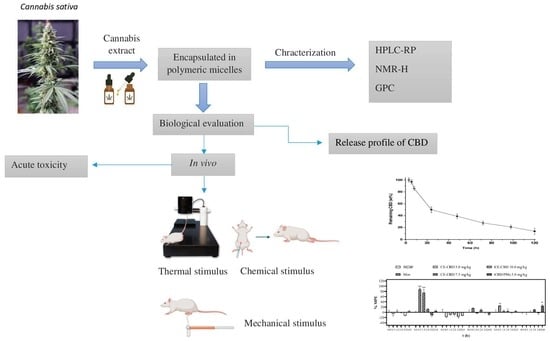
Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of CBD/PMs
2.2. Acute Toxicity Test
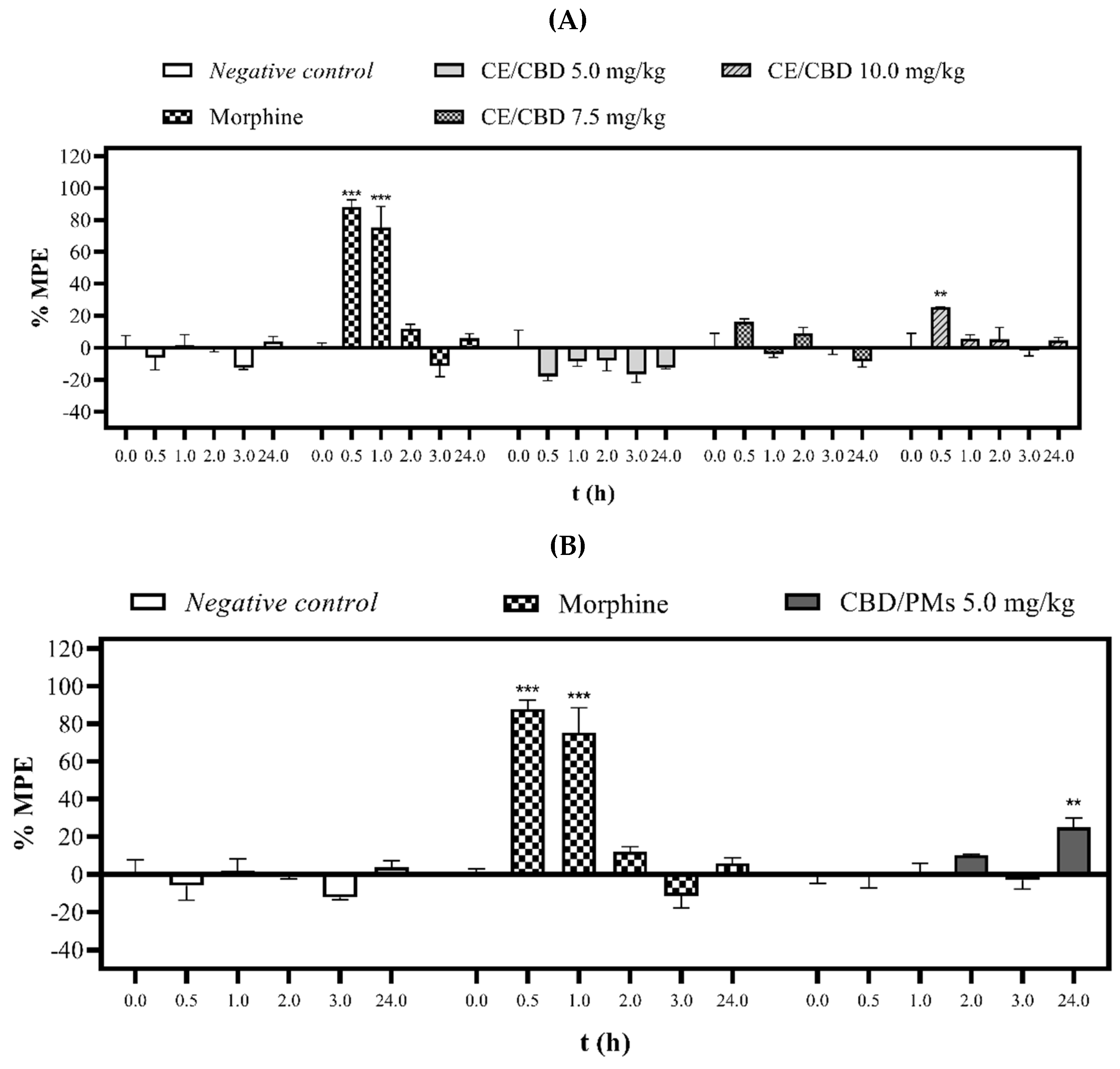
2.3. Evaluation of Analgesic Activity
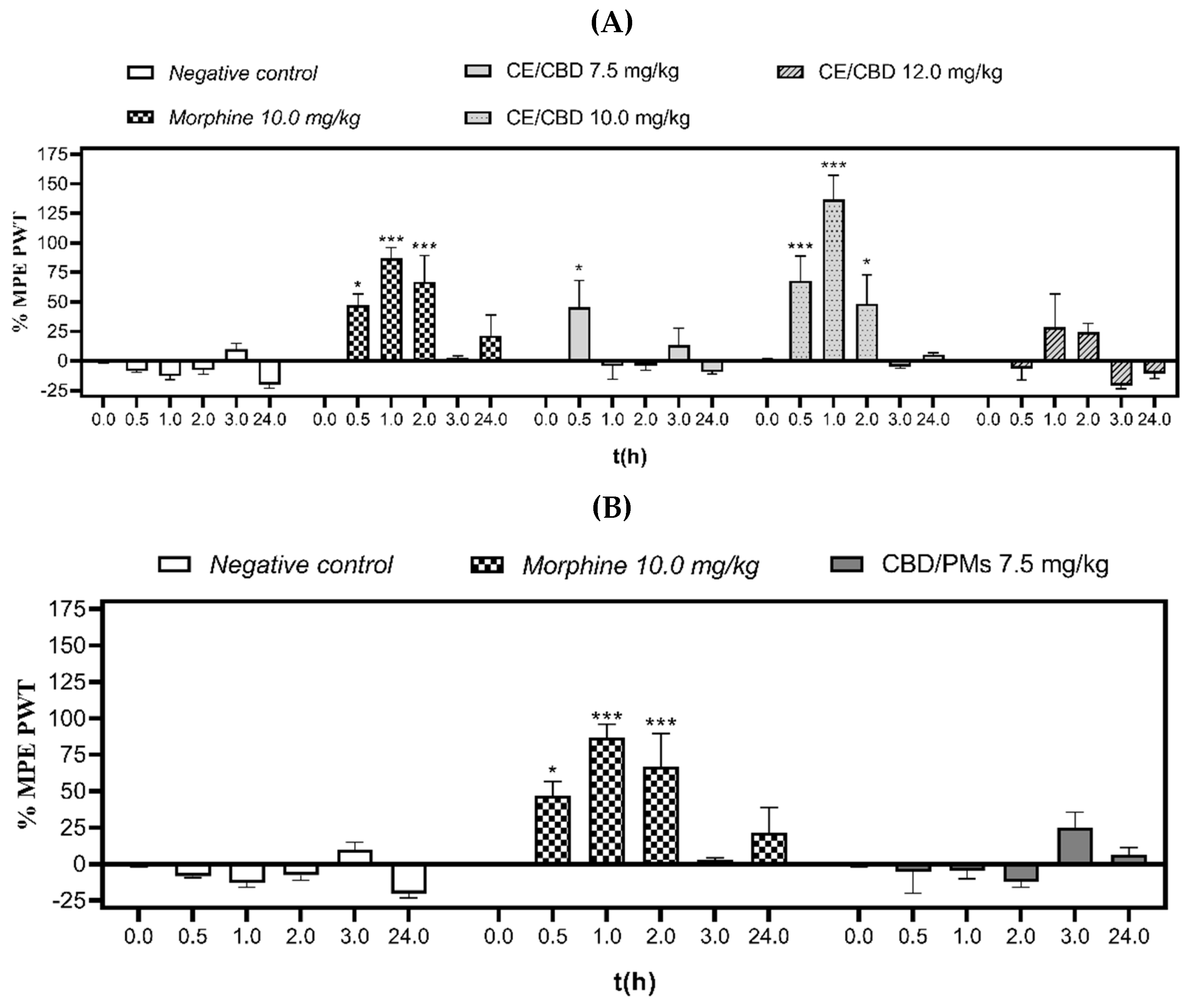
2.3.1. Tail-Flick Test
2.3.2. Electronic von Frey Test
2.3.3. Phenylquinone-Induced Writhing Test
3. Materials and Methods
3.1. Drugs and Chemicals
3.2. Synthesis and Characterization of PEG-Block-PCL Copolymer (PEG-b-PCL)
3.3. Preparation of CBD/PMs
3.4. Drug-Load Determination
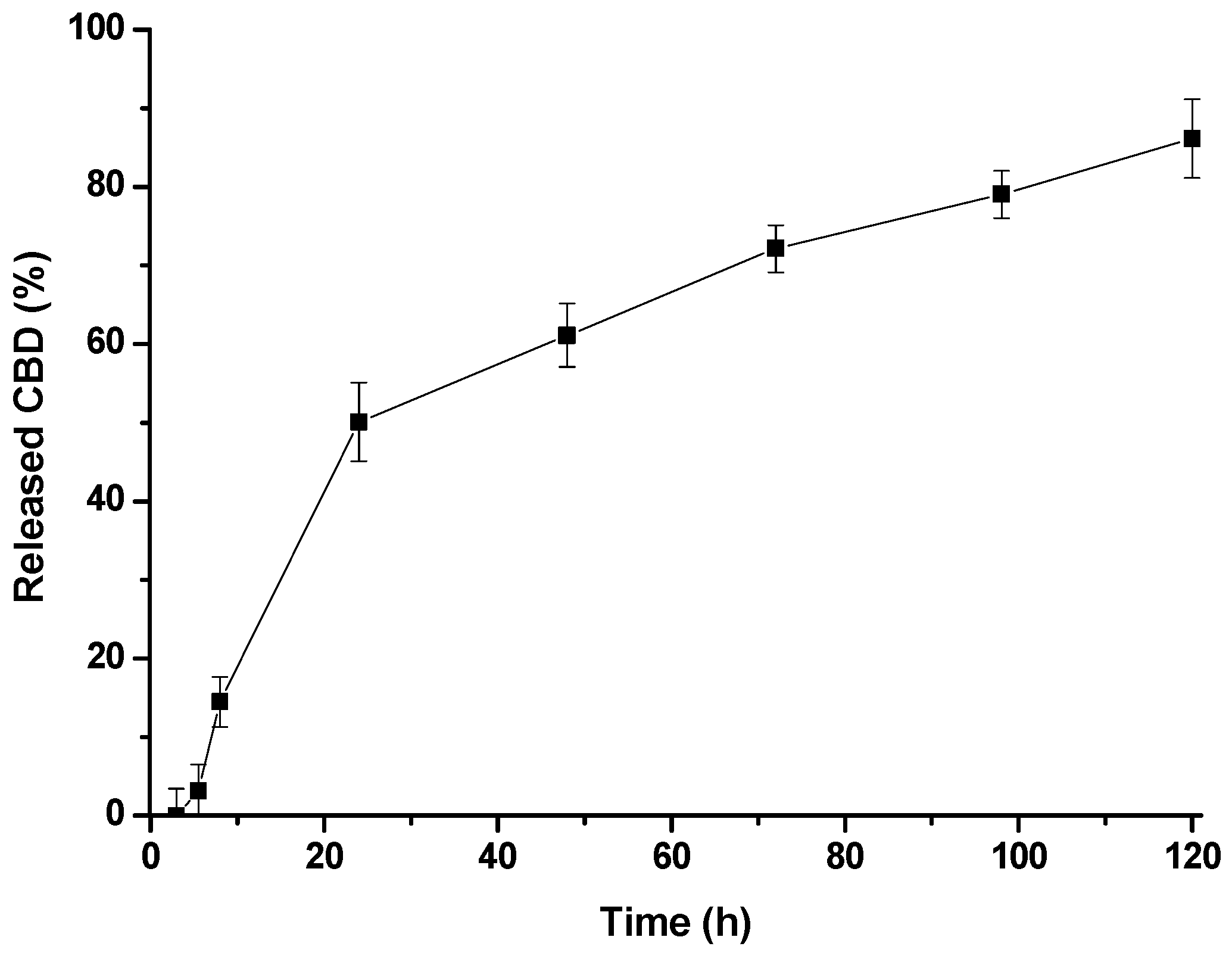
3.5. CBD Release Assay
3.6. Animals
3.7. Acute Toxicity Test
3.8. Tail-Flick Test
3.9. Electronic von Frey Behavioral Test
3.10. Phenylquinone-Induced Writhing Test
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Elman, I.; Borsook, D. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev. 2013, 39, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.K.; Tam, K.L.; Bekker, A.; Zuo, W.; Ye, J.-H. Cannabinoids in Opioid Addiction Treatment: Pharmacological Mechanisms. J. Alcohol Drug Depend. 2019, 7, 4. [Google Scholar] [CrossRef]
- Shmagel, A.; Ngo, L.; Ensrud, K.; Foley, R. Prescription Medication Use Among Community-Based U.S. Adults with Chronic Low Back Pain: A Cross-Sectional Population Based Study. J. Pain 2018, 19, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Beyth, R.J.; Shorr, R.I. Epidemiology of adverse drug reactions in the elderly by drug class. Drugs Aging 1999, 14, 231–239. [Google Scholar] [CrossRef]
- Schwan, J.; Sclafani, J.; Tawfik, V.L. Chronic Pain Management in the Elderly. Anesthesiol. Clin. 2019, 37, 547–560. [Google Scholar] [CrossRef]
