Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System
Abstract
1. Introduction
2. Results
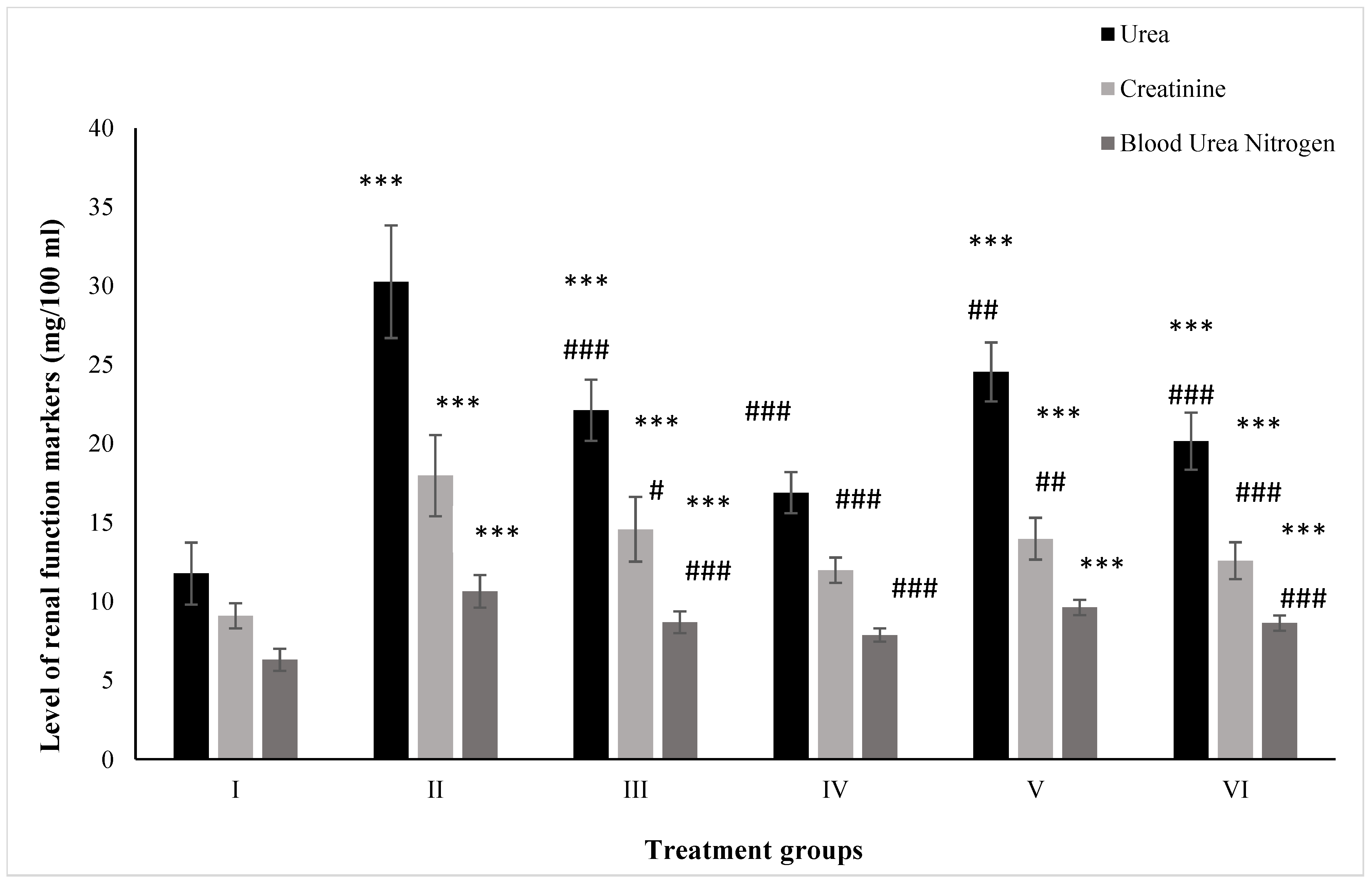
2.1. Effect on Kidney Function Markers
2.1.1. Urea
2.1.2. Creatinine
2.1.3. BUN
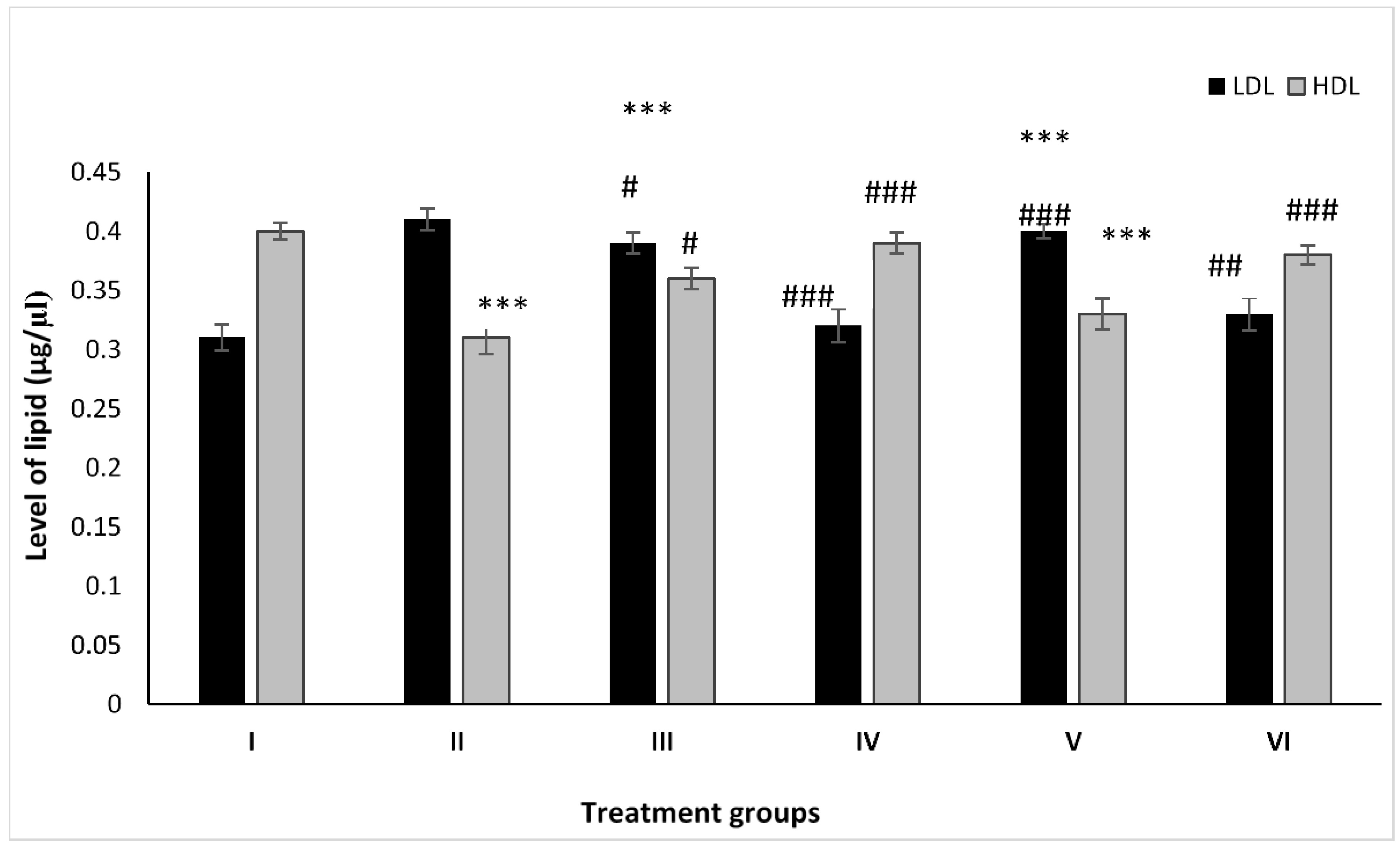
2.2. Effect on Lipid Profile
2.2.1. LDL
