Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile
Abstract
1. Introduction
2. Results and Discussion
2.1. Anti-Candidal Effect of CMJ
2.1.1. Anti-Microbial Effect of CMJ
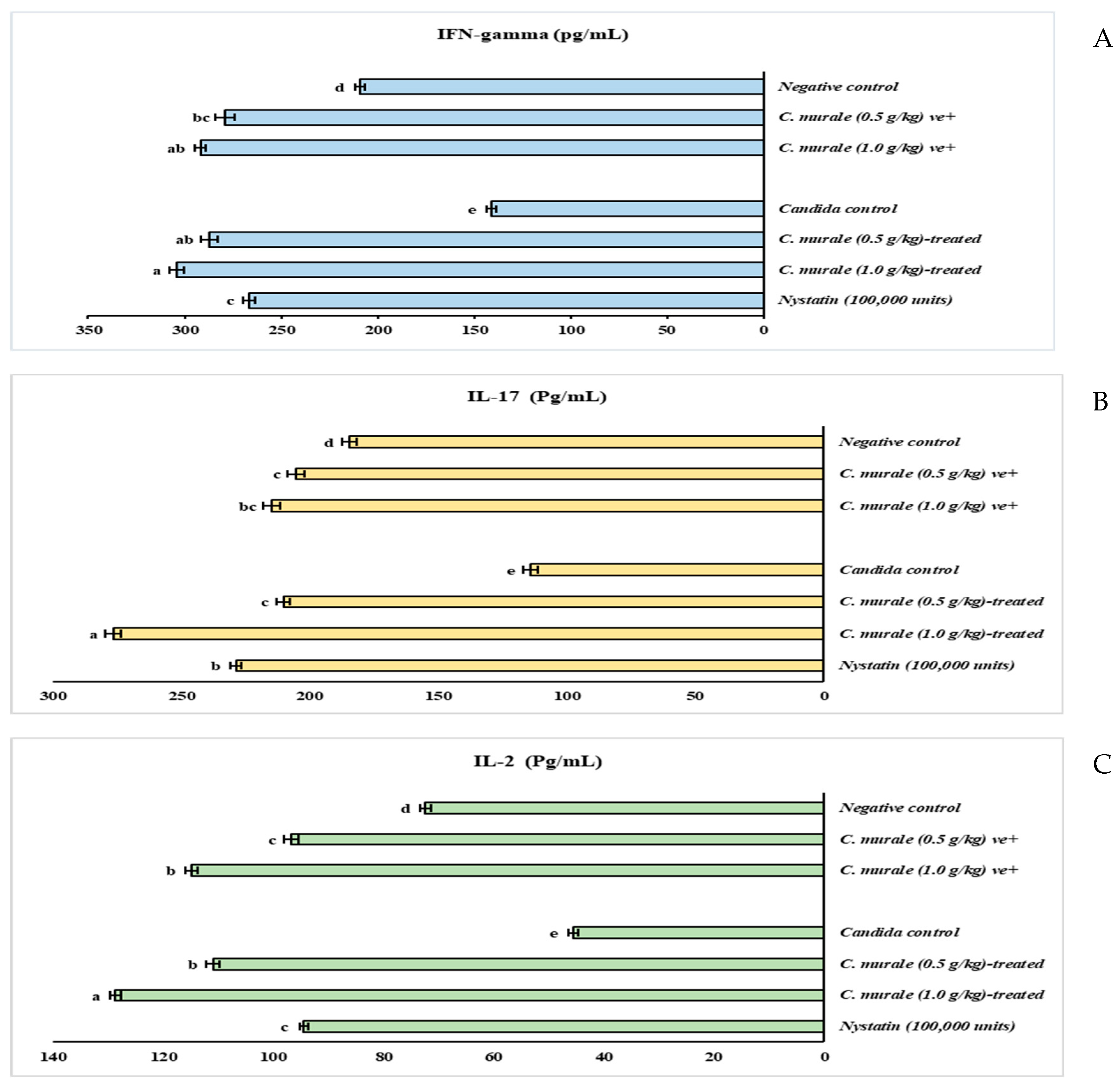
2.1.2. Immunomodulatory Effect of CMJ
2.2. Antioxidant Impact of CMJ
2.3. Chemical Composition of CMJ
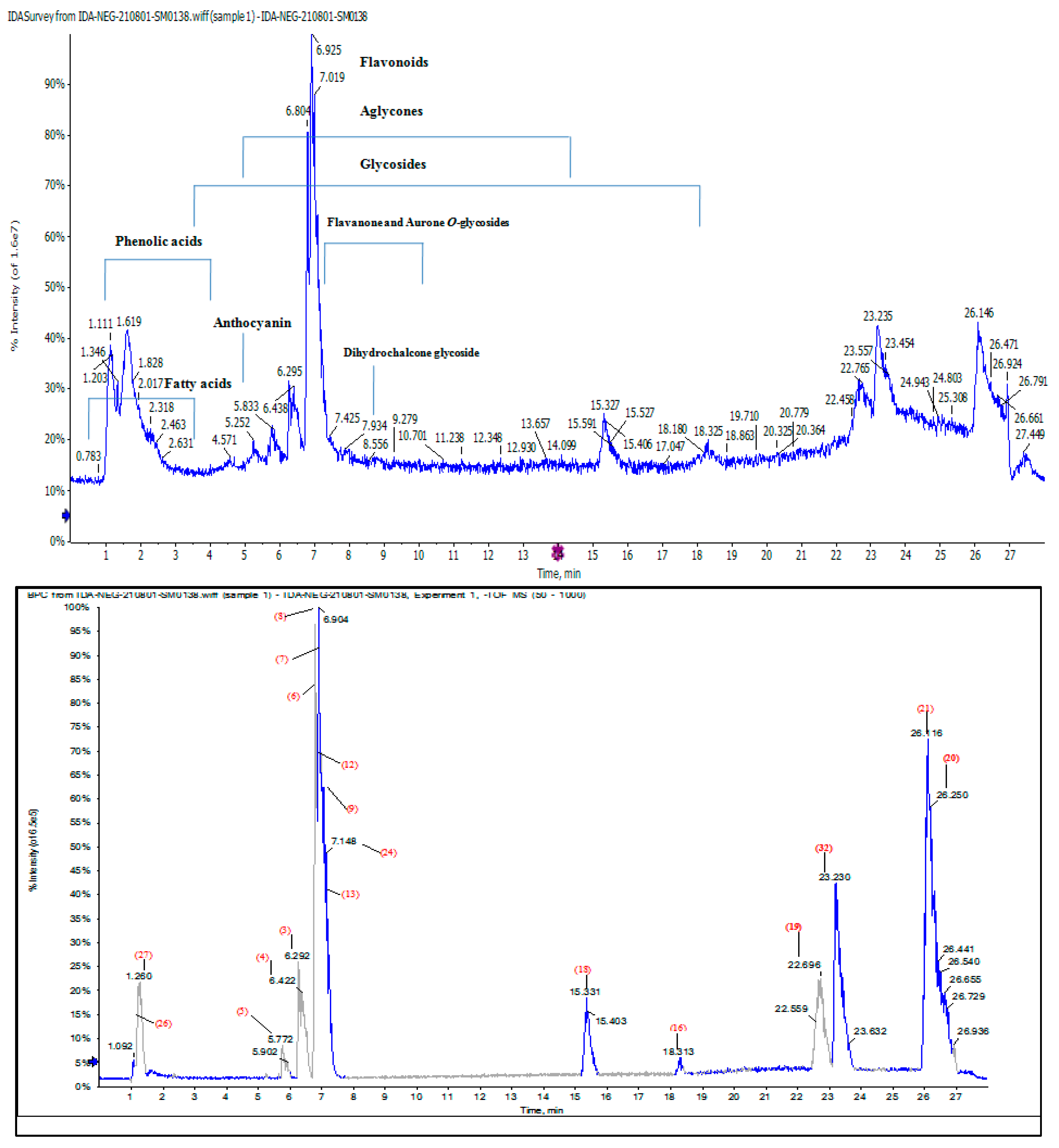
2.4. Characterization of Phytoconstituents in Chenopodium murale Juice (CMJ) by HPLC/QTOF-HR-MS/MS Analysis
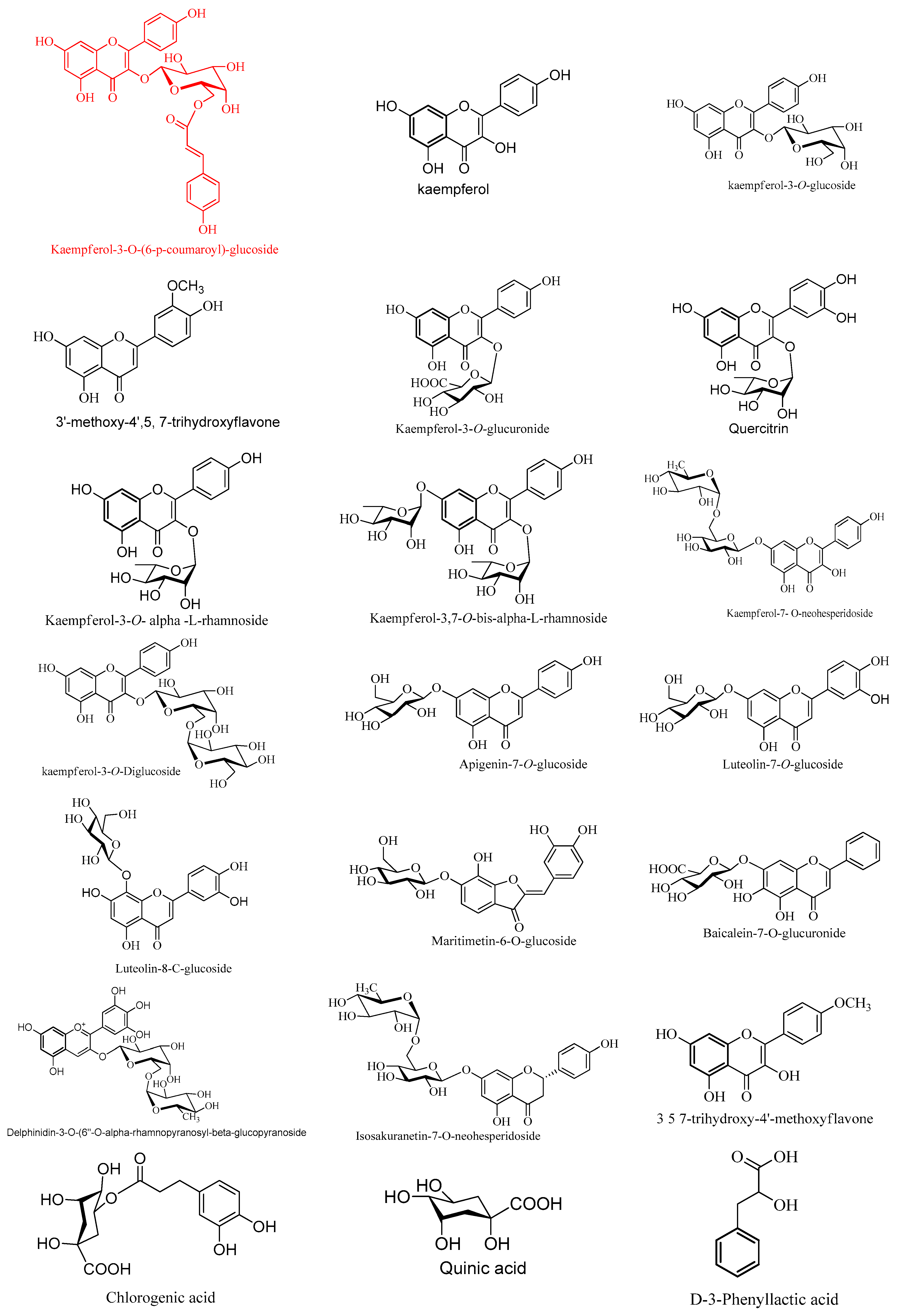
2.4.1. Characterization of the First Detected Kaempferol Derivatives
2.4.2. Characterization of Flavone Derivatives
2.4.3. Characterization of the First Detected Flavanones and Aurone O-Glycosides
2.4.4. Characterization of Phenolics and Fatty Acids
2.4.5. Characterization of Flavonoids Aglycone
2.4.6. Characterization of the First Detected Anthocyanins
3. Materials and Methods
3.1. Chemicals and Drugs
3.2. Collection of C. murale and Preparation of the Juice
3.3. Anti-Candidiasis Activity of CMJ and Nystatin
3.3.1. Assay of Acute Oral Toxicity (LD50)
3.3.2. Microbial Strains and Culture Conditions
3.3.3. Animal Preparation and Oral Infection
3.3.4. Oral Ulcer Induction
3.3.5. The Dosing Protocol of CMJ and Nystatin
3.3.6. Experimental Scheme
3.3.7. Microbiological Evaluation of the Progression of the Infection
3.3.8. Biochemical Analysis
3.4. Chemical Composition of the CMJ
Quantitative Analysis of the CMJ
3.5. Qualitative Analysis of Phytoconstituents in CMJ by HPLC/QTOF-HR-MS/MS
3.5.1. Sample Preparation
3.5.2. Instruments and Acquisition Method
3.5.3. LC-MS Data Processing
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sivapathasundharam, B.; Sundararaman, P.; Kannan, K. Oral Ulcers—A Review. J. Dent. Oral Disord. 2018, 4, 1098. [Google Scholar]
- Scully, C.; Shotts, R. ABC of oral health: Mouth ulcers and other causes of orofacial soreness and pain. Br. Med. J. 2000, 321, 162–165. [Google Scholar] [CrossRef]
- Coronado-Castellote, L.; Jimenez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, 279–286. [Google Scholar] [CrossRef]
- Martinez, A.; Regadera, J.; Jimenez, E.; Santos, I.; Gargallo-Viola, D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob. Agents Chemother. 2001, 45, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Malayeri, F.A.; Rezaei, A.A.; Raiesi, O. Antifungal agents: Polyene, azole, antimetabolite, other and future agents. J. Bas. Res. Med. Sci. 2018, 5, 48–55. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Fuentes-Bazan, S.; Uotila, P.; Borsch, T. A novel phylogeny-based generic classification for Chenopodium sensu lato, and a tribal rearrangement of Chenopodioideae (Chenopodiaceae). Willdenowia 2012, 42, 14. [Google Scholar] [CrossRef]
- Tack Holm, V. Botanical identification of the plants found at the Monastery of Phoebammon. In La Monastere de Phoebammon dans la Thebaide; Bachatly, C., Ed.; de la Societe d’Archeologie Copte: Cairo, Egypt, 1961. [Google Scholar]
- Ahmed, B.; Jan, Q.; Bashir, S.; Choudhary, M.I.; Nisar, M. Phytochemical evaluation of Chenopodium murale Linn. Asian J. Plant Sci. 2003, 2, 1072–1078. [Google Scholar]
- Ibrahim, L.; Kawashty, S.; Baiuomy, A.R.; Shabana, M.; El-Eraky, W.; El-Negoumy, S. A comparative study of the flavonoids and some biological activities of two Chenopodium species. Chem. Nat. Compd. 2007, 43, 24–28. [Google Scholar] [CrossRef]
- Javaid, A.; Amin, M. Antifungal activity of methanol and n-hexane extracts of three Chenopodium species against Macrophomina phaseolina. Nat. Prod. Res. 2009, 23, 1120–1127. [Google Scholar] [CrossRef]