- Sofia, R.D.; Vassar, H.B.; Knobloch, L.C. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacology 1975, 40, 285–295. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- Robson, P.J. Therapeutic potential of cannabinoid medicines. Drug Test. Anal. 2013, 6, 24–30. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Überall, M.A. A review of scientific evidence for THC:CBD oromucosal spray (nabiximols) in the management of chronic pain. J. Pain Res. 2020, 13, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a treatment for arthritis and joint pain: An exploratory cross-sectional study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Monhemius, R.; Azami, J.; Green, D.L.; Roberts, M.H.T. CB1 receptor mediated analgesia from the Nucleus Reticularis Gi-gantocellularis pars alpha is activated in an animal model of neuropathic pain. Brain Res. 2001, 908, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.M.N.; Araújo, T.C.D.L.; Pereira, E.C.V.; Neto, J.A.; Silva, D.S.; Brito, M.C.; Lima, K.S.B.; Monte, A.P.O.D.; de Matos, M.H.T.; de Lima, R.S.; et al. Investigation of antinociceptive, antipyretic, antiasthmatic, and spasmolytic activities of Brazilian Cannabis sativa L. roots in rodents. J. Ethnopharmacol. 2021, 278, 114259. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Maggini, V.; Calvi, L.; Pelagatti, T.; Gallo, E.R.; Civati, C.; Privitera, C.; Squillante, F.; Maniglia, P.; Di Candia, D.; Spampatti, R.; et al. An Optimized Terpene Profile for a New Medical Cannabis Oil. Pharmaceutics 2022, 14, 298. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of Synergistic Interaction between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res. 2018, 3, 120–135. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E. Cannabis and Cannabis Extracts. J. Cannabis Ther. 2001, 1, 103–132. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacol. Pharm. 2015, 06, 75–85. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Domb, A.J.; Ho, A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur. J. Pharm. Sci. 2017, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; A Barrett, D.