The Corey-Seebach Reagent in the 21st Century: A Review
Abstract
1. Introduction
2. Literature Review
2.1. Alkaloid-Based Natural Products Synthesis
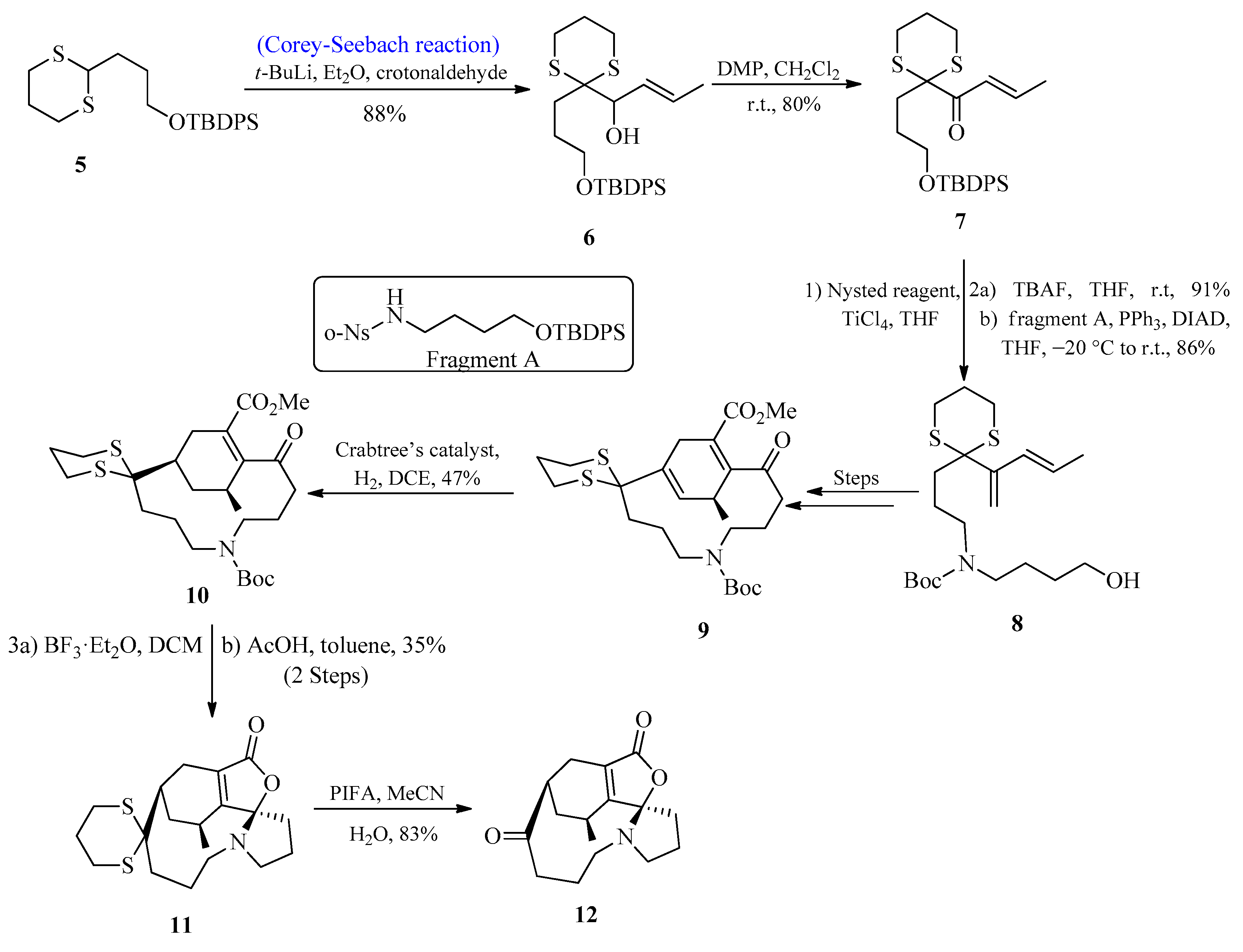
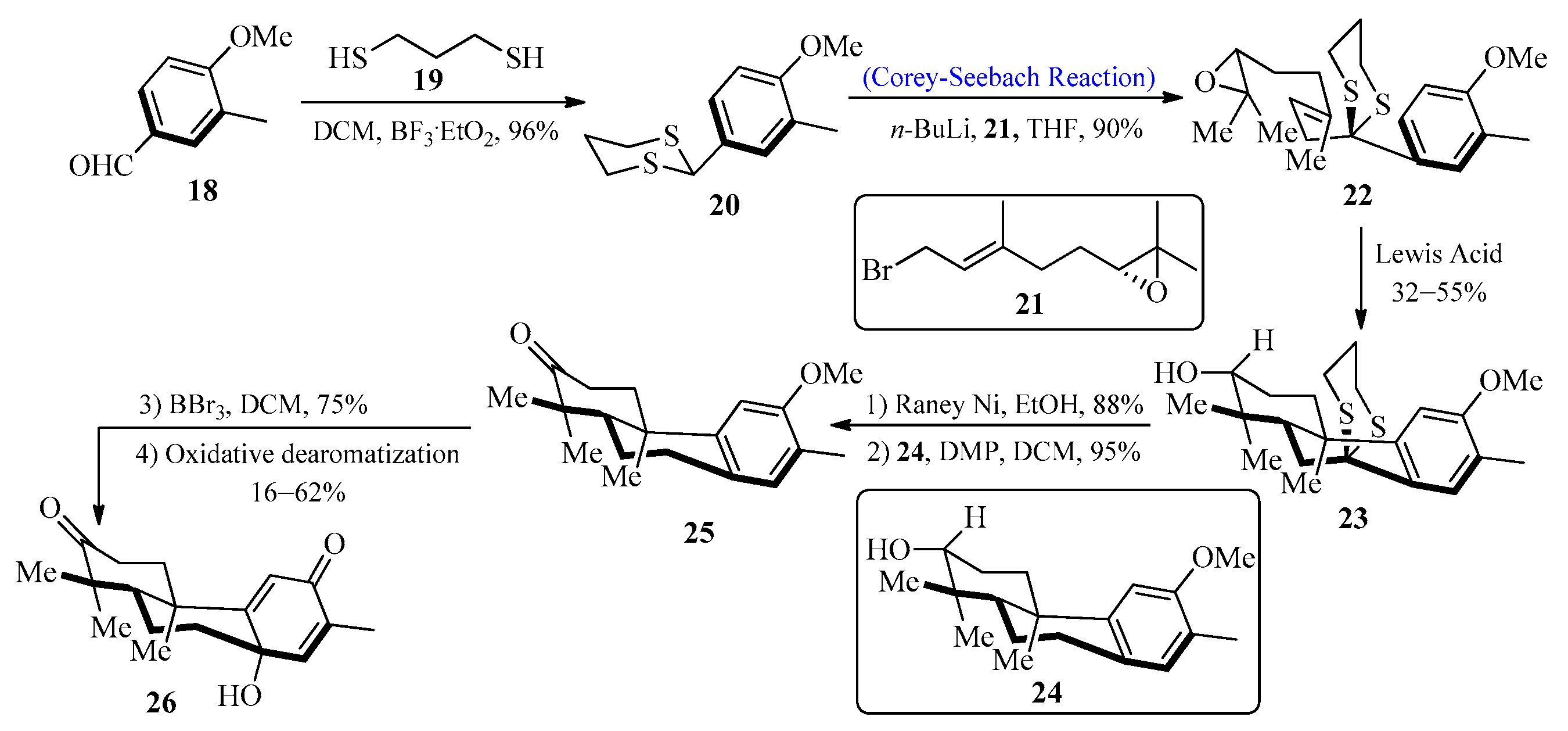
2.1.1. Lycoplanine A Alkaloids
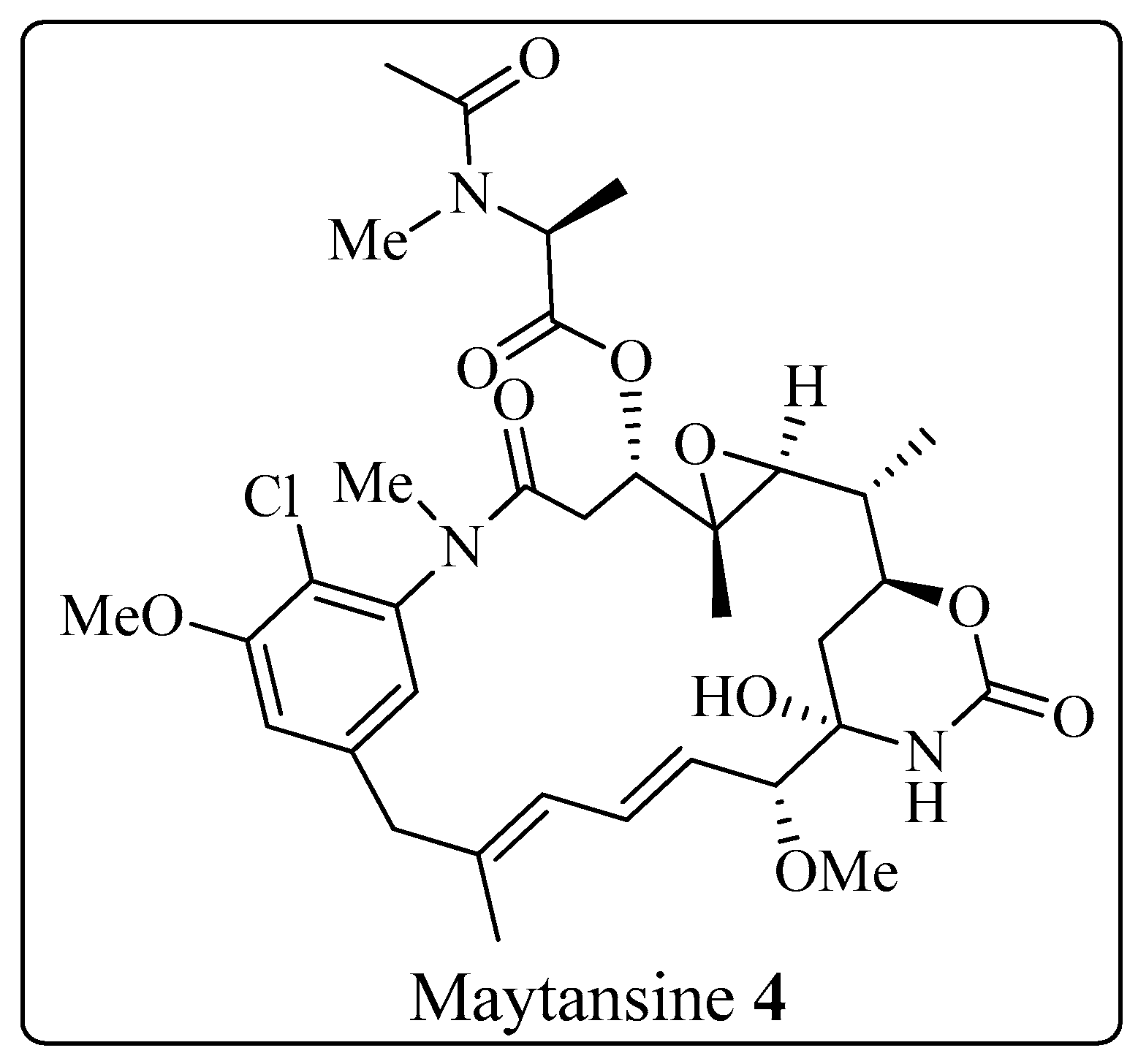
2.1.2. Diterpenoid Alkaloids
2.2. Terpenoids-Based Natural Products Synthesis
2.2.1. Bisnorditerpene
2.2.2. Totarol Synthesis
2.3. Polyketide Based Natural Products
2.3.1. Ambruticin J Synthesis
2.3.2. Biakamides
2.4. Photoinitiators
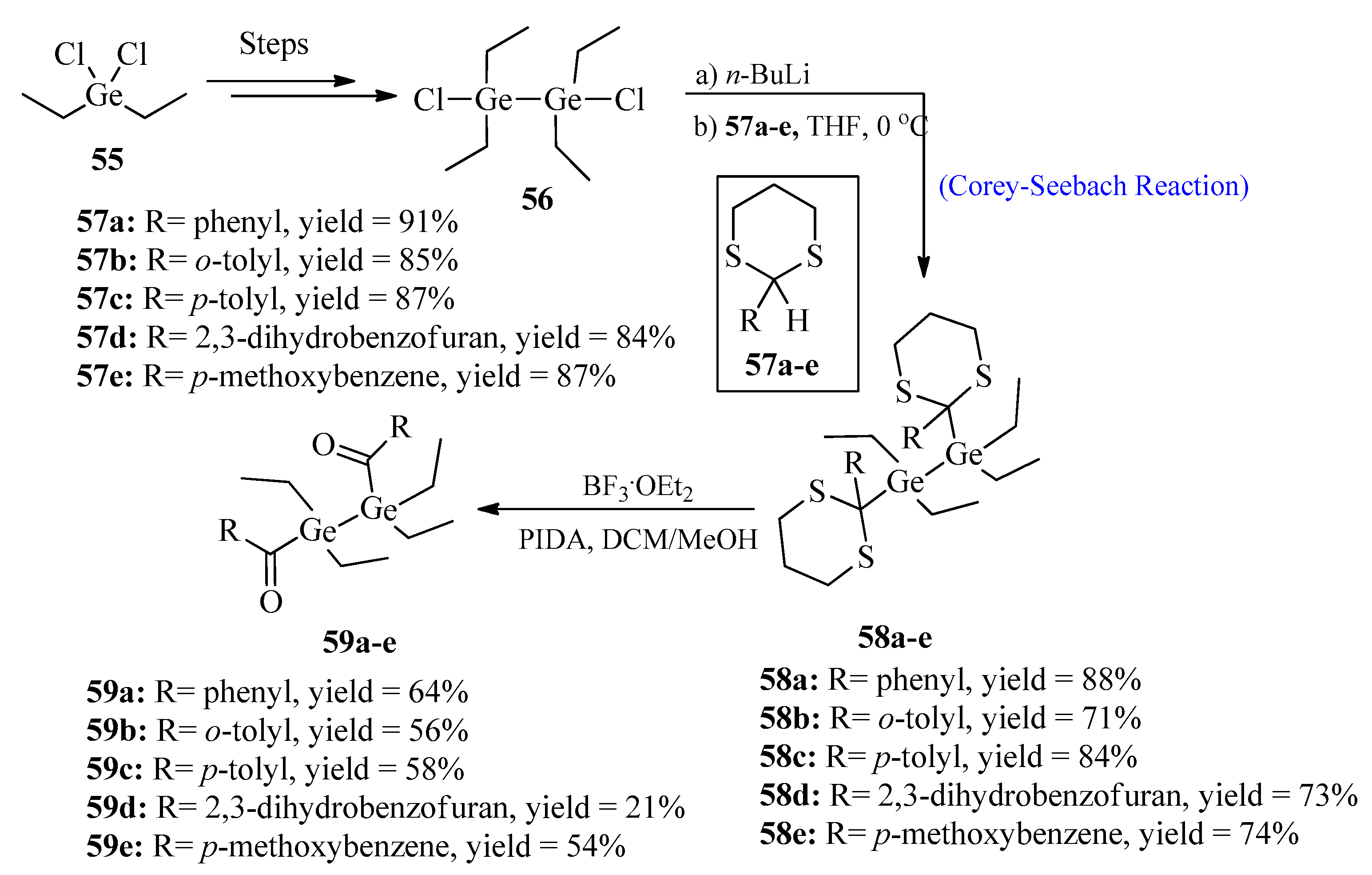
2.4.1. Bisacyldigermanes
2.4.2. Benzoylgermanium Derivatives
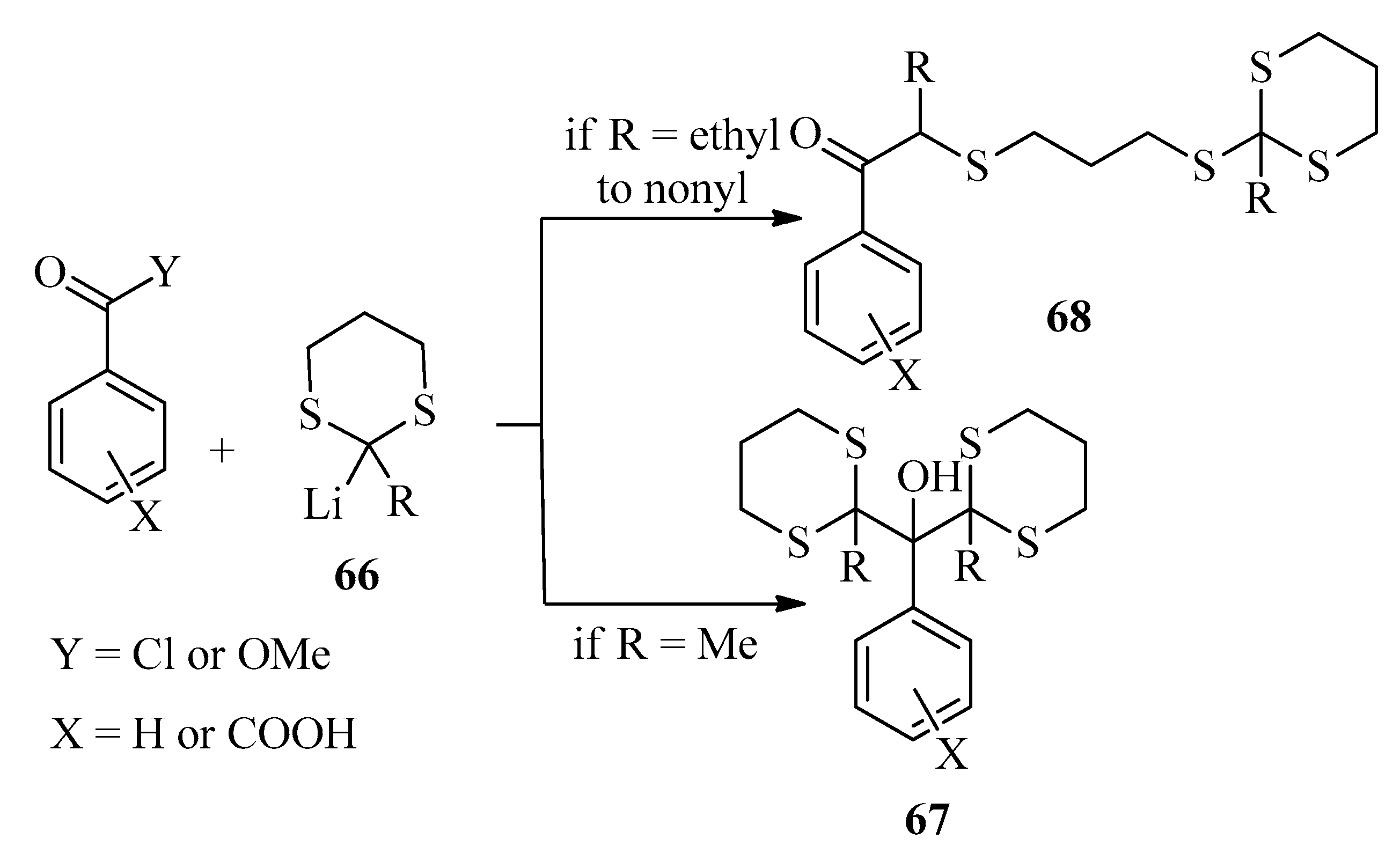
2.4.3. Photoinduced Sensitization
2.4.4. Photoinduced Bis-Addition
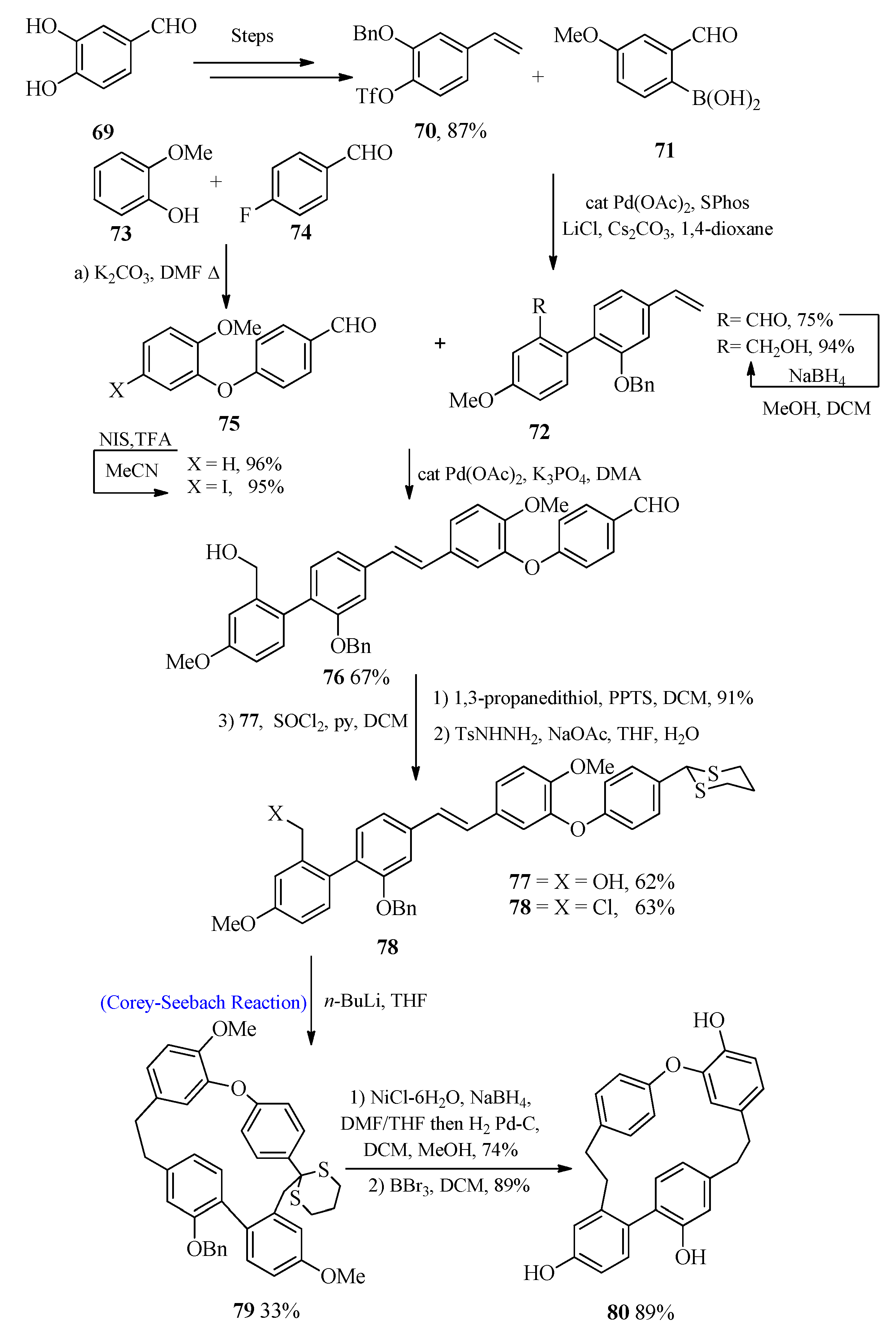
2.5. Bisbibenzyl Analogue
Riccardin C Synthesis
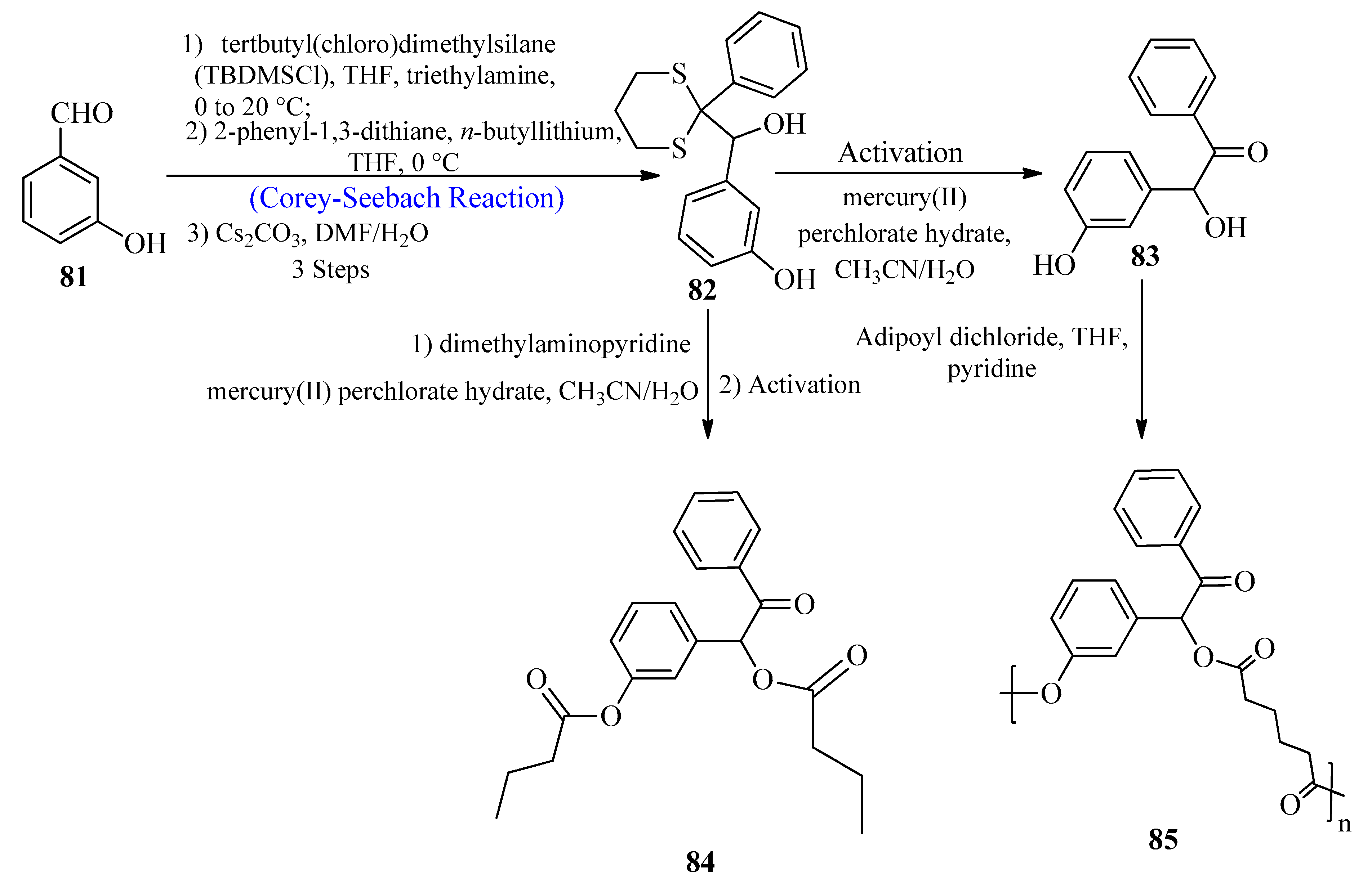
2.6. Biocompatible Polyesters
Benzoin-Derived Diol Linker
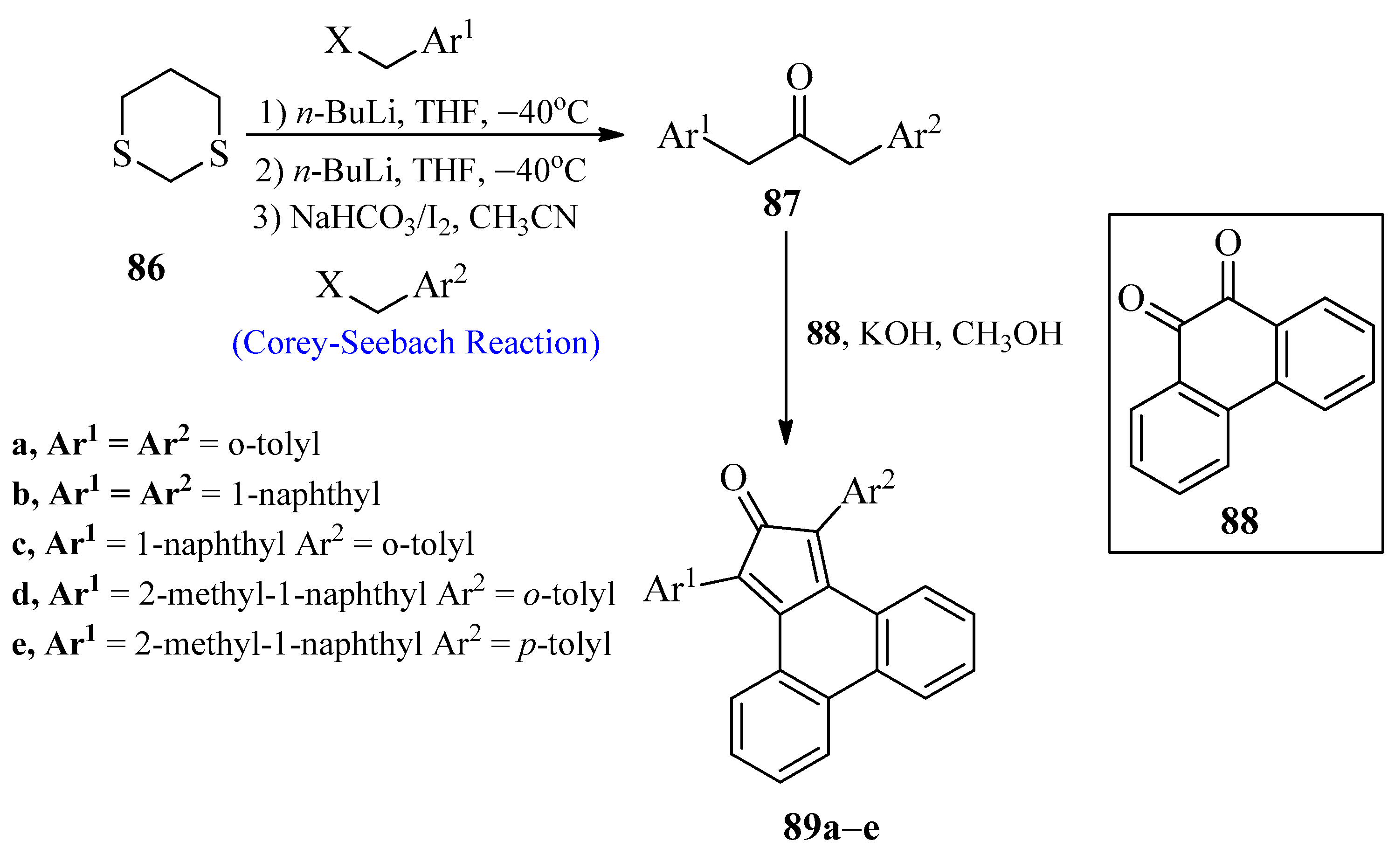
2.7. Tetraphenylcyclopentadienones
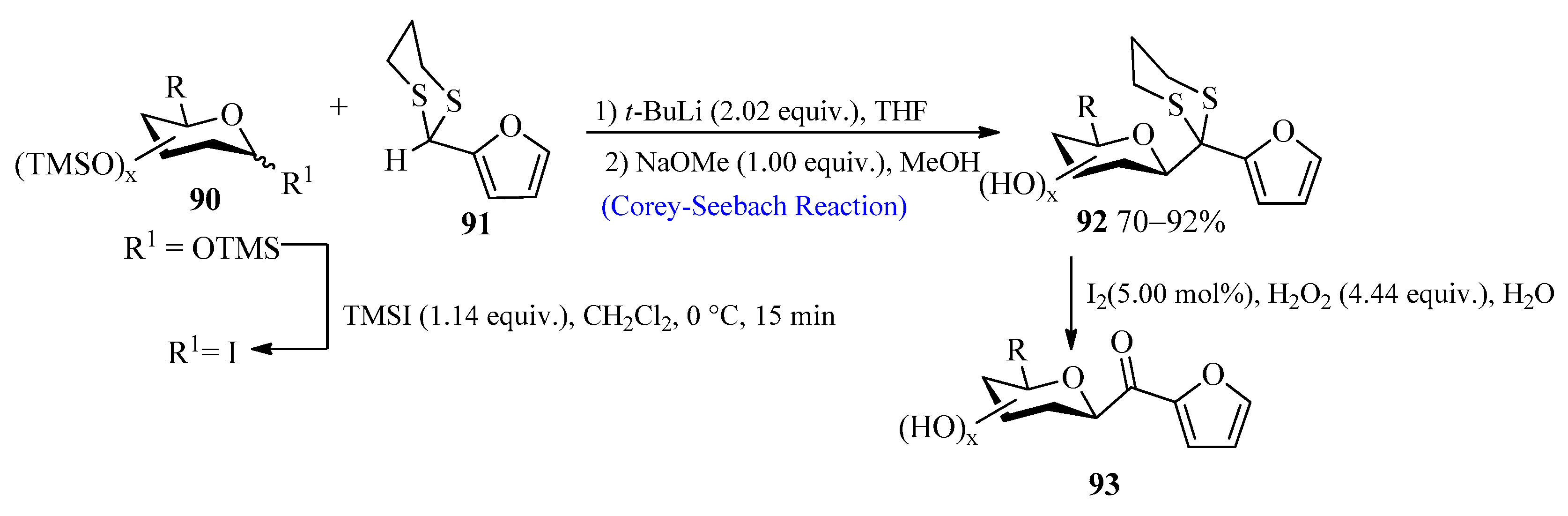
2.8. Scleropentaside A
2.9. Steroids
2.9.1. Withanolide A
2.9.2. Cholanic Acid Derivatives
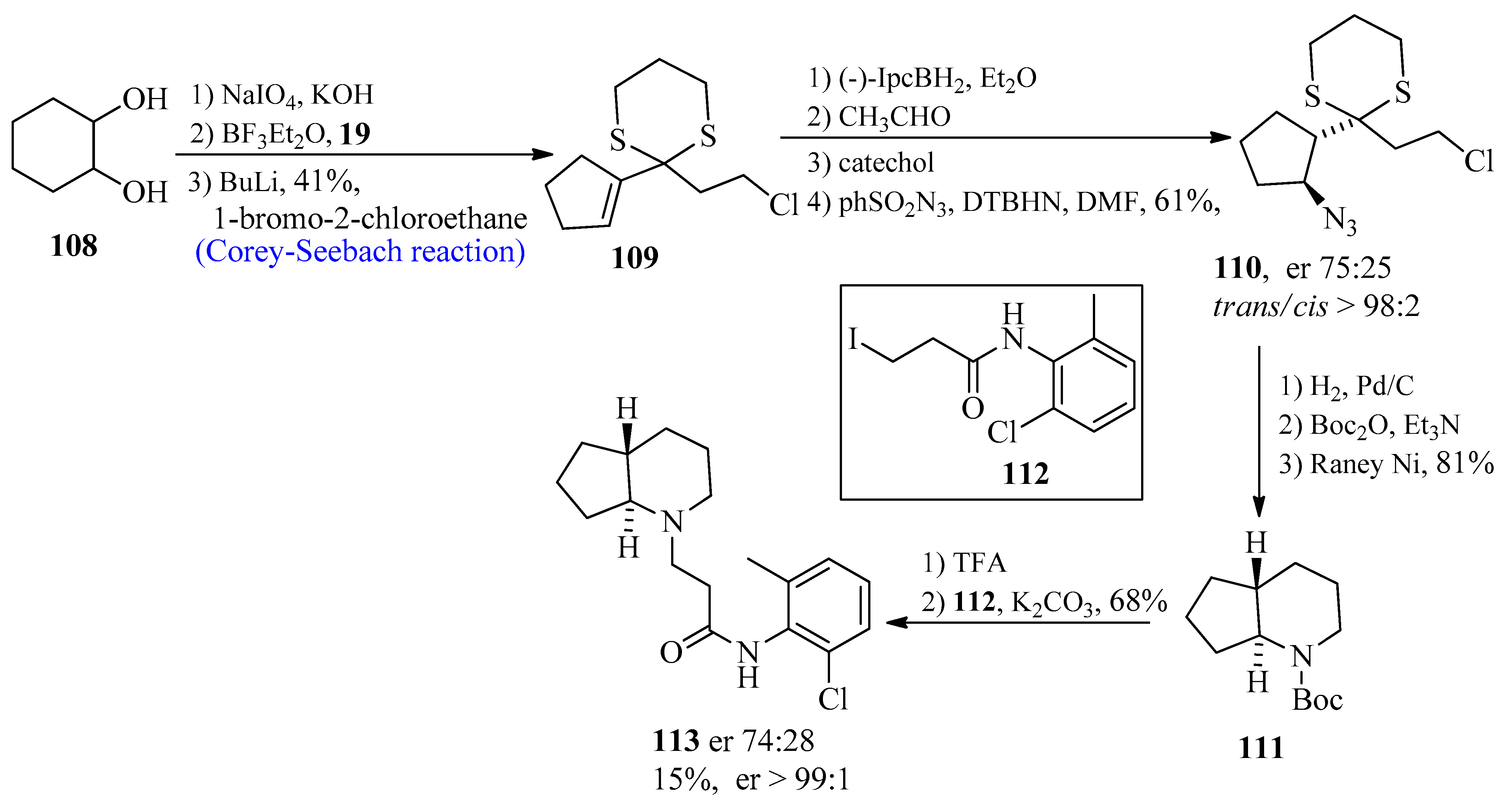
2.10. Rodocaine
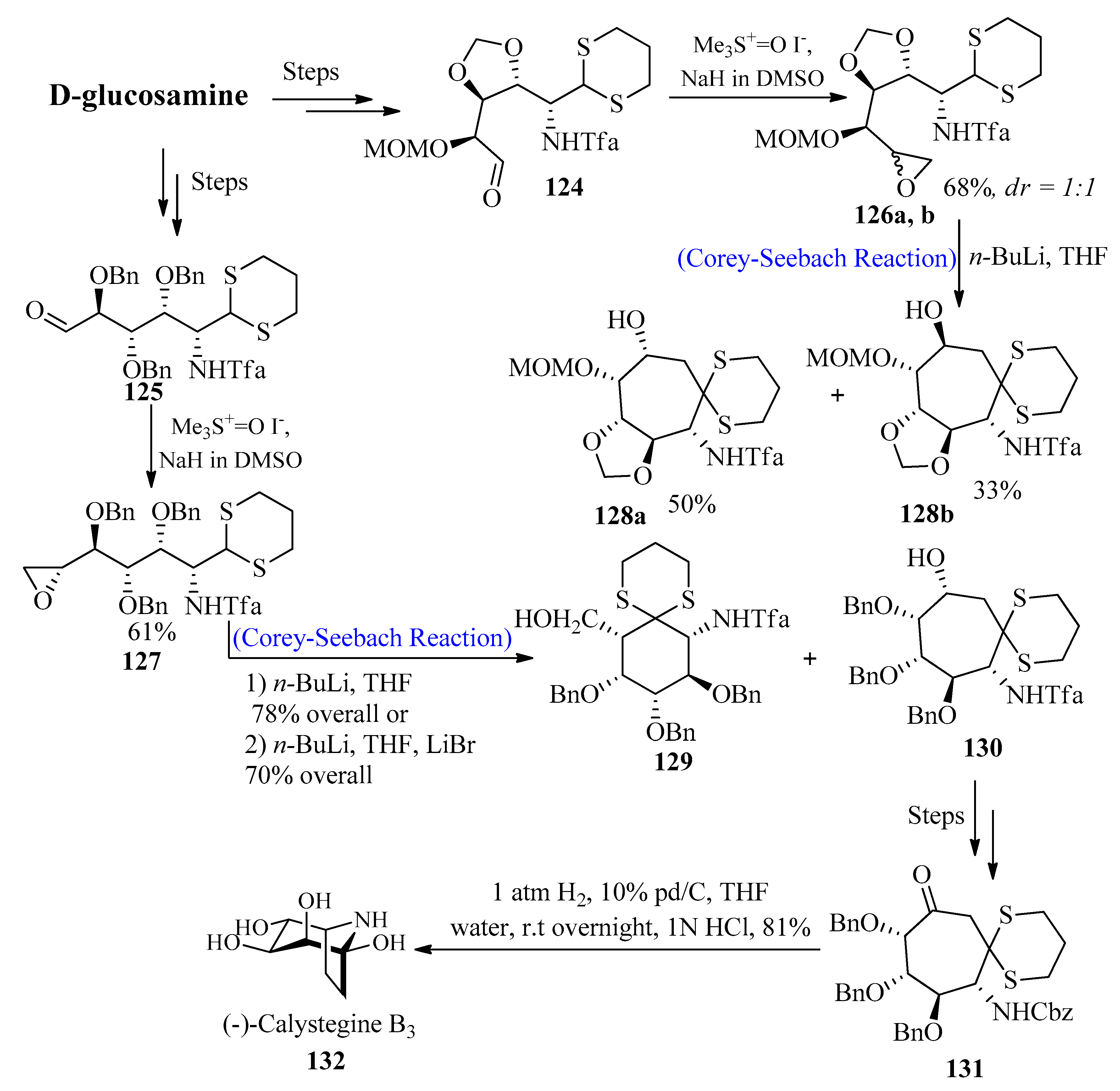
2.11. D-Glucosamine Trimethylene Derivatives
2.12. (−)-Calystegine B3
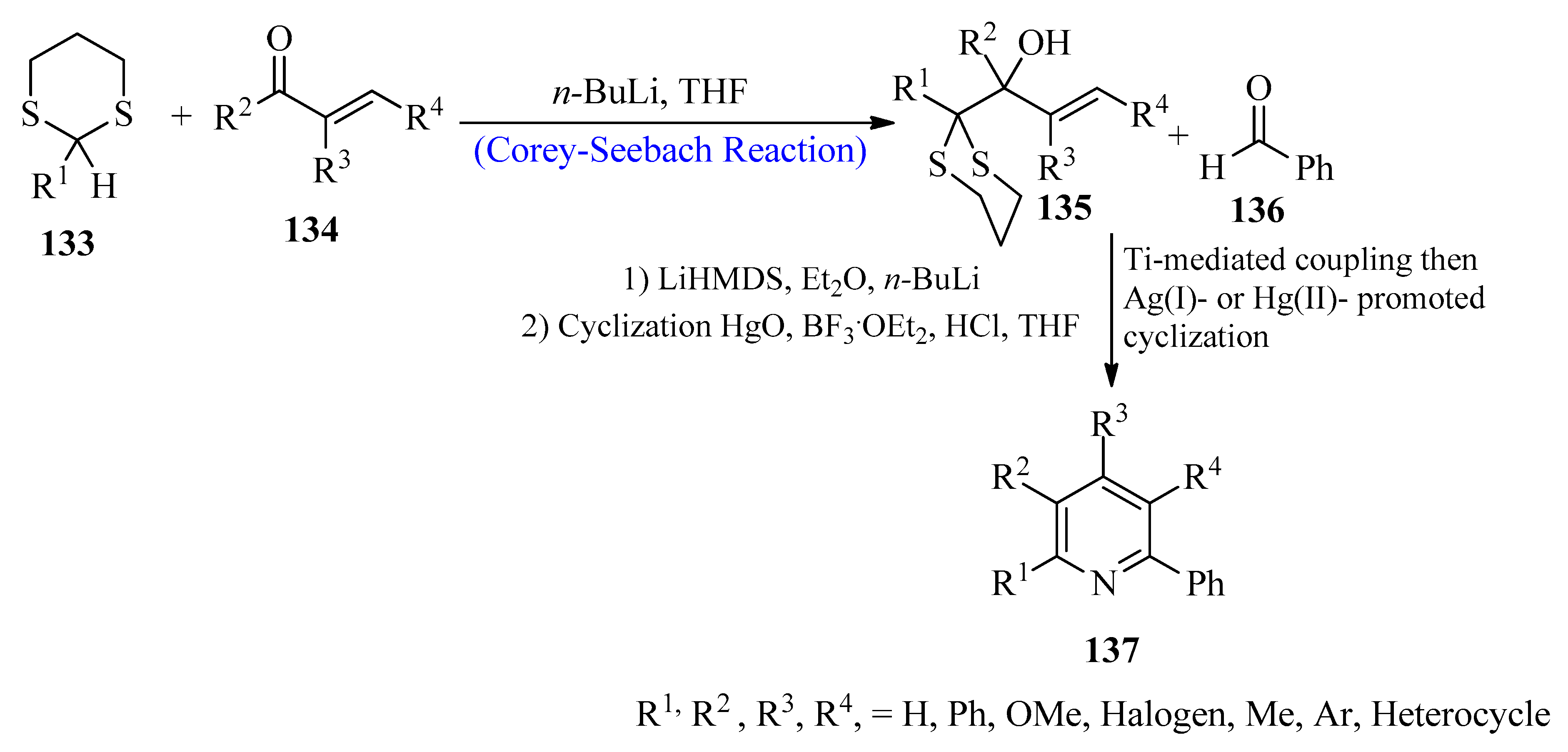
2.13. Substituted Pyridines
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Corey, E.J. The logic of chemical synthesis: Multistep synthesis of complex carbogenic molecules (nobel lecture). Angew. Chem. Int. Ed. 1991, 30, 455–465. [Google Scholar] [CrossRef]
- Corey, E.J.; Seebach, D. Carbanions of 1,f Dithianes. Reagents for C -C Bond Formation by Nucleophilic Displacement and Carbonyl Addition. Angew. Chem. Int. Ed. 1965, 4, 1075–1077. [Google Scholar] [CrossRef]
- Seebach, D.; Corey, E.J. Generation and synthetic applications of 2-lithio-1,3-dithianes. JOC 1975, 40, 231–237. [Google Scholar] [CrossRef]
- Seebach, D.; Kolb, M. Zur Umpolung der Carbonylreaktivität; Deprotonierung von Ketenthioacetalen zu 1, 1-dithiosubstituierten Allyl-und Pentadienyllithiumverbindungen sowie deren Reaktionen mit Elektrophilen. Justus Liebigs Ann. Der Chem. 1977, 1977, 811–829. [Google Scholar] [CrossRef]
- Grobel, B.T.; Seebach, D. Umpolung of the reactivity of carbonyl compounds through sulfur-containing reagents. Synthesis 1977, 1977, 357–402. [Google Scholar] [CrossRef]
- Seebach, D. Methods of reactivity umpolung. Angew. Chem. Int. Ed. 1979, 18, 239–258. [Google Scholar] [CrossRef]
- Donabauer, K.; Murugesan, K.; Rozman, U.; Crespi, S.; König, B. Photocatalytic Reductive Radical-Polar Crossover for a Base-Free Corey–Seebach Reaction. Chem. Eur. J. 2022, 26, 12945–12950. [Google Scholar] [CrossRef]
- Corey, E.J.; Ericson, B.W.J. Oxidative hydrolysis of 1,3-dithiane derivatives to carbonyl compounds using N-halosuccinimide reagents. Org. Chem. 1971, 36, 3553–3560. [Google Scholar] [CrossRef]