- Albaser, N.A.; Thabit, A.A.M.; AL-Ghani, A.M.S. Yemeni medicinal plants having anti-fungal activity: Review study. World J. Pharma. Res. 2020, 9, 212–239. [Google Scholar]
- Gohar, A.A.; Elmazar, M. Isolation of hypotensive flavonoids from Chenopodium species growing in Egypt. Phytother. Res. 1997, 11, 564–567. [Google Scholar] [CrossRef]
- Saleem, M.; Ahmed, B.; Qadir, M.I.; Mahrukh; Rafiq, M.; Ahmad, M.; Ahmad, B. Hepatoprotective effect of Chenopodium murale in mice. Bangladesh J. Pharmacol. 2014, 9, 124–128. [Google Scholar] [CrossRef]
- Abbas, M.; Rana, S.; Shahid, M.; Rana, N.; Mahmood-ul-Hassan, M.; Hussain, M. Chemical evaluation of weed seeds mixed with wheat grains at harvest. J. Animal Plant Sci. 2012, 22, 283–288. [Google Scholar]
- Ahmed, O.H.; Hamad, M.N.; Jaafar, N.S. Phytochemical investigation of Chenopodium Murale (family: Chenopodiaceae) cultivated in Iraq, isolation and identification of scopoletin and gallic acid. Asian J. Pharm Clin. Res. 2017, 10, 70–77. [Google Scholar] [CrossRef]
- Abd Elkarim, A.S.; Ahmed, A.H.; Taie, H.A.A.; Elgamal, A.M.; Abu-elghait, M.; Shabana, S. Synadenium grantii hook f.: HPLC/QTOF-MS/MS tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan. J. Chem. 2021, 14, 811–828. [Google Scholar] [CrossRef]
- Yılmaz, P.K.; Ertaş, A.; Akdeniz, M.; Avcı, M.K.; Kolak, U. Chemical compositions by LC-MS/MS and GC-MS and biological activities of Chenopodium album subsp. Album var. Microphyllum. Ind. Crops Prod. 2019, 141, 111755. [Google Scholar] [CrossRef]
- Podolak, I.; Olech, M.; Galanty, A.; Załuski, D.; Grabowska, K.; Sobolewska, D.; Michalik, M.; Nowak, R. Flavonoid and phenolic acid profile by LC-MS/MS and biological activity of crude extracts from Chenopodium hybridum aerial parts. Nat. Prod. Res. 2016, 30, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef]
- Nasser, N.M.; Al-Araji, M.; Al-Ani, W.M.K. Antifungal activity of mellilotus officinalis of Iraq. J. Chem. Pharm. Rese. 2014, 6, 611–617. [Google Scholar]
- Zhuan-Hong, L.; Han, G.; Wen-Bin, X.; Juan, G.; Xin, L.; Mireguli, A.; Da-Jun, H. Rapid Identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC-ESI-QTOF-MS-MS. J. Chrom. Sci. 2016, 54, 805–810. [Google Scholar]
- Karioti, A.; Bilia, A.R.; Skaltsa, H. Quercus ilex L.: A rich source of polyacylated flavonoid glucosides. Food Chem. 2010, 123, 131–142. [Google Scholar] [CrossRef]
- Felipe, D.F.; Brambilla, L.Z.S.; Porto, C.; Pilau, E.J.; Cortez, D.A.G. Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules 2014, 19, 15720–15734. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Kite, G.C.; Veitch, N.C. Identification of common glycosyl groups of flavonoid O-glycosides by serial mass spectrometry of sodiated species. Rapid Commun. Mass Spectrom. 2011, 25, 2579–2590. [Google Scholar] [CrossRef]
- Enge, C.; Gräter, D.; Esquive, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra-high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Chung, H.J.; Lim, S.Y.; Kim, I.S.; Bu, Y.M.; Kim, H.C.; Kim, D.H.; Yoo, H.H. Simultaneous determination of baicalein, baicalin, wogonin, and wogonoside in rat plasma by LC-MS/MS for studying the pharmacokinetics of the standardized extract of Scutellariae Radix. Bull. Korean Chem. Soc. 2012, 33, 177–182. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.; Areche, C.; Sepúlveda, B.; Simirgiotis, M. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Cao-Ngoc, P.; Leclercq, L.; Rossi, J.C.; Hertzog, J.; Tixier, A.S.; Chemat, F.; Nasreddine, R.; Al Hamoui, G.; Banni, D.; Nehmé, R.; et al. Water-based extraction of bioactive principles from blackcurrant leaves and Chrysanthellum americanum: A comparative study. Foods 2020, 9, 1478. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; Laboratoire de Pharmacognosie, Universite Catholique de Louvain, Brussels, Belgium. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Kazuma, K.; Kogawa, K.; Noda, N.; Kato, N.; Suzuk, M. Identification of delphinidin 3-O-(6-O-Malonyl)-glucoside-3-O-glucoside, a postulated intermediate in the biosynthesis of ternatin C5in the blue petals of Clitoria ternatea (Butterfly Pea). Chem. Biodiv. 2004, 1, 1760–1770. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Arbeiter, A.B.; Peeters, K.; Višnjevec, A.M.; Pražnikar, Z.J. HPLC-DAD-ESI-QTOF-MS Determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T.; Nikolov, S.D. The Genus Chenopodium: Phytochemistry, ethnopharmacology and pharmacology. Phcog Rev. 2009, 3, 280–306. [Google Scholar]
- Mulley, A.G.; Goroll, A.H. Primary Care Medicine: Office Evaluation and Management of Adult Patients; Wolters Kluwer Health: Philadelphia, PA, USA, 2006; pp. 802–803. ISBN 978-0-7817-7456-7. [Google Scholar]
- Paderi, A.; Gambale, E.; Botteri, C.; Giorgione, R.; Lavacchi, D.; Brugia, M.; Mazzoni, F.; Giommoni, E.; Bormioli, S.; Amedei, A.; et al. Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis. Molecules 2021, 26, 5789. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Peterson, A.C.; Brane, L.; Huppler, A.R.; Hernandez-Santos, N.; Whibley, N.; Garg, A.V.; Simpson-Abelson, M.R.; Gibson, G.A.; Mamo, A.J. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic candida albicans infections. J. Exp. Med. 2014, 211, 2075–2084. [Google Scholar] [CrossRef]
- Zucchini, N.; Crozat, K.; Baranek, T.; Robbins, S.H.; Altfeld, M.; Dalod, M. Natural killer cells in immunodefense against infective agents. Expert. Rev. Anti. Infect. Ther. 2008, 6, 867–885. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; My-Chan Dang, P. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- De Luca, A.; Iannitti, R.G.; Bozza, S.; Beau, R.; Casagrande, A.; D’Angelo, C.; Moretti, S.; Cunha, C.; Giovannini, G.; Massi-Benedetti, C. CD4þ T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease. J. Clin. Investig. 2012, 122, 1816–1831. [Google Scholar] [CrossRef]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef]
- Shukla, M.; Chandley, P.; Rohatgi, S. The role of B-cells and antibodies against Candida vaccine antigens in invasive candidiasis. Vaccines 2021, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.L.; Krylov, V.B.; Khlusevich, Y.A.; Baykov, I.K.; Yashunsky, V.; Emelyanova, L.A.; Tsvetkov, Y.E.; Karelin, A.A.; Bardashova, A.V.; Wong, S.S.W. Novel mouse monoclonal antibodies specifically recognizing B-(1–3)-D-glucan antigen. PLoS ONE 2019, 14, e0215535. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Rovasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Sparber, F.; LeibundGut-Landmann, S. Interleukin 17-mediated host defense against Candida albicans. Pathogens 2015, 4, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.; Mrowietz, U.; Puig, L.; Zachariae, C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 2017, 177, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef]
- Conti, H.R.; Bruno, V.M.; Childs, E.E.; Daugherty, S.; Hunter, J.P.; Mengesha, B.G.; Saevig, D.L.; Hendricks, M.R.; Coleman, B.M.; Brane, L. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003, 278, 17036–17043. [Google Scholar] [CrossRef]
