27, 148. [Google Scholar] [CrossRef] [PubMed]
| Effect Size (Percent Inhibition in Decimals) with Combination Indices (CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose PD (μM) | Dose AU (μM) | H2373 | H2452 | H2052 | H2461 | ||||
| Effect | CI | Effect | CI | Effect | CI | Effect | CI | ||
| 0.99 | 1.39 | 0.99 | 1.42 | 0.99 | 4.24 × 1014 | 0.97 | 1.25 | 0.99 | 1.52 |
| 0.99 | 0.69 | 0.99 | 0.71 | 0.79 | 4.65 × 108 | 0.93 | 0.82 | 0.99 | 0.76 |
| 0.89 | 0.76 | 0.89 | 0.74 | 0.4 | 3.26 × 105 | 0.55 | 1.11 | 0.97 | 0.54 |
| 0.63 | 0.69 | 0.63 | 0.67 | 0.13 | 6.14 × 102 | 0.05 | 3367.61 | 0.59 | 0.58 |
| 0.48 | 1.30 | 0.48 | 1.32 | 0.08 | 6.37 × 101 | 0.01 | 502,919.00 | 0.45 | 0.37 |
| 0.99 | 1.39 | 0.99 | 1.42 | 0.99 | 2.12 x 1014 | 0.97 | 1.25 | 0.99 | 1.52 |
| 0.99 | 0.69 | 0.99 | 0.71 | 0.82 | 5.19 x 108 | 0.94 | 0.78 | 0.99 | 0.76 |
| 0.87 | 0.81 | 0.87 | 0.79 | 0.23 | 5.61 × 103 | 0.46 | 1.33 | 0.94 | 0.68 |
| 0.6 | 0.65 | 0.6 | 0.63 | 0.05 | 4.97 | 0.06 | 938.60 | 0.48 | 0.67 |
| 0.46 | 0.92 | 0.46 | 0.91 | 0.01 | 1.03 | 0.04 | 3419.45 | 0.33 | 0.46 |
| 0.99 | 1.39 | 0.99 | 1.42 | 0.99 | 4.24 × 1013 | 0.96 | 1.37 | 0.99 | 1.52 |
| 0.99 | 0.69 | 0.99 | 0.71 | 0.82 | 1.04 × 108 | 0.92 | 0.86 | 0.99 | 0.76 |
| 0.85 | 0.85 | 0.85 | 0.82 | 0.18 | 3.10 × 102 | 0.42 | 1.14 | 0.95 | 0.64 |
| 0.55 | 0.59 | 0.55 | 0.56 | 0.05 | 1.89 | 0.09 | 50.45 | 0.34 | 0.80 |
| 0.42 | 0.51 | 0.42 | 0.49 | 0.02 | 0.81 | 0.02 | 5958.66 | 0.16 | 0.66 |
| 0.99 | 1.39 | 0.99 | 1.42 | 0.99 | 2.12 × 1013 | 0.96 | 1.37 | 0.99 | 1.52 |
| 0.98 | 0.86 | 0.98 | 0.87 | 0.83 | 6.94 × 10 7 | 0.9 | 0.93 | 0.99 | 0.76 |
| 0.83 | 0.89 | 0.83 | 0.86 | 0.19 | 2.05 × 102 | 0.37 | 1.20 | 0.94 | 0.68 |
| 0.49 | 0.63 | 0.49 | 0.60 | 0.05 | 1.51 | 0.01 | 25,147.50 | 0.35 | 0.79 |
| 0.28 | 0.87 | 0.28 | 0.84 | 0.03 | 0.72 | 0.01 | 25,146.70 | 0.06 | 1.65 |
| 0.99 | 1.39 | 0.99 | 1.42 | 0.99 | 4.24 × 1012 | 0.97 | 1.25 | 0.99 | 1.52 |
| 0.99 | 0.69 | 0.99 | 0.71 | 0.88 | 7.65 × 107 | 0.91 | 0.90 | 0.99 | 0.76 |
| 0.8 | 0.94 | 0.8 | 0.91 | 0.3 | 5.12 × 102 | 0.47 | 0.96 | 0.95 | 0.64 |
| 0.36 | 0.73 | 0.36 | 0.69 | 0.16 | 17.7 | 0.01 | 5030.72 | 0.43 | 0.71 |
| 0.14 | 1.45 | 0.14 | 1.41 | 0.13 | 6.52 | 0.01 | 5029.95 | 0.15 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratzke, M.; Scaria, G.; Porter, S.; Kren, B.; Klein, M.A. Inhibition of Mitochondrial Antioxidant Defense and CDK4/6 in Mesothelioma. Molecules 2023, 28, 4380. https://doi.org/10.3390/molecules28114380
Kratzke M, Scaria G, Porter S, Kren B, Klein MA. Inhibition of Mitochondrial Antioxidant Defense and CDK4/6 in Mesothelioma. Molecules. 2023; 28(11):4380. https://doi.org/10.3390/molecules28114380
Chicago/Turabian StyleKratzke, Marian, George Scaria, Stephen Porter, Betsy Kren, and Mark A. Klein. 2023. "Inhibition of Mitochondrial Antioxidant Defense and CDK4/6 in Mesothelioma" Molecules 28, no. 11: 4380. https://doi.org/10.3390/molecules28114380
APA StyleKratzke, M., Scaria, G., Porter, S., Kren, B., & Klein, M. A. (2023). Inhibition of Mitochondrial Antioxidant Defense and CDK4/6 in Mesothelioma. Molecules, 28(11), 4380. https://doi.org/10.3390/molecules28114380








