Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition
Abstract
1. Introduction
2. Results and Discussion
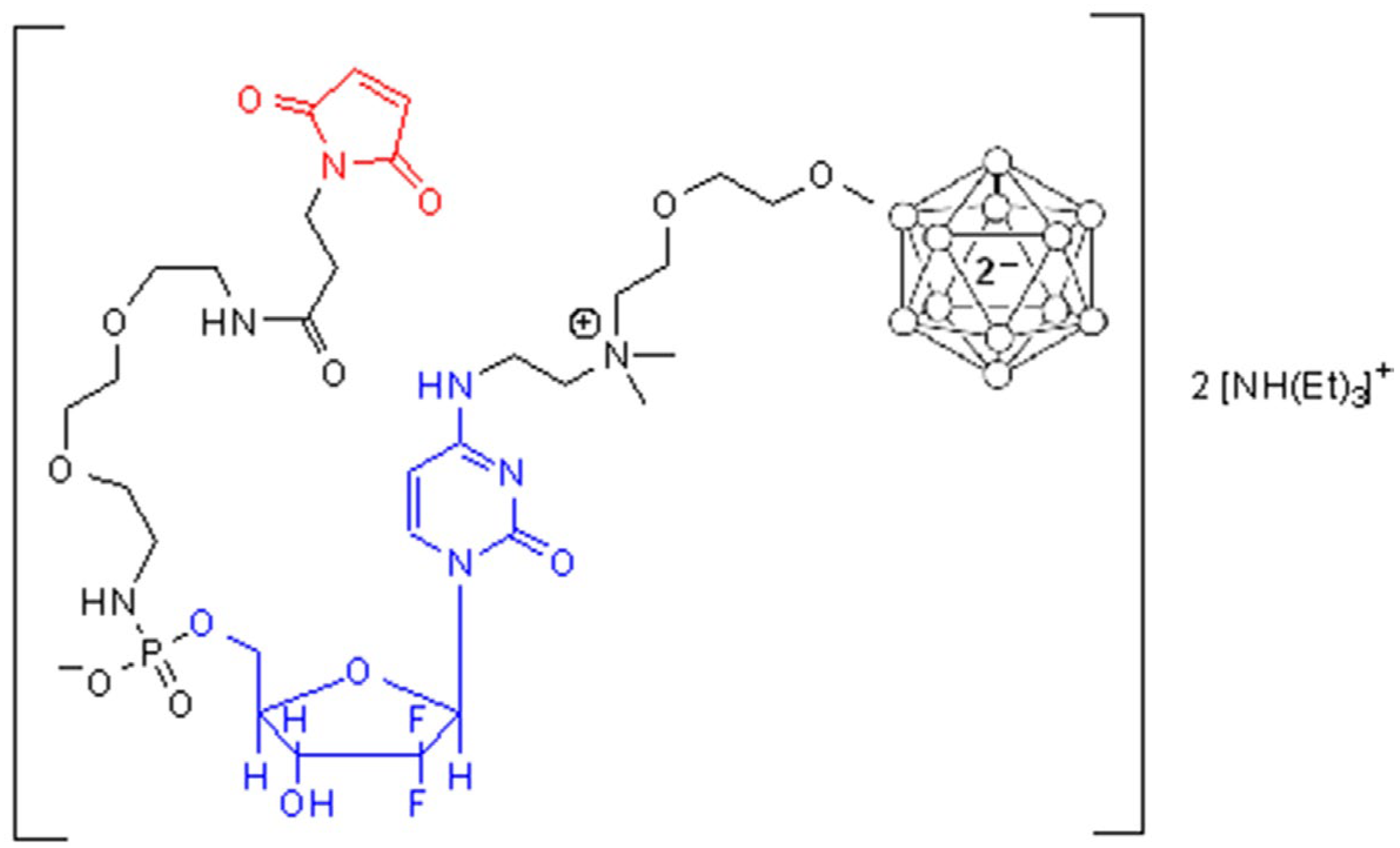
2.1. Synthesis of Maleimide Gemcitabine Boron-Containing Derivative 1
2.2. Bioconjugation
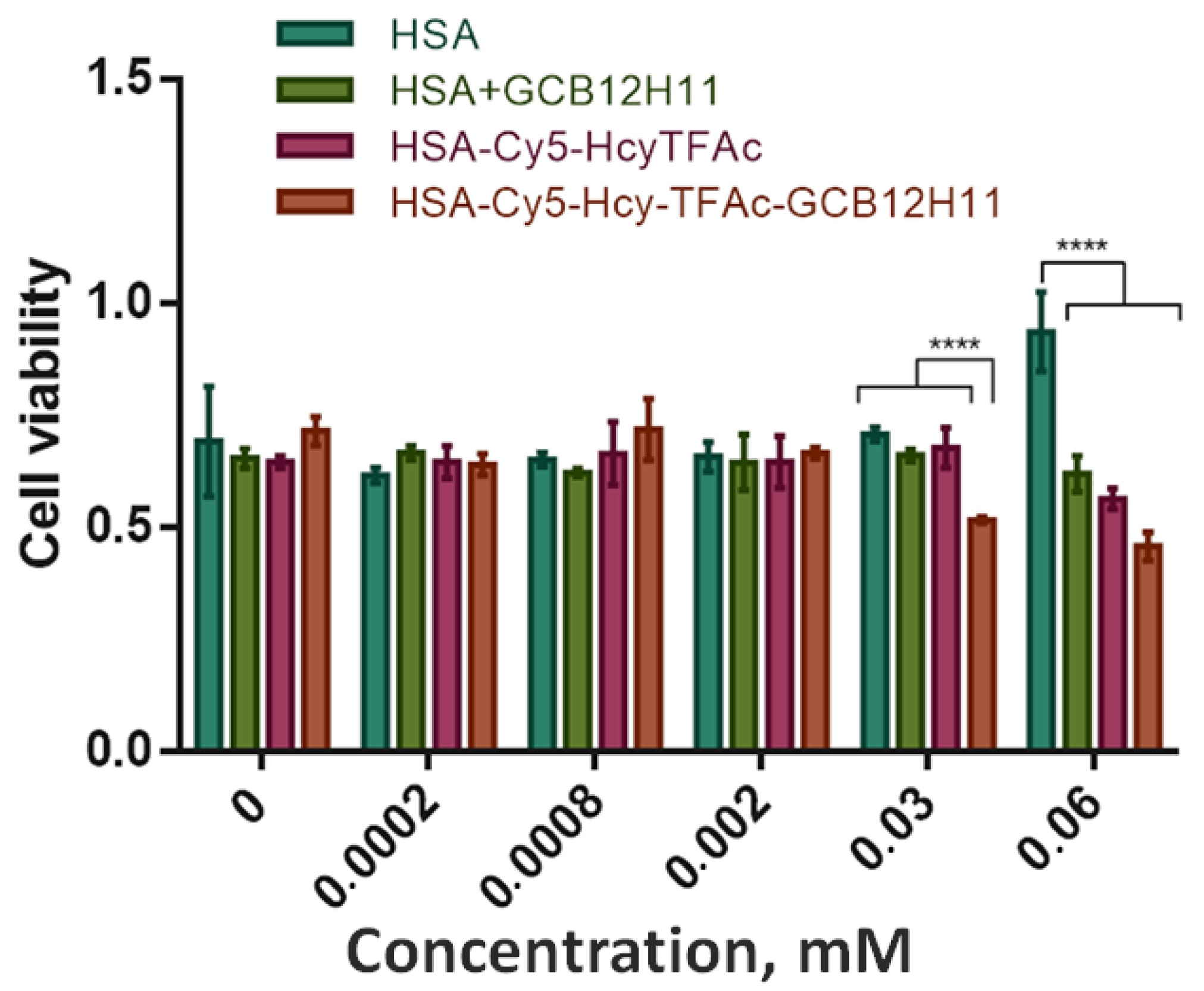
2.3. Cell Viability Assay
2.4. The Usage of the HSA-Cy7-HcyTFAc-GCB12H11 Conjugate as Boron Delivery Agent in BNCT
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. 4-N-Benzoyl-2′-deoxy-2′,2′-Difluorocytidine 2
3.2.2. 4-N-(2-Dimethylaminoethyl)-2′deoxy-2′,2′-Difluorocytidine 3
3.2.3. Compound 5
3.2.4. Compound 6
3.2.5. Compound 7
3.2.6. Compound 1
3.2.7. Synthesis and Characterization of Multifunctional Human Serum Albumin-Therapeutic Conjugates HSA-Cy5-HcyTFAc-GCB12H11 and HSA-Cy7-HcyTFAc-GCB12H11
3.2.8. Cell Viability Assay (MTT Test)
3.2.9. Model BNCT Experiments
Incubation of the Cell Lines and Irradiation
Clonogenic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043–120059. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.A.; Smart, B.E. Conformational energies of 2-fluoroethanol and 2- fluoroacetaldehyde enol—Strength of the internal hydrogen- bond. J. Phys. Chem. 1991, 95, 1609–1612. [Google Scholar] [CrossRef]
- Mohammadian, M.; Kouchakzadeh, H.; Rahmandoust, M.; Mohammadian, T. Targeted albumin nanoparticles for the enhancement of gemcitabine toxicity on cancerous cells. J. Drug Deliv. Sci. Technol. 2020, 56, 101503–101510. [Google Scholar] [CrossRef]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Kulluru, L.P.; Rizvi, S.A.; D’Souza, M.; D’Souza, M. Formulation development of albumin based theranostic nanoparticles as a potential delivery system for tumor targeting. J. Drug Target. 2013, 21, 77–86. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Lee, J.; Chang, H.; Jeewon, L.; Kwon, I.C.; Kim, K. Molecular imaging and targeted drug delivery using albumin-based nanoparticles. Curr. Pharm. Des. 2015, 21, 1889–1898. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, S.N. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Schäffler, M.; Sousa, F.; Wenk, A.; Sitia, L.; Hirn, S.; Schleh, C.; Haberl, N.; Violatto, M.; Canovi, M.; Andreozzi, P.; et al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials 2014, 35, 3435–3466. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H.; Brekken, R.A. Mini review SPARC, a matricellular protein: At the crossroads of cell matrix SPARC, a matricellular protein: At the crossroads of cell matrix communication. Matrix Biol. 2001, 19, 815–827. [Google Scholar] [CrossRef]
- Kouros, M. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol. 1999, 31, 1363–1366. [Google Scholar]