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Durán-Lobato, M.; Martín-Banderas, L.; Lopes, R.; Gonçalves, L.M.D.; Fernández-Arévalo, M.; Almeida, A.J. Lipid nanoparticles as an emerging platform for cannabinoid delivery: Physicochemical optimization and biocompatibility. Drug Dev. Ind. Pharm. 2015, 42, 190–198. [Google Scholar] [CrossRef]
- Hadidi, M.; Rostamabadi, H.; Moreno, A.; Jafari, S.M. Nanoencapsulation of essential oils from industrial hemp (Cannabis sativa L.) by-products into alfalfa protein nanoparticles. Food Chem. 2022, 386, 123765. [Google Scholar] [CrossRef]
- Light, K.; Karboune, S. Emulsion, hydrogel and emulgel systems and novel applications in cannabinoid delivery: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 8199–8229. [Google Scholar] [CrossRef]
- Mihailova, L.; Tchekalarova, J.; Shalabalija, D.; Geskovski, N.; Gjorgievska, V.S.; Stefkov, G.; Krasteva, P.; Crcarevska, M.S.; Dodov, M.G. Lipid nano-carriers loaded with Cannabis sativa extract for epilepsy treatment – in vitro characterization and in vivo efficacy studies. J. Pharm. Sci. 2022, 111, 3384–3396. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Bethanis, K.; Koniari, E.; Christoforides, E.; Nikola, O.; Andreou, A.; Mantzou, A.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Lambrou, G.I. Biophysical Studies and In Vitro Effects of Tumor Cell Lines of Cannabidiol and Its Cyclodextrin Inclusion Complexes. Pharmaceutics 2022, 14, 706. [Google Scholar] [CrossRef]
- Phupaboon, S.; Matra, M.; Prommachart, R.; Totakul, P.; Supapong, C.; Wanapat, M. Extraction, Characterization, and Chitosan Microencapsulation of Bioactive Compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 2022, 11, 2103. [Google Scholar] [CrossRef]