- Cuneyt, V.; Roberts, R.L.; Stiehm, R. The syndrome of chronic mucocutaneous candidiasis with selective antibody deficiency. Ann. Allergy Asthma Immunol. 2003, 90, 259–264. [Google Scholar]
- Lilic, D.; Gravenor, I.; Robson, N.; Lammas, D.A.; Drysdale, P.; Calvert, J.E.; Cant, A.J.; Abinun, M. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect. Immun. 2003, 71, 5690–5699. [Google Scholar] [CrossRef] [PubMed]
- Beno, D.W.A.; Mathews, H.L. Growth inhibition of Candida Albicans by interleukin-2-activated splenocytes. Infect. Immun. 1992, 60, 853–886. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, C.; Graikou, K.; Chinou, I. Chemosystematic Value of Chemical Constituents from Scabiosa hymettia (Dipsacaceae). Chem. Biodivers. 2008, 5, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.; Tamokou Jde, D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, M.; Dimitrova, P.; Patkar, S.; Saso, L.; Ivanovska, N. Inhibition of Candida albicans extracellular enzyme activity by selected natural substances and their application in Candida infection. Can. J. Microbiol. 2008, 54, 435–440. [Google Scholar] [CrossRef]
- Pushkala, V.P.; Sulekha, S.M.P.; Mathukumar, S.; Ragavi, B.; Sowmiya, U. Molecular Docking Analysis of Siddha Formulation Parangipattai Chooranam Against Vaginal Candidiasis. Appl. Biochem. Biotechnol. 2022, 194, 1039–1050. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Da Silva, C.R.; Valente Sa, L.G.A.; Dos Santos, E.V.; Ferreira, T.L.; Coutinho, T.N.P.; Moreira, L.E.A.; Sousa, R.C.; Andrade, C.R.; Silva, W.M.B.; Carneiro, I.; et al. Evaluation of the antifungal effect of chlorogenic acid against strains of Candida spp. resistant to fluconazole: Apoptosis induction and in silico analysis of the possible mechanisms of action. J. Med. Microbiol. 2022, 71, 5. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Flavones, flavonols, and glycosylated derivatives-impact on Candida albicans growth and virulence, expression of CDR1 and ERG11, cytotoxicity. Pharmaceuticals 2021, 14, 27. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, E.R.; Lee, D.G. Effect of apigenin isolated from Aster yomena against Candida albicans: Apigenin-triggered apoptotic pathway regulated by mitochondrial calcium signaling. J. Ethnopharmacol. 2019, 231, 19–28. [Google Scholar] [CrossRef]
- Serpa, R.; France, E.J.G.; Furlaneto-Maia, L.; Andrade, C.G.T.J.; Diniz, A.; Furlaneto, M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. J. Med. Microbiol. 2012, 61, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Mickymaray, S. Antifungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y. Myricetin disturbs the cell wall integrity and increases the membrane permeability of Candida albicans. J. Microbiol. Biotechnol. 2022, 32, 37–45. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals No. 423: Acute Oral Toxicity—Acute Toxic Class Method; OECD: Paris, France, 1996. [Google Scholar]
- Costa, A.C.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O. Recent mouse and rat methods for the study of experimental oral candidiasis. Virulence 2013, 4, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.N. Satistical Analysis, Fundamentals of Experimental Pharmacology; Scientific Book Agency: Calcutta, India, 1984; pp. 189–190. [Google Scholar]
- Garg, R.; Kumar, R.; Nathiya, D.; Goshain, O.V.; Trivedi, V.; Sharma, A.K.; Murti, K. Comparative acute toxicity studies of selected indigenous herbal plants in Swiss albino mice. IOSR J. Pharm. Biol. Sci. 2016, 11, 20–27. [Google Scholar]