- Podhajcer, O.L.; Benedetti, L.G.; Girotti, M.R.; Prada, F.; Salvatierra, E.; Llera, A.S. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008, 27, 691–705. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, S.; Song, L.; Wu, Q.; Bigner, D.D.; Hjelmeland, A.B.; Rich, J.N. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene 2007, 26, 4084–4094. [Google Scholar] [CrossRef]
- Ali, M.S.; Al-Lohedan, H.A. Experimental and Computational Investigation on the Interaction of Anticancer Drug Gemcitabine with Human Plasma Protein: Effect of Copresence of Ibuprofen on the Binding. Molecules 2022, 27, 1635. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, J.L.; Qi, W.X.; Chen, J.Y.; Xu, H.P. A Novel Delivery System of RGD-HSA Loaded GEM/CUR Nanoparticles for the Treatment of Pancreatic Cancer Therapy. Drug. Des. Devel. Ther. 2022, 16, 2395–2406. [Google Scholar] [CrossRef]
- Kong, L.; Du, J.; Gu, J.; Deng, J.; Guo, Y.; Tao, B.; Jin, C.; Fu, D.; Li, J. Gemcitabine-Loaded Albumin Nanoparticle Exerts an Antitumor Effect on Gemcitabine-Resistant Pancreatic Cancer Cells Induced by MDR1 and MRP1 Overexpression in Vitro. Front. Surg. 2022, 9, 890412–890425. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, W.; Di, Y.; Gu, J.; Guo, Z.; Li, H.; Fu, D.; Jin, C. Triple-functional albumin-based nanoparticles for combined chemotherapy and photodynamic therapy of pancreatic cancer with lymphatic metastases. Int. J. Nanomed. 2017, 12, 6771–6785. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Di, Y.; Yao, L.; Yu, X.; Fu, D.; Li, J.; Jin, C. Antitumor effect of gemcitabine-loaded albumin nanoparticle on gemcitabine-resistant pancreatic cancer induced by low hENT1 expression. Int. J. Nanomed. 2018, 13, 4869–4880. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Fei, S.; He, H.; Zhang, Y.; Yin, T.; Tang, X. Formulation and Pharmacokinetics of HSA-core and PLGA-shell Nanoparticles for Delivering Gemcitabine. AAPS Pharm. Sci. Tech. 2018, 19, 812–819. [Google Scholar] [CrossRef]
- Norouzi, P.; Amini, M.; Mottaghitalab, F.; Mirzazadeh Tekie, F.S.; Dinarvand, R.; Mirzaie, Z.H.; Atyabi, F. Design and fabrication of dual-targeted delivery system based on gemcitabine-conjugated human serum albumin nanoparticles. Chem. Biol. Drug. Des. 2020, 96, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, J.; Chen, T.; Yin, L.; Jin, Q.; Ji, J. Enzyme-sensitive gemcitabine conjugated albumin nanoparticles as a versatile theranostic nanoplatform for pancreatic cancer treatment. J. Colloid. Interface Sci. 2017, 507, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ur-Rehman, S.; Khalid, L.; Bhatti, I.A.; Bhatti, H.N.; Iqbal, J.; Bai, F.Q.; Zhang, H.X. Investigation of the adsorption properties of gemcitabine anticancer drug with metal-doped boron nitride fullerenes as a drug-delivery carrier: A DFT study. RSC Adv. 2022, 12, 2873–2887. [Google Scholar] [CrossRef] [PubMed]