- Sharkawy, A.; Silva, A.M.; Rodrigues, F.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/collagen peptides nanoparticles as green topical delivery vehicles for cannabidiol (CBD). Colloids Surfaces a Physicochem. Eng. Asp. 2021, 631, 127677. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Fernández-Arévalo, M.; Martín-Banderas, L. Development of enhanced drug delivery vehicles for three cannabis-based terpenes using poly(lactic-co-glycolic acid) based nanoparticles. Ind. Crop. Prod. 2021, 164. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Jansen, C.; Fernández-Arévalo, M.; Turner, H.; Martín-Banderas, L. Potential use for chronic pain: Poly(Ethylene Glycol)-Poly(Lactic-Co-Glycolic Acid) nanoparticles enhance the effects of Cannabis-Based terpenes on calcium influx in TRPV1-Expressing cells. Int. J. Pharm. 2022, 616, 121524. [Google Scholar] [CrossRef] [PubMed]
- Villamil, J.C.; Parra-Giraldo, C.M.; Pérez, L.D. Enhancing the performance of PEG-b-PCL copolymers as precursors of micellar vehicles for amphotericin B through its conjugation with cholesterol. Colloids Surfaces a Physicochem. Eng. Asp. 2019, 572, 79–87. [Google Scholar] [CrossRef]
- Gaucher, G.; Satturwar, P.; Jones, M.-C.; Furtos, A.; Leroux, J.-C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Deng, P.; Song, Z.; Chu, W.; Zhu, W.; Teng, F.; Zhou, F. Glycyrrhetinic acid-modified PEG-PCL copolymeric micelles for the delivery of curcumin. React. Funct. Polym. 2017, 111, 30–37. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chin. Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef]
- Uziel, A.; Gelfand, A.; Amsalem, K.; Berman, P.; Lewitus, G.M.; Meiri, D.; Lewitus, D.Y. Full-Spectrum Cannabis Extract Microdepots Support Controlled Release of Multiple Phytocannabinoids for Extended Therapeutic Effect. ACS Appl. Mater. Interfaces 2020, 12, 23707–23716. [Google Scholar] [CrossRef]