- Tanemura, K.; Dohya, H.; Imamura, M.; Suzuki, T.; Horaguchi, T. Deprotection of 1,3-dithianes by 2, 3-dichloro-5, 6-dicyano-p-benzoquinone (DDQ). Chem. Lett. 1994, 23, 965–968. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Barik, S.K. A facile mild deprotection protocol for 1,3-dithianes and 1,3-dithiolanes with 30% hydrogen peroxide and iodine catalyst in aqueous micellar system. Synthesis 2009, 2009, 1393–1399. [Google Scholar] [CrossRef]
- Andersen, N.H.; McCrae, D.A.; Grotjahn, D.B.; Gabhe, S.Y.; Theodore, L.J.; Ippolito, R.M.; Sarkar, T.K. The use of metalloids (-SiMe3,-SnR3) as protected carbanions: Selective activation and new cyclization processes. Tetrahedron 1981, 37, 4069–4079. [Google Scholar] [CrossRef]
- Porter, Q.N.; Utley, J.H. Electrochemical removal of 1,3-dithian protecting groups. J. Chem. Soc. Chem. Commun. 1978, 6, 255–256. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Structure of swinholide-A, a new macrolide from the marine sponge Theonella swinhoei. Tetrahedron Lett. 1985, 26, 511–514. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsuchiya, K.; Harada, T.; Nishide, M.; Kurokawa, T.; Nakagawa, T.; Kobayashi, K. Pironetin, a novel plant growth regulator produced by Streptomyces sp. NK10958 I. Taxonomy, production, isolation and preliminary characterization. J. Antibiot. 1994, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Sahar, A.; Khan, Z.A.; Ahmad, M.; Zahoor, A.F.; Mansha, A.; Iqbal, A. Synthesis and antioxidant potential of some biscoumarin derivatives. Trop. J. Pharm. Res. 2017, 16, 203–210. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsuchiya, K.; Kurokawa, T.; Nakagawa, T.; Shimada, N.; Iitaka, Y. Pironetin, a novel plant growth regulator produced by Streptomyces sp. NK10958 II. Structural elucidation. J. Antibiot 1994, 47, 703–707. [Google Scholar] [CrossRef]
- Scheuer, P.J.; Takahashi, W.; Tsutsumi, J.; Yoshida, T. Ciguatoxin: Isolation and chemical nature. Science 1967, 155, 1267–1268. [Google Scholar] [CrossRef]
- Corey, E.J.; Weigel, L.O.; Floyd, D.; Bock, M.G. Total synthesis of (+-)-N-methylmaysenine. J. Am. Chem. Soc. 1978, 100, 2916–2918. [Google Scholar] [CrossRef]
- Corey, E.J.; Weigel, L.O.; Chamberlin, A.R.; Lipshutz, B. Total synthesis of (−)-N-methylmaysenine. J. Am. Chem. Soc. 1980, 102, 1439–1441. [Google Scholar] [CrossRef]
- Michida, M.; Mukaiyama, T. Lewis Base Catalyzed 1,3-Dithiane Addition to Carbonyl and Imino Compounds Using 2-Trimethylsilyl-1,3-dithiane. Chem. Asian J. 2008, 3, 1592–1600. [Google Scholar] [CrossRef]
- Lee, H.B.; Balasubramanian, S. Studies on a dithiane-protected benzoin photolabile safety catch linker for solid-phase synthesis. J. Org. Chem. 1999, 64, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Dewar, G.H.; Marshall, I.G.; Patel, S.S.; Waigh, R.D. Chemodegradable neuromuscular blocking agents-I: Bis 3, 4-dihydroisoquinolinium salts. Pharm. Sci. Commun. 1993, 4, 3–14. [Google Scholar]
- Yus, M.; Nájera, C.; Foubelo, F. The role of 1,3-dithianes in natural product synthesis. Tetrahedron 2003, 59, 6147–6212. [Google Scholar] [CrossRef]