- Farzad, F.; Hashemzadeh, H. Probing the effect of polyethene glycol on the adsorption mechanisms of Gem on the hexagonal boron nitride as a highly efficient polymer-based drug delivery system: DFT, classical MD and Well-tempered Metadynamics simulations. J. Mol. Graph. Model. 2020, 98, 107613–107620. [Google Scholar] [CrossRef]
- Matsushita, K.; Okuda, T.; Mori, S.; Konno, M.; Eguchi, H.; Asai, A.; Koseki, J.; Iwagami, Y.; Yamada, D.; Akita, H.; et al. A Hydrogen Peroxide Activatable Gemcitabine Prodrug for the Selective Treatment of Pancreatic Ductal Adenocarcinoma. Chem. Med. Chem. 2019, 14, 1384–1391. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Lu, W.; Liu, S.; Xiao, Y.; Yu, J. 4-Carboxyphenylboronic acid-decorated, redox-sensitive rod-shaped nano-micelles fabricated through co-assembling strategy for active targeting and synergistic co-delivery of camptothecin and gemcitabine. Eur. J. Pharm. Biopharm. 2019, 144, 193–206. [Google Scholar] [CrossRef]
- Samaniego Lopez, C.; Martínez, J.H.; Acebedo, S.L.; Spagnuolo, C.C. Benzoxaboroles as dynamic covalent receptors for bioconjugation and transport of nucleosides and related drugs: Proof of action in HeLa cells. Bioorg. Chem. 2019, 90, 103059–103064. [Google Scholar] [CrossRef]
- Evens, A.M.; Rosen, S.T.; Helenowski, I.; Kline, J.; Larsen, A.; Colvin, J.; Winter, J.N.; van Besien, K.M.; Gordon, L.I.; Smith, S.M. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br. J. Haematol. 2013, 163, 55–61. [Google Scholar] [CrossRef]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Moshkin, M.P.; et al. Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorg. Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef]
- Popova, T.V.; Dymova, M.A.; Koroleva, L.S.; Zakharova, O.D.; Lisitskiy, V.A.; Raskolupova, V.I.; Sycheva, T.V.; Taskaev, S.Y.; Silnikov, V.N.; Godovikova, T.S. Homocystamide Conjugates of Human Serum Albumin as A Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy. Molecules 2021, 26, 6537. [Google Scholar] [CrossRef]
- Raskolupova, V.I.; Popova, T.V.; Zakharova, O.D.; Nikotina, A.E.; Abramova, T.V.; Silnikov, V.N. Human Serum Albumin Labelling with a New BODIPY Dye Having a Large Stokes Shift. Molecules 2021, 26, 2679. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Pyshnaya, I.A.