- De la Ossa, D.H.P.; Ligresti, A.; Gil-Alegre, M.; Aberturas, M.; Molpeceres, J.; Di Marzo, V.; Suárez, A.T. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control. Release 2012, 161, 927–932. [Google Scholar] [CrossRef]
- Berrocoso, E.; Rey-Brea, R.; Fernández-Arévalo, M.; Micó, J.A.; Martín-Banderas, L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2623–2632. [Google Scholar] [CrossRef]
- Maharajan, M.K.; Yong, Y.J.; Yip, H.Y.; Woon, S.S.; Yeap, K.M.; Yap, K.Y.; Yip, S.C.; Yap, K.X. Medical cannabis for chronic pain: Can it make a difference in pain management? J. Anesth. 2019, 34, 95–103. [Google Scholar] [CrossRef]
- Duan, B.; Cheng, L.; Ma, Q. Spinal Circuits Transmitting Mechanical Pain and Itch. Neurosci. Bull. 2017, 34, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Černe, K. Toxicological properties of Δ9-tetrahydrocannabinol and cannabidiol. Arch. Ind. Hyg. Toxicol. 2020, 71, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williford, J.-M.; Archang, M.M.; Minn, I.; Ren, Y.; Wo, M.; Vandermark, J.; Fisher, P.B.; Pomper, M.G.; Mao, H.-Q. Critical Length of PEG Grafts on lPEI/DNA Nanoparticles for Efficient in Vivo Delivery. ACS Biomater. Sci. Eng. 2016, 2, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, L.; Xiao, C.; Chen, L.; Zhuang, X.; Chen, X. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun. 2014, 50, 11274–11290. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; El-Demerdash, F.M.; Radwan, F.M. Sodium arsenite induced biochemical perturbations in rats: Ameliorating effect of curcumin. Food Chem. Toxicol. 2008, 46, 3506–3511. [Google Scholar] [CrossRef]
- A Comparison of Selected Organ Weights and Clinical Pathology Parameters in Male and Female CD-1 and CByB6F1 Hybrid Mice 12-14 Weeks in Age. Available online: https://www.criver.com/sites/default/files/resources/doc_a/AComparisonofSelectedOrganWeightsandClinicalPathologyParametersinMaleandFemaleCD-1andCByB6F1HybridMice12-14WeeksinAge.pdf (accessed on 12 May 2023).