- Liu, J.S.; Zhu, Y.L.; Yu, C.M.; Zhou, Y.Z.; Han, Y.Y.; Wu, F.W.; Qi, B.F. The str uctures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Morita, H.; Shiro, M.; Kobayashi, J.I. Sieboldine A, a Novel Tetracyclic Alkaloid from Lycopodium s ieboldii, Inhibiting Acetylcholinesterase. Org. Lett. 2003, 5, 3991–3993. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, X.Q.; Li, M.M.; Zhao, Y.; Xu, G.; Cheng, X.; Zhao, Q.S. Lycojapodine A, a novel alkaloid from Lycopodium japonicum. Org. Lett. 2009, 11, 1397–1400. [Google Scholar] [CrossRef]
- Aver, W.A.; Trifonov, L.S. Lycopodium alkaloids. Alkaloids Chem. Pharmacol. 1994, 45, 233–266. [Google Scholar] [CrossRef]
- Heathcock, C.H.; Smith, K.M.; Blumenkopf, T.A. Total synthesis of (+-)-fawcettimine (Burnell’s base A). J. Am. Chem. Soc. 1986, 108, 5022–5024. [Google Scholar] [CrossRef]
- Linghu, X.; Kennedy-Smith, J.J.; Toste, F.D. Total synthesis of (+)-fawcettimine. Angew. Chem. 2007, 119, 7815–7817. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Nian, Y.; Zhu, Q.F.; Li, X.N.; Su, J.; Wu, X.D.; Zhao, Q.S. Lycoplanine A, a C16N Lycopodium alkaloid with a 6/9/5 tricyclic skeleton from Lycopodium complanatum. Org. Lett. 2017, 19, 4668–4671. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Yao, L.; Tang, B.; Mu, G.; Shi, T.; Wang, Z. Synthesis of an isomer of lycoplanine A via cascade cyclization to construct the spiro-N, O-acetal moiety. Org. Biomol. Chem. 2021, 19, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Chen, Q.H.; Liu, X.Y. Diterpenoid alkaloids. Nat. Prod. Rep. 2010, 27, 529–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Chen, Q.H.; Liang, X.T. The C18-diterpenoid alkaloids. Alkaloids Chem. Biol. 2009, 67, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, X.; Song, X.; Wang, Z.; Liu, X.; Song, H.; Qin, Y. Studies towards Bioinspired Synthesis of Hetidine-Type C20-Diterpenoid Alkaloids. Chin. J. Chem. 2017, 35, 991–1000. [Google Scholar] [CrossRef]
- Hanson, J.R.; Nichols, T.; Mukhrish, Y.; Bagley, M.C. Diterpenoids of Terrestrial Origin. Nat. Prod. Rep. 2019, 36, 1499–1512. [Google Scholar] [CrossRef]
- Torres, M.C.M.; Braz-Filho, R.; Silveira, E.R.; Diniz, J.C.; Viana, F.A.; Pessoa, O.D.L. Terpenoids from Croton regelianus. Helv. Chim. Acta 2010, 93, 375–381. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Zhang, W.; Xie, X.; She, X. Concise Total Synthesis of the Bisnorditerpene (+)-(5β, 8α, 10α)-8-hydroxy-13-methylpodocarpa-9 (11), 13-diene-3, 12-dione. Asian J. Org. Chem. 2016, 5, 986–990. [Google Scholar] [CrossRef]
- Ahmad, S.; Zahoor, A.F.; Naqvi, S.A.R.; Akash, M. Recent trends in ring opening of epoxides with sulfur nucleophiles. Mol. Divers. 2018, 22, 191–205. [Google Scholar] [CrossRef]
- Bendall, J.G.; Cambie, R.C. Totarol: A non-conventional diterpenoid. Aust. J. Chem. 1995, 48, 883–917. [Google Scholar] [CrossRef]
- Muroi, H.; Kubo, I. Bactericidal effects of anacardic acid and totarol on methicillin-resistant Staphylococcus aureus (MRSA). Biosci. Biotechnol. Biochem. 1994, 58, 1925–1926. [Google Scholar] [CrossRef]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillinresistant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Oike, S.; Muroi, H.; Kubo, I. Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med. 1996, 62, 122–125. [Google Scholar] [CrossRef]