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Poletaeva, J.; Zavjalov, E.L.; Silnikov, V.N.; Ryabchikova, E.I.; Godovikova, T.S. Rational design of albumin theranostic conjugates for gold nanoparticles anticancer drugs: Where the seed meets the soil? Biomedicines 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Krumkacheva, O.A.; Burmakova, A.S.; Spitsyna, A.S.; Zakharova, O.D.; Lisitskiy, V.A.; Kirilyuk, I.A.; Silnikov, V.N.; Bowman, M.K.; Bagryanskaya, E.G.; et al. Protein modification by thiolactone homocysteine chemistry: A multifunctionalized human serum albumin theranostic. RSC Med. Chem. 2020, 11, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Khan, H.; Chubarov, A.S.; Lisitskiy, V.A.; Antonova, N.M.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Silnikov, V.N.; Ahmad, S.; et al. Biotin-decorated anti-cancer nucleotide theranostic conjugate of human serum albumin: Where the seed meets the soil? Bioorg. Med. Chem. Lett. 2018, 28, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef]
- Moysan, E.; Bastiat, G.; Benoit, J.P. Gemcitabine versus modified gemcitabine: A review of several promising chemical modifications. Mol. Pharm. 2013, 10, 430–444. [Google Scholar] [CrossRef]
- Pereira, M.; Vale, N. Two Possible Strategies for Drug Modification of Gemcitabine and Future Contributions to Personalized Medicine. Molecules 2022, 27, 291. [Google Scholar] [CrossRef]
- Dosio, F.; Brusa, P.; Crosasso, P.; Arpicco, S.; Cattel, L. Preparation, characterization and properties in vitro and in vivo of a paclitaxel–albumin conjugate. J. Control. Release 1997, 47, 293–304. [Google Scholar] [CrossRef]
- Duncan, R.; Kopecková-Rejmanová, P.; Strohalm, J.; Hume, I.; Cable, H.C.; Pohl, J.; Lloyd, J.B.; Kopecek, J. Anticancer agents coupled to N-(2-hydroxypropyl) methacrylamide copolymers. I. Evaluation of daunomycin and puromycin conjugates in vitro. Br. J. Cancer 1987, 55, 165–174. [Google Scholar] [CrossRef]
- Immordino, M.L.; Brusa, P.; Rocco, F.; Arpicco, S.; Ceruti, M.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Release 2004, 100, 331–346. [Google Scholar] [CrossRef]
- Plunkett, W.; Huang, P.; Gandhi, V. Preclinical characteristics of gemcitabine. Anticancer Drugs 1995, 6, 7–13. [Google Scholar] [CrossRef]
- Huang, P.; Chubb, S.; Hertel, L.W.; Grindey, G.B.; Plunkett, W. Action of 2’2’-Difluorodeoxycytidine on DNA Synthesis. Cancer Res. 1991, 51, 6110–6117. [Google Scholar]
- Peters, G.J.; van der Wilt, C.L.; van Moorsel, C.J.; Kroep, J.R.; Bergman, A.M.; Ackland, S.P. Basis for effective combination cancer chemotherapy with antimetabolites. Pharm. Ther. 2000, 87, 227–253. [Google Scholar] [CrossRef]
- Mini, E.; Nobili, S.; Casiagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17, 7–12. [Google Scholar] [CrossRef]