- Urits, I.; Gress, K.; Charipova, K.; Habib, K.; Lee, D.; Lee, C.; Jung, J.W.; Kassem, H.; Cornett, E.; Paladini, A.; et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 463–477. [Google Scholar] [CrossRef]
- Beers, J.L.; Fu, D.; Jackson, K.D. Cytochrome P450–Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol. Drug Metab. Dispos. 2021, 49, 882–891. [Google Scholar] [CrossRef]
- Nadulski, T.; Sporkert, F.; Schnelle, M.; Stadelmann, A.M.; Roser, P.; Schefter, T.; Pragst, F. Simultaneous and Sensitive Analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in Plasma after Oral Application of Small Doses of THC and Cannabis Extract. J. Anal. Toxicol. 2005, 29, 782–789. [Google Scholar] [CrossRef]
- Izgelov, D.; Shmoeli, E.; Domb, A.J.; Hoffman, A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int. J. Pharm. 2020, 580, 119201. [Google Scholar] [CrossRef] [PubMed]
- Meymandi, M.-S.; Keyhanfar, F.; Yazdanpanah, O.; Heravi, G. The Role of NMDARs Ligands on Antinociceptive Effects of Pregabalin in the Tail Flick Test. Anesthesiol. Pain Med. 2015, 5, e28968. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, E.R.; Schneider, G.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef]
- Abraham, A.D.; Leung, E.J.Y.; Wong, B.A.; Rivera, Z.M.G.; Kruse, L.C.; Clark, J.J.; Land, B.B. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 2019, 45, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Brunt, T.M.; Bossong, M.G. The neuropharmacology of cannabinoid receptor ligands in central signaling pathways. Eur. J. Neurosci. 2020, 55, 909–921. [Google Scholar] [CrossRef]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: Possible role of CB1 and TRPV1 receptors. Neuropharmacology 2021, 197, 108712. [Google Scholar] [CrossRef]
- Bornheim, L.M.; Grillo, M.P. Characterization of Cytochrome P450 3A Inactivation by Cannabidiol: Possible Involvement of Cannabidiol-Hydroxyquinone as a P450 Inactivator. Chem. Res. Toxicol. 1998, 11, 1209–1216. [Google Scholar] [CrossRef]
- Klein, C.; Karanges, E.; Spiro, A.; Wong, A.; Spencer, J.; Huynh, T.; Gunasekaran, N.; Karl, T.; Long, L.E.; Huang, X.-F.; et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 2011, 218, 443–457. [Google Scholar] [CrossRef]
- Britch, S.C.; Wiley, J.L.; Yu, Z.; Clowers, B.H.; Craft, R.M. Cannabidiol-Δ 9-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend. 2017, 175, 187–197. [Google Scholar] [CrossRef]
- Casey, S.L.; Atwal, N.; Vaughan, C.W. Cannabis constituent synergy in a mouse neuropathic pain model. Pain 2017, 158, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.S.; Marshall, A.G.; Makdani, A.; Jarocka, E.; Liljencrantz, J.; Ridderström, M.; Shaikh, S.; O’neill, F.; Saade, D.; Donkervoort, S.; et al. An ultrafast system for signaling mechanical pain in human skin. Sci. Adv. 2019, 5, eaaw1297. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.M.P.; Aguiar, A.F.L.; Paes-Colli, Y.; Trindade, P.M.P.; Ferreira, B.K.; Reis, R.A.D.M.; Sampaio, L.S. Cannabinoid Therapeutics in Chronic Neuropathic Pain: From Animal Research to Human Treatment. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; de Novellis, V.; Di Marzo, V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2010, 162, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef]
- Pavao-De-Souza, G.F.; Zarpelon, A.C.; Tedeschi, G.C.; Mizokami, S.S.; Sanson, J.S.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Casagrande, R.; Verri, W.A. Acetic acid- and phenyl-p-benzoquinone-induced overt pain-like behavior depends on spinal activation of MAP kinases, PI3K and microglia in mice. Pharmacol. Biochem. Behav. 2012, 101, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Cannabinoid Receptors and Their Signaling Mechanisms. In The Endocannabinoid System; Academic Press: Cambridge, MA, USA, 2017; pp. 25–62. [Google Scholar] [CrossRef]
- Jung, S.-M.; Peyton, L.; Essa, H.; Choi, D.-S. Adenosine receptors: Emerging non-opioids targets for pain medications. Neurobiol. Pain 2022, 11, 100087. [Google Scholar] [CrossRef] [PubMed]
- Vacca, V.; Marinelli, S.; Pieroni, L.; Urbani, A.; Luvisetto, S.; Pavone, F. Higher pain perception and lack of recovery from neuropathic pain in females: A behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Pain 2014, 155, 388–402. [Google Scholar] [CrossRef]
- Blanton, H.L.; Pietrzak, A.; McHann, M.C.; Guindon, J. Sex and dose-dependent antinociceptive effects of the JNK (c-Jun N-terminal kinase) inhibitor SU 3327 are mediated by CB2 receptors in female, and CB1/CB2 receptors in male mice in an inflammatory pain model. Brain Res. Bull. 2021, 177, 39–52. [Google Scholar] [CrossRef]
- Olfert, E.D.; Cross, B.M.; McWilliam, A.A. Manual Sobre el Cuidado y uso de los Animales de Experimentación. Available online: http://viceinvestigacion.unal.edu.co/fileadmin/recursos/focos/etica-investigacion-animal/docs/manual_cuidado_uso_animales_consejo_canadiense.pdf (accessed on 8 May 2023).