- Constantine, G.H.; Karchesy, J.J.; Franzblau, S.G.; LaFleur, L.E. (+)− Totarol from Chamaecyparis nootkatensis and activity against Mycobacterium tuberculosis. Fitoterapia 2001, 72, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Shaw, J.T. Synthesis of antimicrobial natural products targeting FtsZ:(+)-totarol and related totarane diterpenes. Org. Lett. 2010, 12, 3324–3327. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Guth, F.M. The ambruticins and jerangolids–chemistry, biology and chemoenzymatic synthesis of potent antifungal drug candidates. Nat. Prod. Rep. 2020, 37, 1300–1315. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Galgiani, J.N.; Tian, Z.Q.; Zhong, Z.; Timmermans, P.; Katz, L. Efficacy of ambruticin analogs in a murine model of coccidioidomycosis. Antimicrob. Agents Chemother. 2006, 50, 3467–3469. [Google Scholar] [CrossRef]
- Connor, D.T.; Greenough, R.C.; Von Strandtmann, M. W-7783, a unique antifungal antibiotic. J. Org. Chem. 1977, 42, 3664–3669. [Google Scholar] [CrossRef]
- Just, G.; Potvin, P. Synthesis of ring A of ambruticin and proof of its absolute stereochemistry. Can. J. Chem. 1980, 58, 2173–2177. [Google Scholar] [CrossRef]
- Zahoor, A.F.; Yousaf, M.; Siddique, R.; Ahmad, S.; Naqvi, S.A.R.; Rizvi, S.M.A. Synthetic strategies toward the synthesis of enoxacin-, levofloxacin-, and gatifloxacin-based compounds: A review. Synth. Commun. 2017, 47, 1021–1039. [Google Scholar] [CrossRef]
- Michelet, V.; Genet, J.P. An overview of synthetic and biological studies of ambruticin and analogues. Curr. Org. Chem. 2005, 9, 405–418. [Google Scholar] [CrossRef]
- Dongo, A.; Bataille-Simoneau, N.; Campion, C.; Guillemette, T.; Hamon, B.; Iacomi-Vasilescu, B.; Simoneau, P. The group III two-component histidine kinase of filamentous fungi is involved in the fungicidal activity of the bacterial polyketide ambruticin. AEM 2009, 75, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vetcher, L.; Menzella, H.G.; Kudo, T.; Motoyama, T.; Katz, L. The antifungal polyketide ambruticin targets the HOG pathway. Antimicrob. Agents Chemother. 2007, 51, 3734–3736. [Google Scholar] [CrossRef] [PubMed]
- Trentadue, K.; Chang, C.F.; Nalin, A.; Taylor, R.E. Enantioselective Total Synthesis of the Putative Biosynthetic Intermediate Ambruticin J. Chem. Eur. J. 2021, 27, 11126–11131. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef]
- Kotoku, N.; Ishida, R.; Matsumoto, H.; Arai, M.; Toda, K.; Setiawan, A.; Kobayashi, M. Biakamides A–D, unique polyketides from a marine sponge, act as selective growth inhibitors of tumor cells adapted to nutrient starvation. J. Org. Chem. 2017, 82, 1705–1718. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym Sci. A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Wiesner, T.; Haas, M. Do germanium-based photoinitiators have the potential to replace the well-established acylphosphine oxides? Dalton Trans. 2021, 50, 12392–12398. [Google Scholar] [CrossRef]