2.2.2. HDL
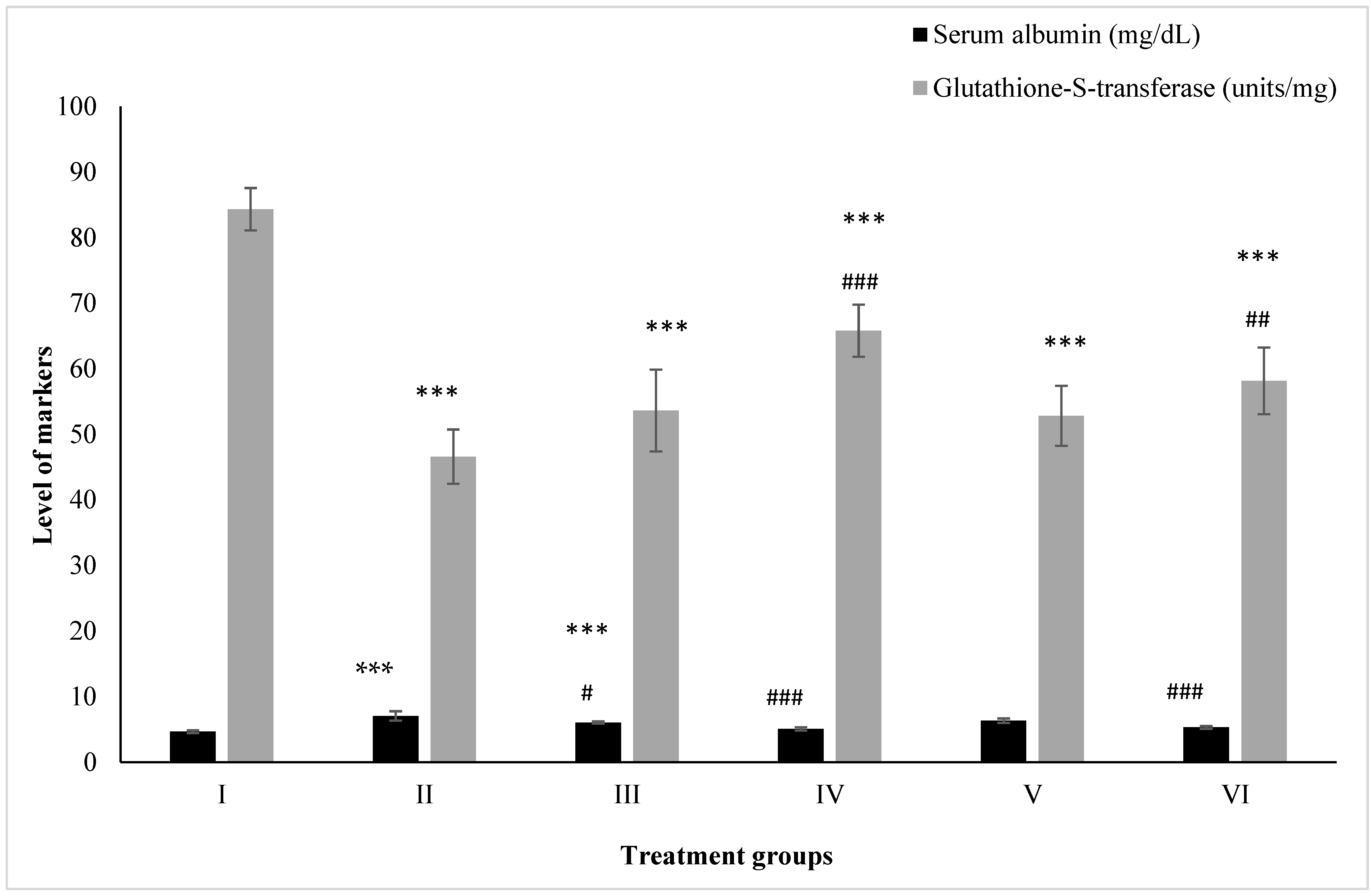
2.3. Effect on Kidney Toxicity Markers
2.3.1. Albumin
2.3.2. Glutathione-S-Transferase
2.4. Effect on Antioxidant Parameters
2.4.1. CAT
2.4.2. SOD
2.4.3. GR
2.4.4. GSH
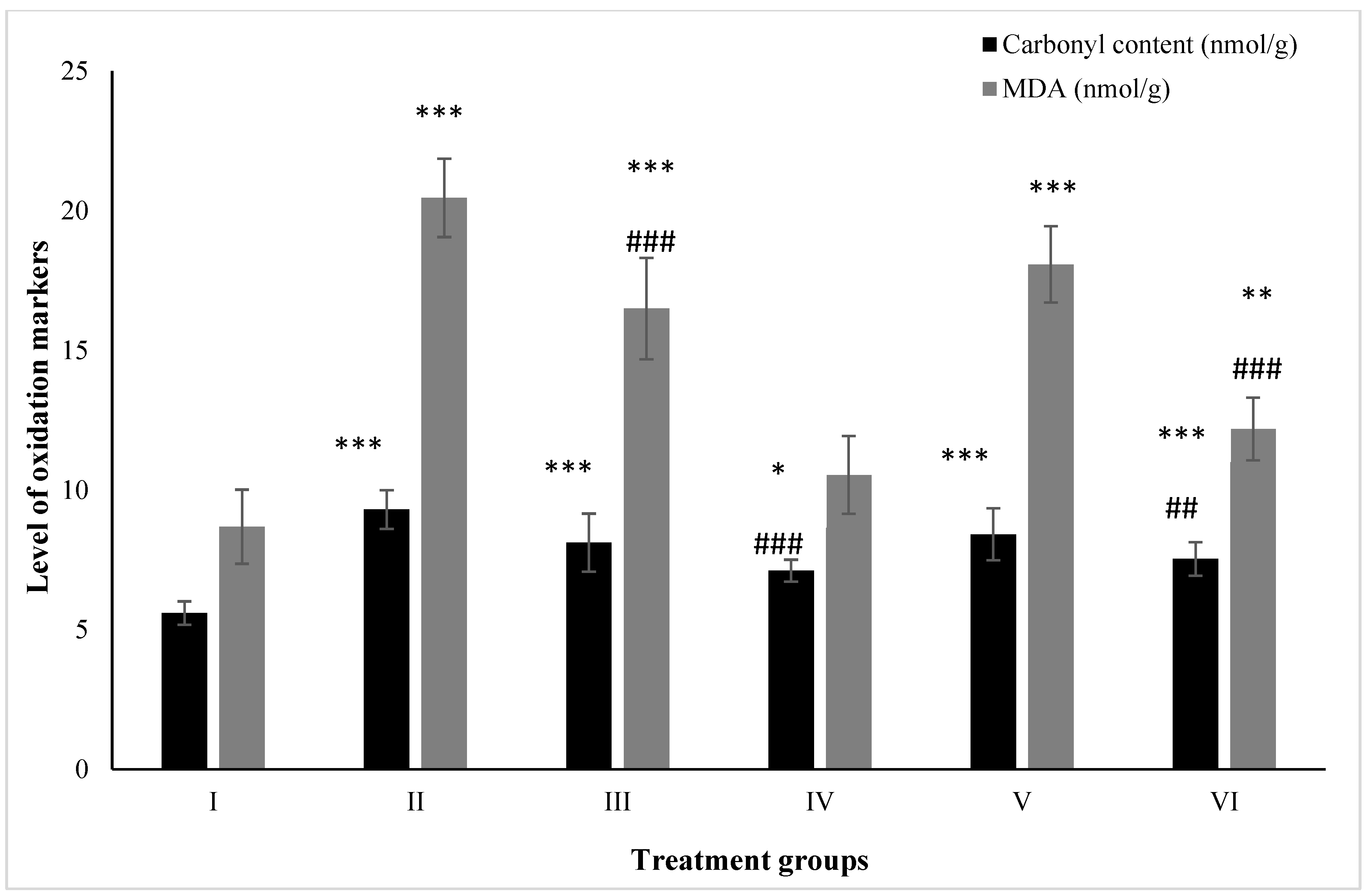
2.5. Effect on Macromolecular Oxidation
2.5.1. Carbonyl Content
2.5.2. MDA
2.6. Effect on Nuclear DNA
2.7. Histological Evaluation of Kidney Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Animal Husbandry
- Group I: Control treated with saline only;
- Group III: ZnO at a dose of 5 mg/kg body weight twice a week for a month;
- Group IV: ZnO-NP at a dose of 5 mg/kg body weight twice a week for a month;
- Group V: A single dose of KBrO3 at a dose of 100 mg/kg + 5 mg/kg of ZnO administered twice a week for a month;
- Group VI: A single dose of KBrO3 at a dose of 100 mg/kg + 5 mg/kg of ZnO-NP administered twice a week for a month.