- Chami, N.; Chami, F.; Bennis, S.; Trouillas, J.; Remmal, A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz. J. Infect. Dis. 2004, 8, 217–226. [Google Scholar] [CrossRef]
- Van Pelt, L.F. Ketamine and xylazine for surgical anesthesia in rats. J. Am. Vet. Med. Assoc. 1977, 171, 842–844. [Google Scholar]
- Dacie, J.V.; Lewis, S.M. Laboratory investigation in hemolytic anemia in practical haematology. In Practical Haematology, 5th ed.; Churchill Livingstone: Edinburgh, Scotland, 1975; p. 40. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of H2O2 by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Adv. Enzymol. Relat. Areas. J. Mol. Biol. 1974, 41, 35–97. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological samples. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 11, 764–775. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteureagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effect on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Onwuka, G.I. Soaking, boiling and anti-nutritional factors in pigeon pea (Cajanus cajan) and cowpea (Vigna unguiculata). J. Food Process. Preserv. 2006, 30, 616–630. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 48, 788–794. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical profiling, in vitro and in silico anti-microbial and anti-cancer activity evaluations and staph gyraseb and h-top-iiβ receptor-docking studies of major constituents of Zygophyllum coccineum L. aqueous-ethanolic extract and its subsequent fractions: An approach to validate traditional phytomedicinal knowledge. Molecules 2021, 26, 577. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some African plants belonging to Kalanchoe species: Antitrypanosomal, cytotoxic, antitopoisomerase activities and chemical profiling using. Pharmacogn. Mag. 2021, 17, 6–14. [Google Scholar] [CrossRef]
| Groups | Strength | CFU/Petri |
|---|---|---|
| Negative control | +ve | 11.33 ± 1.53 |
| C. murale (0.5 g/kg) ve+ | −ve | 1.00 ± 0.09 |
| C. murale (1.0 g/kg) ve+ | −ve | 0.87 ± 0.07 |
| Candida control | +++ | 5.86 × 104 ± 1.21 |
| C. murale (0.5 g/kg)-treated | ++ | 236.67 ± 37.86 |
| C. murale (1.0 g/kg)-treated | + | 4.33 ± 0.58 |
| Nystatin (100,000 units) | −ve | 1.67 ± 0.29 |
| Groups | Hemoglobin (g/dL) | RBC (×1012/L) | WBC (×109/L) | Platelets (×109/L) | Lymphocyte % | Neutrophil % | Monocytes % | Eosinophil % | Basophil % |
|---|---|---|---|---|---|---|---|---|---|
| Negative control | 11.87 ± 0.16 ab | 5.89 ± 0.06 a | 8.60 ± 0.38 b | 436.50 ± 6.43 b | 63.60 ± 0.29 a | 27.25 ± 1.14 d | 6.07 ± 0.55 d | 2.69 ± 0.24 cd | 0.40 ± 0.04 c |
| C. murale (0.5 g/kg) ve+ | 12.83 ± 0.32 ab | 6.09 ± 0.16 ab | 6.97 ± 0.18 d | 448.35 ± 15.21 b | 59.17 ± 3.67 b | 30.73 ± 2.29 c | 6.99 ± 0.78 c | 2.66 ± 0.30 cd | 0.44 ± 0.05 c |
| C. murale (1.0 g/kg) ve+ | 11.80 ± 0.24 ab | 5.93 ± 0.03 ab | 7.23 ± 0.44 d | 525.00 ± 1.48 a | 54.99 ± 0.81 c | 35.07 ± 1.01 a | 6.88 ± 0.51 c | 2.62 ± 0.19 d | 0.43 ± 0.03 c |
| Candida control | 12.68 ± 0.96 ab | 5.43 ± 0.34 b | 13.30 ± 1.50 a | 194.75 ± 5.14 e | 59.07 ± 4.22 b | 26.50 ± 2.44 d | 9.56 ± 0.79 a | 4.24 ± 0.35 a | 0.63 ± 0.05 a |
| C. murale (0.5 g/kg)-treated | 10.13 ± 0.29 b | 5.19 ± 0.07 b | 4.50 ± 0.23 e | 234.99 ± 5.06 d | 55.65 ± 0.17 c | 32.92 ± 1.92 b | 8.03 ± 0.90 b | 2.90 ± 0.33 c | 0.50 ± 0.06 b |
| C. murale (1.0 g/kg)-treated | 12.70 ± 0.15 ab | 5.98 ± 0.12 ab | 4.77 ± 0.64 e | 321.98 ± 8.33 c | 55.18 ± 0.66 c | 35.68 ± 1.77 a | 6.42 ± 0.59 cd | 2.32 ± 0.21 e | 0.40 ± 0.04 c |