- Wiesner, T.; Glotz, G.; Wunnicke, O.; Bleger, D.; Knezevic, I.; Torvisco, A.; Haas, M. The Road to Bisacyldigermanes: A New Compound Class Suitable as Visible Light Photoinitiators. Chem. Photo. Chem. 2022, 6, e202200107. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Restor. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Ullrich, G.; Ganster, B.; Salz, U.; Moszner, N.; Liska, R. Photoinitiators with functional groups. IX. Hydrophilicbisacylphosphine oxides for acidic aqueous formulations. J. Polym. Sci. A Polym. Chem. 2006, 44, 1686–1700. [Google Scholar] [CrossRef]
- Moszner, N.; Zeuner, F.; Lamparth, I.; Fischer, U.K. Benzoylgermanium derivatives as novel visible-light photoinitiators for dental composites. Macromol. Mater. Eng. 2009, 294, 877–886. [Google Scholar] [CrossRef]
- Gravel, D.; Farmer, L.; Denis, R.C.; Schultz, E. Photoinduced rearrangement of carbocyclic 2-phenylthio-1,3-diols to deoxysugars. Tetrahedron Lett. 1994, 35, 8981–8984. [Google Scholar] [CrossRef]
- McHale, W.A.; Kutateladze, A.G. An Efficient Photo-SET-Induced Cleavage of Dithiane− Carbonyl Adducts and Its Relevance to the Development of Photoremovable Protecting Groups for Ketones and Aldehydes. J. Org. Chem. 1998, 63, 9924–9931. [Google Scholar] [CrossRef]
- Vath, P.; Falvey, D.E.; Barnhurst, L.A.; Kutateladze, A.G. Photoinduced CC bond cleavage in dithiane-carbonyl adducts: A laser flash photolysis study. J. Org. Chem. 2001, 66, 2887–2890. [Google Scholar] [CrossRef]
- Gustafson, T.P.; Kurchan, A.N.; Kutateladze, A.G. Externally sensitized mesolytic fragmentations in dithiane–ketone adducts. Tetrahedron 2006, 62, 6574–6580. [Google Scholar] [CrossRef]
- Kita, Y.; Sekihachi, J.; Hayashi, Y.; Da, Y.Z.; Yamamoto, M.; Akai, S. An efficient synthesis of. alpha.-silylacetates having various types of functional groups in the molecules. J. Org. Chem. 1990, 55, 1108–1112. [Google Scholar] [CrossRef]
- Li, Z.; Kutateladze, A.G. Anomalous C− C Bond Cleavage in Sulfur-Centered Cation Radicals Containing a Vicinal Hydroxy Group. J. Org. Chem. 2003, 68, 8236–8239. [Google Scholar] [CrossRef] [PubMed]
- Valiulin, R.A.; Kottani, R.; Kutateladze, A.G. When ethyl is infinitely different from methyl: Double addition of lithiated dithianes to aromatic carboxylates revisited. J. Org. Chem. 2006, 71, 5047–5049. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemosystematics of the Hepaticae. Phytochemistry 2004, 65, 623–669. [Google Scholar] [CrossRef]
- Zinsmeister, H.D.; Becker, H.; Eicher, T. Bryophytes, a source of biologically active, naturally occurring material? Angewandte Chemie Int. Ed. 1991, 30, 130–147. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Kostiuk, S.L. Macrocylic bisbibenzyl natural products and their chemical synthesis. Nat. Prod. Rep. 2012, 29, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.F.; Qu, J.B.; Wu, X.Z.; Liu, N.; Ji, M.; Lou, H.X. Antifungal macrocyclic bis (bibenzyls) from the Chinese liverwort Ptagiochasm intermedlum L. Nat. Prod. Res. 2010, 24, 515–520. [Google Scholar] [CrossRef]