4.2.2. Preparation of Nanoparticles
4.2.3. Preparation of Biological Samples
4.2.4. Assessment of Kidney Function Markers
4.2.5. Estimation of Toxicity Burden on the Kidney
4.2.6. Activity Assay of Antioxidant Enzymes
4.2.7. Estimation of Reduced Glutathione Level
4.2.8. Assessment of Macromolecular Oxidative Damage
4.2.9. Comet Assay
4.2.10. Histopathological Evaluation
4.2.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nkwatoh, T.N.; Fon, T.P.; Navti, L.K. Potassium bromate in bread, health risks to bread consumers and toxicity symptoms amongst bakers in Bamenda, North West Region of Cameroon. Heliyon 2023, 9, e13146. [Google Scholar] [CrossRef] [PubMed]
- El Ati-Hellal, M.; Doggui, R.; Krifa, Y.; El Ati, J. Potassium bromate as a food additive: A case study of Tunisian breads. Environ. Sci. Pollut. Res. 2018, 25, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- Fielding, M.; Hutchison, J. Bromate and Water Treatment. In Proceedings of the International Workshop, International Water Supply Association, Paris, France, 22–24 November 1993. [Google Scholar]
- Ben Saad, H.; Driss, D.; Ben Amara, I.; Boudawara, O.; Boudawara, T.; Ellouz Chaabouni, S.; Mounir Zeghal, K.; Hakim, A. Altered hepatic m RNA expression of immune response-associated DNA damage in mice liver induced by potassium bromate: Protective role of vanillin. Environ. Toxicol. 2016, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.K.; Mahmood, R. Oral administration of potassium bromate, a major water disinfection by-product, induces oxidative stress and impairs the antioxidant power of rat blood. Chemosphere 2012, 87, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Alhazza, I.M.; Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Alwasel, S.H. Chemopreventive effect of riboflavin on the potassium bromate–induced renal toxicity in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavel, V.; Santhi, K.K.; Kurup, A.H.; Kalakandan, S.; Anandharaj, A.; Rawson, A. Potassium bromate: Effects on bread components, health, environment and method of analysis: A review. Food Chem. 2020, 311, 125964. [Google Scholar] [CrossRef]
- Ajarem, J.; Altoom, N.G.; Allam, A.A.; Maodaa, S.N.; Abdel-Maksoud, M.A.; Chow, B.K. Oral administration of potassium bromate induces neurobehavioral changes, alters cerebral neurotransmitters level and impairs brain tissue of swiss mice. Behav. Brain Funct. 2016, 12, 14. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Yang, D. Application of nanotechnology in the COVID-19 pandemic. Int. J. Nanomed. 2021, 16, 623. [Google Scholar] [CrossRef]
- Abdel-Latif, A.S.; Abu-Risha, S.E.; Bakr, S.M.; El-Kholy, W.M.; El-Sawi, M.R. Potassium bromate-induced nephrotoxicity and potential curative role of metformin loaded on gold nanoparticles. Sci. Prog. 2021, 104, 00368504211033703. [Google Scholar] [CrossRef]
- De Souza Rebouças, J.; Esparza, I.; Ferrer, M.; Sanz, M.L.; Irache, J.M.; Gamazo, C. Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J. Biomed. Biotechnol. 2012, 2012, 474605. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Osuchowski, F.; Latos, W.; Kawczyk-Krupka, A. The use of upconversion nanoparticles in prostate cancer photodynamic therapy. Life 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Abbasalipourkabir, R.; Moradi, H.; Zarei, S.; Asadi, S.; Salehzadeh, A.; Ghafourikhosroshahi, A.; Mortazavi, M.; Ziamajidi, N. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem. Toxicol. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A. Neuroscience nanotechnology: Progress, opportunities and challenges. Nat. Rev. Neurosci. 2006, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Chang, C.-C.; Wang, T.-H.; Lam, P.K.; Wei, M.-Y.; Chen, C.-T.; Chen, C.-Y.; Chou, L.-Y.; Shieh, F.-K. Rapid fabrication of biocomposites by encapsulating enzymes into Zn-MOF-74 via a mild water-based approach. ACS Appl. Mater. Interfaces 2021, 13, 52014–52022. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef]
- Attia, J.V.; Dessens, C.E.; van de Water, R.; Houvast, R.D.; Kuppen, P.J.; Krijgsman, D. The molecular and functional characteristics of HLA-G and the interaction with its receptors: Where to intervene for cancer immunotherapy? Int. J. Mol. Sci. 2020, 21, 8678. [Google Scholar] [CrossRef]
- Sharma, N.; Tanwer, B.S.; Vijayvergia, R. Study of medicinal plants in Aravali regions of Rajasthan for treatment of kidney stone and urinary tract troubles. Int. J. PharmTech Res. 2011, 3, 110–113. [Google Scholar]