| Nystatin (100,000 units) | 13.33 ± 0.59 a | 6.43 ± 0.18 a | 7.85 ± 0.74 c | 320.76 ± 2.72 c | 57.17 ± 3.97 bc | 31.27 ± 2.55 bc | 7.66 ± 0.68 b | 3.40 ± 0.37 b | 0.51 ± 0.05 b |
| Groups | Catalase (CAT) | Superoxide Dismutase (SOD) | ||||
|---|---|---|---|---|---|---|
| Serum (U/L). | Mucosa (U/mg Protein) | Spleen (U/mg Protein) | Serum (U/mL). | Mucosa (U/mg Protein) | Spleen (U/mg Protein) | |
| Negative control | 250.42 ± 2.02 f | 12.98 ± 0.58 d | 12.77 ± 0.42 b | 272.08 ± 3.43 c | 8.00 ± 0.08 c | 5.72 ± 0.16 bc |
| C. murale (0.5 g/kg) ve+ | 353.92 ± 3.17 e | 12.76 ± 0.18 d | 13.79 ± 1.03 b | 287.18 ± 3.43 ab | 8.83 ± 0.07 bc | 6.09 ± 0.16 b |
| C. murale (1.0 g/kg) ve+ | 379.85 ± 2.85 d | 14.29 ± 0.33 c | 19.16 ± 0.39 a | 300.98 ± 0.48 a | 13.73 ± 0.36 a | 6.14 ± 0.36 b |
| Candida control | 202.43 ± 8.80 g | 7.67 ± 0.39 e | 12.74 ± 0.16 b | 226.52 ± 3.94 d | 6.00 ± 0.05 d | 5.01 ± 0.05 c |
| C. murale (0.5 g/kg)-treated | 498.50 ± 4.30 b | 17.16 ± 0.19 ab | 15.81 ± 0.47 b | 289.16 ± 1.57 ab | 9.23 ± 0.07 b | 6.29 ± 0.06 b |
| C. murale (1.0 g/kg)-treated | 550.86 ± 3.61 a | 18.09 ± 0.80 a | 20.87 ± 1.19 a | 297.55 ± 2.27 a | 13.88 ± 0.16 a | 5.79 ± 0.10 bc |
| Nystatin (100,000 units) | 406.36 ± 4.53 c | 15.75 ± 0.19 bc | 13.50 ± 0.32 b | 281.37 ± 1.98 bc | 8.81 ± 0.17 bc | 7.49 ± 0.30 a |
| Groups | Hydrogen Peroxide (H2O2) | Malondialdehyde (MDA) | Nitric Oxide Concentration (NO) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum (mM/L) | Mucosa (mM/g) | Spleen (mM/g) | Serum (nmol/mL) | Spleen (nmol/g) | Mucosa (nmol/g) | Serum (µmol/L) | Mucosa (µmol/g) | Spleen (µmol/g) | |
| Negative control | 30.80 ± 0.32 d | 1.42 ± 0.03 d | 0.655 ± 0.02 b | 1.88 ± 0.06 b | 1.17 ± 0.04 bc | 2.52 ± 0.08 b | 19.77 ± 1.66 f | 2.91 ± 0.24 c | 0.87 ± 0.06 d |
| C. murale (0.5 g/kg) ve+ | 27.11 ± 0.60 e | 1.44 ± 0.04 d | 0.40 ± 0.01 de | 1.56 ± 0.04 bc | 0.95 ± 0.03 d | 1.95 ± 0.05 d | 32.97 ± 2.16 d | 3.19 ± 0.52 bc | 0.73 ± 0.04 e |
| C. murale (1.0 g/kg) ve+ | 26.45 ± 0.59 e | 1.34 ± 0.03 d | 0.37 ± 0.01 e | 1.47 ± 0.03 c | 0.98 ± 0.02 cd | 1.77 ± 0.04 c | 37.05 ± 2.02 c | 3.06 ± 0.12 bc | 0.82 ± 0.03 de |
| Candida control | 35.15 ± 0.93 c | 3.82 ± 0.13 a | 1.80 ± 0.03 a | 6.32 ± 0.11 a | 4.85 ± 0.08 a | 3.53 ± 0.14 a | 28.73 ± 2.11 e | 3.52 ± 0.25 c | 1.22 ± 0.07 b |
| C. murale (0.5 g/kg)-treated | 42.359 ± 0.61 b | 2.45 ± 0.08 bc | 0.47 ± 0.02 cd | 1.75 ± 0.09 bc | 1.23 ± 0.06 b | 2.45 ± 0.12 b | 42.843 ± 1.06 b | 3.38 ± 0.2 bc | 1.09 ± 0.02 c |
| C. murale (1.0 g/kg)-treated | 63.31 ± 0.92 a | 2.68 ± 0.04 b | 0.40 ± 0.02 de | 1.80 ± 0.03 b | 1.22 ± 0.02 b | 2.48 ± 0.05 b | 57.60 ± 2.40 a | 5.94 ± 0.21 a | 1.41 ± 0.04 a |
| Nystatin (100,000 units) | 26.51 ± 0.36 e | 2.22 ± 0.11 c | 0.53 ± 0.01 c | 1.75 ± 0.02 bc | 1.31 ± 0.02 b | 2.45 ± 0.03 b | 44.66 ± 1.83 b | 5.55 ± 0.32 a | 1.47 ± 0.11 a |
| Yield and Physical Characters | Ethanol 70% |
|---|---|
| Yield (g, %) | 10.00 g extract/100 g dried powder |
| Color | Light green |
| Odor | Like-spinach |
| Condition | powder |
| Phenols | 66.33 ± 0.88 mg gallic acid/g extract |
| Flavonoids | 44.45 ± 1.45 mg quercetin /g extract |
| Tannins | 14.49 ± 0.70 mg tannic acid/g extract |
| Alkaloids | 200.43 ± 8.60 mg alkaloid/g extract |
| No | RT(min) | Name | Class | [M − H]− m/z | Diff (ppm) | MF | MS2 | Rel. Conc. (%) |
|---|---|---|---|---|---|---|---|---|
| Flavonol glycosides | ||||||||