- Semioshkin, A.; Nizhnik, E.; Godovikov, I.; Starikova, Z.; Bregadze, V. Reactions of oxonium derivatives of [B12H12]2− with amines: Synthesis and structure of novel B12-based ammonium salts and amino acids. J. Organomet. Chem. 2007, 692, 4020–4028. [Google Scholar] [CrossRef]
- Semioshkin, A.; Laskova, J.; Zhidkova, O.; Godovikov, I.; Starikova, Z.; Bregadze, V.; Gabel, D. Synthesis and structure of novel closo-dodecaborate-based glycerols. J. Organomet. Chem. 2010, 2010695, 370–374. [Google Scholar] [CrossRef]
- Bhat, V.; Ugarkar, B.G.; Sayeed, V.A.; Grimm, K.; Kosora, N.; Domenico, P.A.; Stocker, E. A Simple and Convenient Method for the Selective N-Acylations of Cytosine. Nucleos. Nucleot. Nnucl. 1989, 8, 179–183. [Google Scholar] [CrossRef]
- Abramova, T.V.; Vasil’eva, S.V.; Ivanova, T.M.; Shishkin, G.V.; Sil’nikov, V.N. Monomers for Oligonucleotide Synthesis with Linkers Carrying Reactive Residues: I. The Synthesis of Deoxynucleoside Derivatives with Methoxyoxalamide Groups in Heterocyclic Bases. J. Bioorganic Chem. 2004, 30, 224–233. [Google Scholar] [CrossRef]
- Wang, Y.; Rösner, D.; Grzywa, M.; Marx, A. Chain-Terminating and Clickable NAD+ Analogues for Labeling the Target Proteins of ADP-Ribosyltransferases. Angew. Chem. Int. Ed. 2014, 53, 8159–8162. [Google Scholar] [CrossRef]
- Kovács, T.; Ötvös, L. Simple synthesis of 5-vinyl- and 5-ethynyl-2′-deoxyuridine-5′-triphosphates. Tetrahedron Lett. 1988, 29, 4525–4528. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kato, T.; Takenishi, T. A novel method for phosphorylation of nucleosides to 5′-nucleotides. Tetrahedron Lett. 1967, 8, 5065–5068. [Google Scholar] [CrossRef] [PubMed]
- Barradas, R.G.; Fletcher, S.; Porter, J.D. The hydrolysis of maleimide in alkaline solution. Can. J. Chem. 1976, 54, 1400–1404. [Google Scholar] [CrossRef]
- Sherstyuk, Y.V.; Ivanisenko, N.V.; Zakharenko, A.L.; Sukhanova, M.V.; Peshkov, R.Y.; Eltsov, I.V.; Kutuzov, M.M.; Kurgina, T.A.; Belousova, E.A.; Ivanisenko, V.A.; et al. Design, Synthesis and Molecular Modeling Study of Conjugates of ADP and Morpholino Nucleosides as A Novel Class of Inhibitors of PARP-1, PARP-2 and PARP-3. Int. J. Mol. Sci. 2019, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Danilin, N.A.; Koroleva, L.S.; Novopashina, D.S.; Venyaminova, A.G. RNase P-Guiding Peptide Conjugates of Oligo(2’-O-methylribonucleotides) as Prospective Antibacterial Agents. Russ. J. Bioorganic Chem. 2019, 45, 825–832. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival–application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Taskaev, S.; Berendeev, E.; Bikchurina, M.; Bykov, T.; Kasatov, D.; Kolesnikov, I.; Koshkarev, A.; Makarov, A.; Ostreinov, G.; Porosev, V.; et al. Neutron Source Based on Vacuum Insulated Tandem Accelerator and Lithium Target. Biology 2021, 10, 350. [Google Scholar] [CrossRef]