- Fujii, K.; Morita, D.; Onoda, K.; Kuroda, T.; Miyachi, H. Minimum structural requirements for cell membrane leakage-mediatedanti-MRSA activity of macrocyclic bis (bibenzyl). Bioorg. Med. Chem. Lett. 2016, 26, 2324–2327. [Google Scholar] [CrossRef] [PubMed]
- Almalki, F.A.; Harrowven, D.C. A Corey–Seebach Macrocyclisation Strategy for the Synthesis of Riccardin C and an Unnaural Macrocyclic Bis (bibenzyl) Analogue. Eur. JOC. 2016, 2016, 5738–5746. [Google Scholar] [CrossRef]
- Peach, J.M.; Pratt, A.J.; Snaith, J.S. Photolabile benzoin and furoin esters of a biologically active peptide. Tetrahedron 1995, 51, 10013–10024. [Google Scholar] [CrossRef]
- Patchornik, A.; Amit, B.W.R.B.; Woodward, R.B. Photosensitive protecting groups. J. Am. Chem. Soc. 1970, 92, 6333–6335. [Google Scholar] [CrossRef]
- Englert, C.; Nischang, I.; Bader, C.; Borchers, P.; Alex, J.; Pröhl, M.; Schubert, U.S. Photocontrolled release of chemicals from nano-and microparticle containers. Angew. Chem. Int. Ed. 2018, 57, 2479–2482. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Xie, H.; Lu, H.; Zhao, S.; Feng, S. Unexpected SiMe 3 effect on color-tunable and fluorescent probes of dendritic polyphenyl naphthalimides with aggregation-induced emission enhancement. J. Mater. Chem. C 2016, 4, 745–750. [Google Scholar] [CrossRef]
- Mandal, S.; Parida, K.N.; Samanta, S.; Moorthy, J.N. Influence of (2,3,4,5,6-pentamethyl/phenyl) phenyl scaffold: Stereoelectronic control of the persistence of o-quinonoid reactive intermediates of photochromic chromenes. J. Org. Chem. 2011, 76, 7406–7414. [Google Scholar] [CrossRef]
- Prati, L.; Mancinelli, M.; Ciogli, A.; Mazzanti, A. Tetrasubstituted cyclopentadienones as suitable enantiopure ligands with axial chirality. Org. Biomol. Chem. 2017, 15, 8720–8728. [Google Scholar] [CrossRef] [PubMed]
- Disadee, W.; Mahidol, C.; Sahakitpichan, P.; Sitthimonchai, S.; Ruchirawat, S.; Kanchanapoom, T. Unprecedented furan-2-carbonyl C-glycosides and phenolic diglycosides from Scleropyrum pentandrum. Phytochemistry 2012, 74, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.; Zahoor, A.F.; Ajmal, M.; Kamal, S.; Ahmad, S.; Abdelgawad, A.M.; Elnaggar, M.E. Design, synthesis, antimicrobial evaluation, and laccase catalysis effect of novel benzofuran–oxadiazole and benzofuran–triazole hybrids. J. Heterocycl. Chem. 2019, 56, 2839–2852. [Google Scholar] [CrossRef]
- Boehlich, G.J.; Schützenmeister, N. β-Selective C-Glycosylation and its Application in the Synthesis of Scleropentaside A. Angew. Chem. Int. Ed. 2019, 58, 5110–5113. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.S.; Sethi, P.D.; Glotter, E.; Kirson, I.; Lavie, D. 5, 20α (R)-dihydroxy-6α, 7α-epoxy-1-oxo-(5α) witha-2, 24-dienolide, a new steroidal lactone from Withania coagulans. Phytochemistry 1971, 10, 685–688. [Google Scholar] [CrossRef]