| 1 | 5.073 | Quercetin 3-O-rhamnoside (Quercitrin) | Flavonol mono-glycosides | 447.0901 | −1 | C21H20O11 | 301.0344, 273.0396, 151.0023 | 0.07528807 |
| 2 | 5.230 | Kaempferol-3-O-glucuronide | Flavonol glycosides | 461.0726 | 0.7 | C21H18O12 | 285.0389 | 0.020850167 |
| 3 | 6.256 | Kaempferol-7-O-neohesperidoside | Flavonol di-glycosides | 593.1327 | C27H30O15 | 431.0858 285.0331 | 0.34005973 * | |
| 4 | 6.506 | kaempferol-3-O-robinoside-7-O-rhamnoside | Flavonol glycosides | 739.2022 | 1.7 | C33H40O19 | 593.1429, 431.0969, 285.0380 | 0.26219089 * |
| 5 | 5.559 | Kaempferol-3-O-di-glucoside | Flavonol di-glycosides | 609.1412 | - | C27H30O16 | 447.0953, 285.0495 | 0.32304453 * |
| 6 | 6.865 | Kaempferol-3-O-glucoside | Flavonol glycosides | 447.0907 | C21H20O11 | 285.0373, 284.0337 | 0.19927864 * | |
| 7 | 6.902 | Kaempferol-3,7-O-bis-α-L-rhamnoside | Flavonol mono glycosides | 577.01543 | 0.7 | C27H30O14 | 431.0969, 285.0405 | 0.24868134 * |
| 8 | 6.941 | Kaempferol-3-O-(6-p-coumaroyl)-glucoside | Flavonol glycosides | 593.1433 | 1.8 | C30H26O13 | 447.1257, 431.1092, 307.000, 285.0409 | 1.49217077 ** |
| 9 | 6.973 | Kaempferol-3-O-α-L-rhamnoside (Afzelin) | Flavonol mono-glycosides | 431.0859 | −1 | C21H19O10 | 284.9331, 255.0280, 227.0275 | 0.21337071 * |
| 10 | 10.062 | 3,5,7-trihydroxy-4′-methoxyflavone (Kaempferide) | methoxy flavonol | 299.0561 | −2.5 | C16H12O6 | 284.0330,253.2070, 271.0268, 183.488 | 0.04408804 |
| Flavones glycosides | ||||||||
| 11 | 5.856 | Baicalein-7-O-glucuronide | Flavone | 445.0777 | 0 | C21H18O11 | 269.0482, 175.0253, 113.0260 | 0.06855429 |
| 12 | 6.939 | Luteolin-8-C-glucoside | Flavone | 447.0964 | C21H20O11 | 327.0544, 285.0401 | 0.39543838 * | |
| 13 | 7.730 | Apigenin-7-O-glucoside | Flavone | 431.0980 | 00 | C21H20O10 | 269.0482, 225.0428, 151.0160 | 0.1863942 * |
| 14 | 12.590 | 3′-methoxy-4′,5, 7-trihydroxyflavonol | Flavone | 315.1239 | C16H12O6 | 300.236, 246.8960, 163.0411 | 0.264702 * | |
| 15 | 14.036 | Acacetin | 4′-O-methylated flavone | 283.0618 | 5.8 | C16H12O5 | 268.0374, 252.0737, 151, 171, 211, 240, 239 | 0.02014899 |
| 16 | 18.155 | Luteolin-7-O-glucoside | Flavone | 447.887 | −1.7 | C21H20O11 | 285.0400, 242.9547,151.05012 | 0.17331039 * |
| Aglycones | ||||||||
| 17 | 9.254 | Luteolin | Flavone | 285.415 | −28.6 | C15H10O6 | 217.0430, 199.0415, 175.0441, 151.0134 | 0.04887441 |
| 18 | 15.073 | Kaempferol | Flavonol | 285.0401 | C15H10O6 | 151.0122,133.0329, 216.9352 | 0.18664631 * | |
| 19 | 22.721 | Quercetin | Flavonol | 301.328 | 0.5 | C15H10O7 | 255.2321, 178.9976,151.0041, 121.0311 | 0.171138974 * |
| 20 | 26.325 | Myricetin | Flavonol | 316.1284 | 7.4 | C15H10O8 | 301.0313, 248.9645, 151.00, 112.9864 | 0.2735515 * |
| 21 | 26.168 | Apigenin | Flavone | 269.0440 | 33.3 | C15H10O5 | 151.0035, 117.0290, 107.0172 | 0.252827 * |
| Anthocyanin, flavanones and Aurone O-glycosides | ||||||||
| 22 | 5.750 | Delphinidin-3-O-(6″-O-alpha-rhamnopyranosyl-β-glucopyranoside | Anthocyanin | 609.1418 | 40.3 | C27H31O+16 | 447.0959, 463.0886, 301.0399, 299.0234 | 0.09779782 * |
| 23 | 9.390 | Isosakuranetin-7-O-neohesperidoside (Poncirin) | Flavanones | 593. 1327 | 1 | C28H34O14 | 447.0835, 285.0331 | 0.01449699 |
| 24 | 7.180 | Maritimetin-6-O-glucoside | Flavonoid (Aurone O-glycosides) | 447.0932 | −0.7 | C21H20O11 | 285.0400, 151.0000, 133.0000 | 0.44026256 * |
| 25 | 10.001 | Naringenin | Flavanones | 271.0602 | −1.2 | C15H12O5 | 151.0032, 177.071 | 0.06571804 |
| Phenolic acids | ||||||||
| 26 | 1.129 | Quinic acid | Phenolic acid | 191.0549 | 16.4 | C7H12O6 | 173.0448, 109.0276 | 0.2669481 * |