- Fuentes, E.; Araya-Maturana, R.; Urra, F.A. Regulation of mitochondrial function as a promising target in platelet activation-related diseases. Free Radic. Biol. Med. 2019, 136, 172–182. [Google Scholar] [CrossRef]
- Peters, R.A. Mechanism of the toxicity of the active constituent of Dichapetalum cymosum and related compounds. In Advances in Enzymology; Nord, F.F., Ed.; Interscience Publishers, Inc.: Geneva, Switzerland, 1957; Volume 18, pp. 113–159. [Google Scholar]
- Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Understanding ribonucleotide reductase inactivation by gemcitabine. Chemistry 2007, 13, 8507–8515. [Google Scholar] [CrossRef]
- Alvarellos, M.L.; Lamba, J.; Sangkuhl, K.; Thorn, C.F.; Wang, L.; Klein, D.J.; Altman, R.B.; Klein, T.E. PharmGKB summary: Gemcitabine pathway. Pharm. Genom. 2014, 24, 564–574. [Google Scholar] [CrossRef]
- Peters, T., Jr. The Albumin molecule: Its structure and chemical properties. In All about Albumin: Biochemistry, Genetics, and Molecular Applications; Academic Press: San Diego, CA, USA, 1996; p. 432. [Google Scholar]
- Sivaev, I.B.; Kulikova, N.Y.; Nizhnik, E.A.; Vichuzhanin, M.V.; Starikova, Z.A.; Semioshkin, A.A.; Bregadze, V.I. Practical synthesis of 1,4-dioxane derivative of the closo-dodecaborate anion and its ring opening with acetylenic alkoxides. J Organomet. Chem. 2008, 693, 519–525. [Google Scholar] [CrossRef]
- Franken, N.; Rodermond, H.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raskolupova, V.I.; Wang, M.; Dymova, M.A.; Petrov, G.O.; Shchudlo, I.M.; Taskaev, S.Y.; Abramova, T.V.; Godovikova, T.S.; Silnikov, V.N.; Popova, T.V. Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules 2023, 28, 2672. https://doi.org/10.3390/molecules28062672
Raskolupova VI, Wang M, Dymova MA, Petrov GO, Shchudlo IM, Taskaev SY, Abramova TV, Godovikova TS, Silnikov VN, Popova TV. Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules. 2023; 28(6):2672. https://doi.org/10.3390/molecules28062672
Chicago/Turabian StyleRaskolupova, Valeria I., Meiling Wang, Maya A. Dymova, Gleb O. Petrov, Ivan M. Shchudlo, Sergey Yu. Taskaev, Tatyana V. Abramova, Tatyana S. Godovikova, Vladimir N. Silnikov, and Tatyana V. Popova. 2023. "Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition" Molecules 28, no. 6: 2672. https://doi.org/10.3390/molecules28062672
APA StyleRaskolupova, V. I., Wang, M., Dymova, M. A., Petrov, G. O., Shchudlo, I. M., Taskaev, S. Y., Abramova, T. V., Godovikova, T. S., Silnikov, V. N., & Popova, T. V. (2023). Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules, 28(6), 2672. https://doi.org/10.3390/molecules28062672