- National Research Council. Guide for the Care and Use of Laboratory Animals, 9th ed.; EE.UU; The National Academies Press: Washington, DC, USA, 2011; pp. 1–246. [Google Scholar]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Rodriguez, Y.J.; Quejada, L.F.; Villamil, J.C.; Baena, Y.; Parra-Giraldo, C.M.; Perez, L.D. Development of Amphotericin B Micellar Formulations Based on Copolymers of Poly(ethylene glycol) and Poly(ε-caprolactone) Conjugated with Retinol. Pharmaceutics 2020, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. Experiment 2020, 373, 416–428. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; Silva, R.F.D.O.; Frohlich, E.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002; p. 14. [Google Scholar]
- D’amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp.Ther. 1941, 72, 74–79. [Google Scholar]
- Vivanco-Estela, A.N.; Dos-Santos-Pereira, M.; Guimaraes, F.S.; Del-Bel, E.; Nascimento, G.C. Cannabidiol has therapeutic potential for myofascial pain in female and male parkinsonian rats. Neuropharmacology 2021, 196, 108700. [Google Scholar] [CrossRef]
- Wong, H.; Cairns, B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef]
- Deuis, J.R.; Lim, Y.L.; de Sousa, S.R.; Lewis, R.J.; Alewood, P.F.; Cabot, P.J.; Vetter, I. Analgesic effects of clinically used compounds in novel mouse models of polyneuropathy induced by oxaliplatin and cisplatin. Neuro-Oncology 2014, 16, 1324–1332. [Google Scholar] [CrossRef]
- Siegmund, E.; Cadmus, R.; Lu, G. A Method for Evaluating both Non-Narcotic and Narcotic Analgesics. Exp. Biol. Med. 1957, 95, 729–731. [Google Scholar] [CrossRef]
- Miranda, H.F.; Pinardi, G. Lack of effect of naltrindole on the spinal synergism of morphine and non-steroidal an-ti-inflammatory drugs (NSAIDS). J. Physiol. Pharmacol. 2009, 60, 71–76. [Google Scholar]
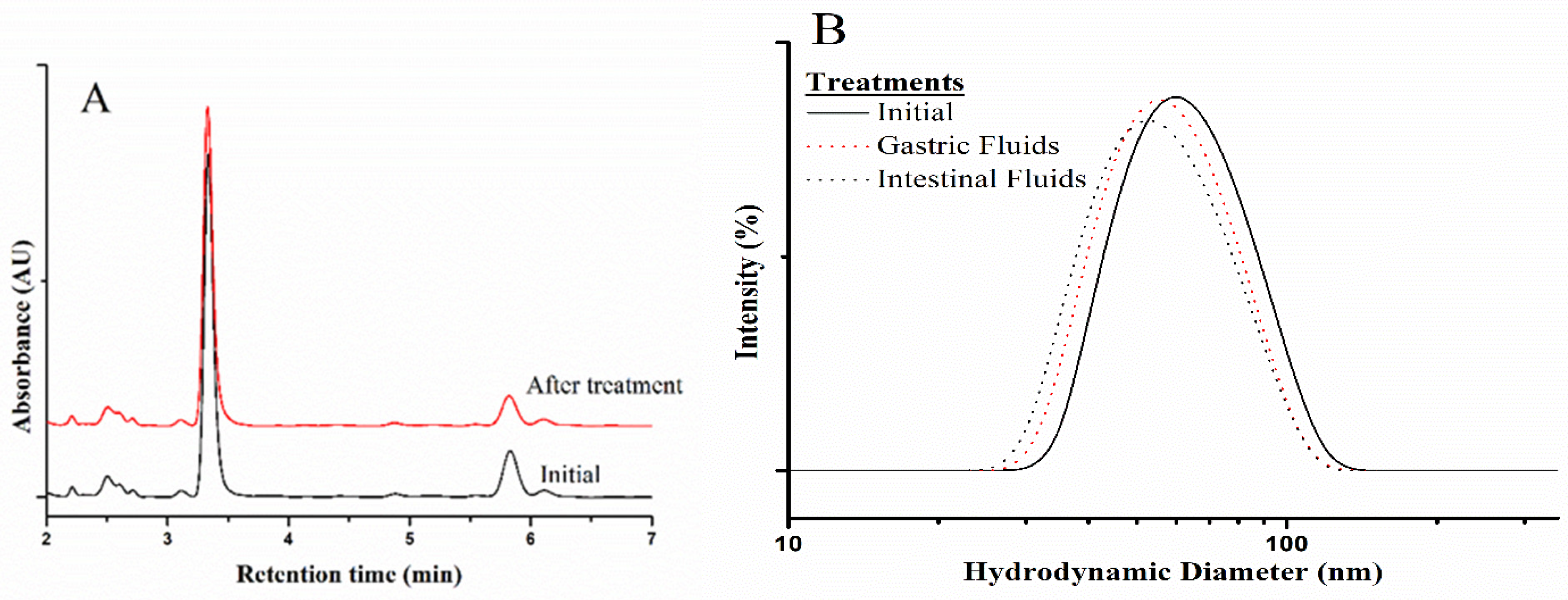
| PEG (Mn) kDa | PCL Mn (NMR-H) kDa | GPC (Mn) kDa | D (GPC) | EE% (CBD) | Dh (nm) | PDI |
|---|---|---|---|---|---|---|
| 2.0 | 1.87 | 4.34 | 1.20 | 91.92 | 83.00 | 0.27 |
| 2.0 | 4.31 | 5.50 | 1.17 | 97.66 | 66.50 | 0.21 |
| 5.0 | 4.00 | 11.8 | 1.21 | 98.31 | 52.60 | 0.23 |
| Mass Ratio of Organic Phase to Water | Dh (nm) | PDI | Physical Appearance |