| 27 | 1.259 | Chlorogenic acid | Phenolic acid | 353.0887 | 2.7 | C16H18O9 | 191.0557, 173.0461 | 0.38734244 * |
| 28 | 2.257 | Caffeic acid | Phenolic acid | 179.0276 | −0.3 | C9H8O4 | 135.0455 | 0.03597749 |
| 29 | 2.953 | Salicylic acid | Phenolic acid | 137.035 | −0.9 | C7H6O3 | 93.0339 | 0.04471586 |
| 30 | 4.173 | P-Hydroxybenzoic acid | Phenolic acid | 137.0258 | −0.5 | C7H6O3 | 119.0111, 93.0000 | 0.02646657 |
| 31 | 4.559 | 3,4-Dihydroxybenzoic acid | Phenolic acid | 153.0199 | 1.7 | C7H6O4 | 135.0092, 109.0300 | 0.21523038 |
| Fatty acids | ||||||||
| 32 | 23. 293 | Lactic acid | Fatty acids | 89.0245 | 2.3 | C3H6O3 | Not fragment | 0.48174368 * |
| 33 | 1.522 | Citraconic acid | Fatty acids | 128.9882 | 1.9 | C5H6O4 | Not fragment | 0.3839927 * |
| 34 | 1.91 | D-3-Phenyllactic acid | Fatty acids | 165.0403 | 2.8 | C9H10O3 | 147.0330 | 0.11360878 * |
| 35 | 2.252 | Glutaric acid | Fatty acids | 131.0695 | −7.3 | C5H8O4 | 86.9960 | 0.00505673 |
| 36 | 2.271 | Suberic acid | Fatty acids | 173.1171 | 0.2 | C8H14O4 | Not fragment | 0.01325589 |
| Sugars | ||||||||
| 37 | 1.045 | L-(+)-Tartrate | Sugars | 148.9536 | −1.7 | C4H6O6 | Not fragment | 0.02088193 |
| 38 | 4.0873 | D-(+)-Raffinose | Oligosaccharides | 503.0786 | −2.8 | C18H32O16 | Not fragment | 0.00989468 |
| 39 | 8.792 | D-(+)-Galacturonic acid | Sugars | 193.0507 | −7.7 | C6H10O7 | 133.0303, 121.0302 | 0.12838032 * |
| Organic compounds and dihydrochalcone glucosides | ||||||||
| 40 | 3.210 | Xanthine | Xanthinones | 151.387 | C5H4N4O2 | 107.418 | 0.00863697 | |
| 41 | 7.775 | P-Nitro phenol | phenols | 138.202 | C6H5NO3 | 108.0224, 92.02 | 0.01166895 | |
| 42 | 7.996 | Phlorizin | dihydrochalcone glucosides | 435.1151 | C21H24O10 | 273.0760 | 0.00015185 | |
| Stages | Experimental Days | Protocols |
|---|---|---|
| Adaptation period | 1st–7th days |
|
| Immunosuppression stage | 8th–14th days | Animals received dexamethasone (0.5 mg/ L) and 0.1% tetracycline in drinking water |
| Infection stage | 15th day |
|
| 1st insemination | 16th day | |
| 17th day | ||
| 2nd insemination | 18th day | |
| 19th day | ||
| 3rd insemination | 20th day | |
| 1st Sampling for Candida detection | In day 21 | |
| Treatment stage | In day 22 |
|
| In day 23 | ||
| In day 24 | ||
| In day 25 | ||
| In day 26 | ||
| In day 27 | ||
| In day 28 | ||
| 2nd Sampling for Candida detection | In day 29 | The end of experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Newary, S.A.; Abd Elkarim, A.S.; Abdelwahed, N.A.M.; Omer, E.A.; Elgamal, A.M.; ELsayed, W.M. Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile. Molecules 2023, 28, 4304. https://doi.org/10.3390/molecules28114304
El-Newary SA, Abd Elkarim AS, Abdelwahed NAM, Omer EA, Elgamal AM, ELsayed WM. Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile. Molecules. 2023; 28(11):4304. https://doi.org/10.3390/molecules28114304
Chicago/Turabian StyleEl-Newary, Samah A., Asmaa S. Abd Elkarim, Nayera A. M. Abdelwahed, Elsayed A. Omer, Abdelbaset M. Elgamal, and Wael M. ELsayed. 2023. "Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile" Molecules 28, no. 11: 4304. https://doi.org/10.3390/molecules28114304
APA StyleEl-Newary, S. A., Abd Elkarim, A. S., Abdelwahed, N. A. M., Omer, E. A., Elgamal, A. M., & ELsayed, W. M. (2023). Chenopodium murale Juice Shows Anti-Fungal Efficacy in Experimental Oral Candidiasis in Immunosuppressed Rats in Relation to Its Chemical Profile. Molecules, 28(11), 4304. https://doi.org/10.3390/molecules28114304