- Jain, S.; Shukla, S.D.; Sharma, K.; Bhatnagar, M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother. Res. 2001, 15, 544–548. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Zhao, J.; Nakamura, N.; Hattori, M.; Komatsu, K. Axon-or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport 2002, 13, 1715–1720. [Google Scholar] [CrossRef]
- Zhao, J.; Nakamura, N.; Hattori, M.; Kuboyama, T.; Tohda, C.; Komatsu, K. Withanolide derivatives from the roots of Withaniasomnifera and their neurite outgrowth activities. Chem. Pharm. Bull. 2002, 50, 760–765. [Google Scholar] [CrossRef]
- Tohda, C.; Kuboyama, T.; Komatsu, K. Search for natural products related to regeneration of the neuronal network. Neurosignals 2005, 14, 34–45. [Google Scholar] [CrossRef]
- Liffert, R.; Hoecker, J.; Jana, C.K.; Woods, T.M.; Burch, P.; Jessen, H.J.; Gademann, K. Withanolide A: Synthesis and structural requirements for neurite outgrowth. Chem. Sci. 2013, 4, 2851–2857. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Djerassi, C. Novel marine sterols with modified bile acid side chain from the sea pen Ptilosarcus gurneyi. Tetrahedron Lett. 1977, 18, 683–686. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Djerassi, C. Marine natural products. Synthesis of four naturally occurring 20. beta.-H cholanic acid derivatives. J. Org. Chem. 1978, 43, 1442–1448. [Google Scholar] [CrossRef]
- Shingate, B.B.; Hazra, B.G.; Pore, V.S.; Gonnade, R.G.; Bhadbhade, M.M. Stereoselective syntheses of 20-epi cholanic acid derivatives from 16-dehydropregnenolone acetate. Tetrahedron 2007, 63, 5622–5635. [Google Scholar] [CrossRef]
- Henshall, T.; Parnell, E.W. Application of acrylonitrile to the synthesis of some reduced heterocyclic compounds. J. Chem. Soc. 1962, 123, 661–667. [Google Scholar] [CrossRef]
- Van Bever, W.F.; Knaeps, A.G.; Willems, J.J.; Hermans, B.K.; Janssen, P.A. Local anesthetic azabicyclo-N-alkylanilides. J. Med. Chem. 1973, 16, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Joosten, A.; Lambert, E.; Vasse, J.L.; Szymoniak, J. Diastereoselective access to trans-2-substituted cyclopentylamines. Org. Lett. 2010, 12, 5128–5131. [Google Scholar] [CrossRef]
- Meyer, D.; Renaud, P. Enantioselective Hydroazidation of Trisubstituted Non-Activated Alkenes. Angew. Chem. Int. Ed. 2017, 56, 10858–10861. [Google Scholar] [CrossRef]
- Anaya, J.; Barton, D.H.; Gero, S.D.; Grande, M.; Hernando, J.M.; Laso, N.M. Different approaches to the asymmetric synthesis of 1,3,6-trisubstituted and 1,2,3,6-tetrasubstituted carbapenems1 from D-glucosamine. Tetrahedron Asymmetry 1995, 6, 609–624. [Google Scholar] [CrossRef]
- Chen, Y.L.; Leguijt, R.; Redlich, H.; Fröhlich, R. Trimethylene dithioacetals of carbohydrates, part 6: CC coupling reactions of dilithiated N-acetyl-d-glucosamine trimethylene dithioacetal derivatives. Synthesis 2006, 24, 4212–4218. [Google Scholar] [CrossRef]
- Kapferer, P.; Birault, V.; Poisson, J.F.; Vasella, A. Synthesis and Evaluation as Glycosidase Inhibitors of Carbasugar-Derived Spirodiaziridines, Spirodiazirines, and Spiroaziridines. Helv. Chim. Acta 2003, 86, 2210–2227. [Google Scholar] [CrossRef]