|---|---|---|---|
| 1:10 | 44.66 ± 0.97 | 0.21 ± 0.01 | Homogenous suspension |
| 1:5 | 86.74 ± 2.41 | 0.22 ± 0.01 | Homogenous suspension |
| 1:3 | 118.85 ± 3.50 | 0.25 ± 0.01 | Homogenous suspension |
| 1:1 | 94.04 ± 1.65 | 0.19 ± 0.02 | Phase separation |
| Sex | CBD/PMs Dose (mg/kg) | Body Weight (g) | Dead/Total (Number) | Symptoms | ||
|---|---|---|---|---|---|---|
| 1st Day | 7th Day | 14th Day | ||||
| Female | 20.0 | 19.6 ± 0.3 | 21.0 ± 0.5 | 21.5 ± 0.6 | 0/3 | None |
| Organs | BW% (Mean ± SEM) | p-Value 1 |
|---|---|---|
| Liver | 4.98 ± 0.10 | 0.09 |
| Kidneys | 1.65 ± 0.01 | 0.11 |
| Heart | 0.70 ± 0.09 | 0.84 |
| Spleen | 0.48 ± 0.02 | 0.17 |
| CE/CBD | CBD/PMs | |||||||
|---|---|---|---|---|---|---|---|---|
| Morphine | 1 (h) | 24 (h) | 3 (h) | 24 (h) | ||||
| Measures | Negative Control | 10.0 mg/kg | 2.5 mg/kg | 5.0 mg/kg | 10.0 mg/kg | 2.5 mg/kg | 2.5 mg/kg | 2.5 mg/kg |
| N | 45 ± 2 | 0 ± 0 *** | 29 ± 1 ** | 26 ± 3 *** | 26 ± 1 *** | 36 ± 2 | 31 ± 4 | 24 ± 5 *** |
| p% | 0 ± 4 | 100 ± 0 *** | 37 ± 2 ** | 43 ± 6 *** | 42 ± 3 *** | 8 ± 5 | 8 ± 12 | 42 ± 13 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Vargas, Y.; Porras-Arguello, J.D.; Blandón-Naranjo, L.; Pérez-Pérez, L.D.; Benjumea, D.M. Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles. Molecules 2023, 28, 4299. https://doi.org/10.3390/molecules28114299
Román-Vargas Y, Porras-Arguello JD, Blandón-Naranjo L, Pérez-Pérez LD, Benjumea DM. Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles. Molecules. 2023; 28(11):4299. https://doi.org/10.3390/molecules28114299
Chicago/Turabian StyleRomán-Vargas, Yoreny, Julián David Porras-Arguello, Lucas Blandón-Naranjo, León Darío Pérez-Pérez, and Dora María Benjumea. 2023. "Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles" Molecules 28, no. 11: 4299. https://doi.org/10.3390/molecules28114299
APA StyleRomán-Vargas, Y., Porras-Arguello, J. D., Blandón-Naranjo, L., Pérez-Pérez, L. D., & Benjumea, D. M. (2023). Evaluation of the Analgesic Effect of High-Cannabidiol-Content Cannabis Extracts in Different Pain Models by Using Polymeric Micelles as Vehicles. Molecules, 28(11), 4299. https://doi.org/10.3390/molecules28114299