- Ogawa, S.; Funayama, S.; Okazaki, K.; Ishizuka, F.; Sakata, Y.; Doi, F. Synthesis of 5a-carba-hexopyranoses and hexopyranosylamines, as well as 5a, 5a′-dicarbadisaccharides, from 3, 8-dioxatricyclo [4.2. 1.02, 4] nonan-9-ol: Glycosidase inhibitory activity of N-substituted 5a-carba-β-gluco-and β-galactopyranosylamines, and derivatives thereof. Bioorg. Med. Chem. Lett. 2004, 14, 5183–5188. [Google Scholar] [CrossRef] [PubMed]
- Duclos, O.; Duréault, A.; Depezay, J.C. Access to polyhydroxylated cycloheptane derivatives through stereoselective nitrile oxide intramolecular cycloaddition. Synthesis of an analogue of calystegine B2. Tetrahedron Lett. 1992, 33, 1059–1062. [Google Scholar] [CrossRef]
- Chen, Y.L.; Redlich, H.; Bergander, K.; Fröhlich, R. d-Glucosamine trimethylene dithioacetal derivatives: Formation of six-and seven-membered ring amino carbasugars. Synthesis of (–)-calystegine B3. Org. Biomol. Chem. 2007, 5, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Zahoor, A.F.; Ahmad, S.; Shahzadi, I.; Irfan, A.; Faiz, S. Novel chiral ligands for palladium-catalyzed asymmetric allylic alkylation/asymmetric Tsuji-Trost reaction: A review. Curr. Org. Chem. 2019, 23, 1168–1213. [Google Scholar] [CrossRef]
- Aziz, H.; Zahoor, A.F.; Ahmad, S. Pyrazole bearing molecules as bioactive scaffolds: A review. J. Chil. Chem. 2020, 65, 4746–4753. [Google Scholar] [CrossRef]
- Henry, G.D. De novo synthesis of substituted pyridines. Tetrahedron 2004, 29, 6043–6061. [Google Scholar] [CrossRef]
- Movassaghi, M.; Hill, M.D. Synthesis of substituted pyridine derivatives via the ruthenium-catalyzed cycloisomerization of 3-azadienynes. J. Am. Soc. 2006, 128, 4592–4593. [Google Scholar] [CrossRef]
- Chen, M.Z.; Micalizio, G.C. Three-component coupling sequence for the regiospecific synthesis of substituted pyridines. J. Am. Chem. Soc. 2012, 134, 1352–1356. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroon, M.; Zahoor, A.F.; Ahmad, S.; Mansha, A.; Irfan, M.; Mushtaq, A.; Akhtar, R.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. The Corey-Seebach Reagent in the 21st Century: A Review. Molecules 2023, 28, 4367. https://doi.org/10.3390/molecules28114367
Haroon M, Zahoor AF, Ahmad S, Mansha A, Irfan M, Mushtaq A, Akhtar R, Irfan A, Kotwica-Mojzych K, Mojzych M. The Corey-Seebach Reagent in the 21st Century: A Review. Molecules. 2023; 28(11):4367. https://doi.org/10.3390/molecules28114367
Chicago/Turabian StyleHaroon, Muhammad, Ameer Fawad Zahoor, Sajjad Ahmad, Asim Mansha, Muhammad Irfan, Aqsa Mushtaq, Rabia Akhtar, Ali Irfan, Katarzyna Kotwica-Mojzych, and Mariusz Mojzych. 2023. "The Corey-Seebach Reagent in the 21st Century: A Review" Molecules 28, no. 11: 4367. https://doi.org/10.3390/molecules28114367
APA StyleHaroon, M., Zahoor, A. F., Ahmad, S., Mansha, A., Irfan, M., Mushtaq, A., Akhtar, R., Irfan, A., Kotwica-Mojzych, K., & Mojzych, M. (2023). The Corey-Seebach Reagent in the 21st Century: A Review. Molecules, 28(11), 4367. https://doi.org/10.3390/molecules28114367