- Kim, K.-S.; Lee, Y.-M.; Kim, S.G.; Lee, I.-K.; Lee, H.-J.; Kim, J.-H.; Kim, J.; Moon, H.-B.; Jacobs Jr, D.R.; Lee, D.-H. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 2014, 94, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Workman, C.J.; Vignali, D.A. Targeting regulatory T cells in tumors. FEBS J. 2016, 283, 2731–2748. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.; Ebaid, H.; Moussa, S.A.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Al Tamimi, J. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef]
- Akbar, N.; Aslam, Z.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Zinc oxide nanoparticles conjugated with clinically-approved medicines as potential antibacterial molecules. AMB Express 2021, 11, 104. [Google Scholar] [CrossRef]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421. [Google Scholar] [CrossRef]
- Yalçin, E.; Çavuşoğlu, K. Toxicity assessment of potassium bromate and the remedial role of grape seed extract. Sci. Rep. 2022, 12, 20529. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Maekawa, A.; Takahashi, M.; Hayashi, Y. Toxicity and carcinogenicity of potassium bromate—A new renal carcinogen. Environ. Health Perspect. 1990, 87, 309–335. [Google Scholar]
- Ahmad, M.K.; Khan, A.A.; Ali, S.N.; Mahmood, R. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PLoS ONE 2015, 10, e0119137. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, V.; Fischer, U.; Weighardt, H.; Schulze-Osthoff, K.; Nickel, C.; Stahlmecke, B.; Kuhlbusch, T.A.; Scherbart, A.M.; Esser, C.; Schins, R.P. Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox-and Nrf2-independent manner. PLoS ONE 2013, 8, e65704. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Ebaid, H.; Alhazza, I.M.; Al-Tamimi, J.; Aman, S.; Abdel-Mageed, A.M. Copper mediates anti-inflammatory and antifibrotic activity of Gleevec in hepatocellular carcinoma-induced male rats. Can. J. Gastroenterol. Hepatol. 2019, 2019, 9897315. [Google Scholar] [CrossRef]
- Naseer, F.; Ahmed, M.; Majid, A.; Kamal, W.; Phull, A.R. Green nanoparticles as multifunctional nanomedicines: Insights into anti-inflammatory effects, growth signaling and apoptosis mechanism in cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Quan, J.-H.; Gao, F.F.; Ismail, H.A.H.A.; Yuk, J.-M.; Cha, G.-H.; Chu, J.-Q.; Lee, Y.-H. Silver nanoparticle-induced apoptosis in ARPE-19 cells is inhibited by Toxoplasma gondii pre-infection through suppression of NOX4-dependent ROS generation. Int. J. Nanomed. 2020, 15, 3695–3716. [Google Scholar] [CrossRef]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L. Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water 2022, 14, 1297. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U.T. The lysosomal–mitochondrial axis theory of postmitotic aging and cell death. Chem. -Biol. Interact. 2006, 163, 29–37. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Khan, A.A.; Naseem, I. Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PLoS ONE 2012, 7, e36273. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Klotz, L.-O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, C.; Liu, L.; Xu, J.; Jiang, H.; Li, D.; Lan, J.; Li, J.; Yang, J.; Tu, Q. ZnO-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics 2020, 10, 11197. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Husain, F.M.; Khan, R.A.; Ebaid, H.; Al-Tamimi, J.; Alhazza, I.M.; Aman, S.; Ibrahim, K.E. Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 9966–9980. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Carlberg, I.; Mannervik, B. [59] Glutathione reductase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Jollow, D.; Mitchell, J.; Zampaglione, N.; Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Levine, N. The Protozoan Phylum Apicomplexa: Volume 1; Chemical Rubber Company Press, Inc.: Boca Raton, FL, USA, 1988. [Google Scholar]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Joko, W.B. Glutathione S-transferase: The firstenzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.; Stadman, E.R.; Shacter, E. Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar]
- Naseem, I.; Hassan, I.; Alhazza, I.M.; Chibber, S. Protective effect of riboflavin on cisplatin induced toxicities: A gender-dependent study. J. Trace Elem. Med. Biol. 2015, 29, 303–314. [Google Scholar] [CrossRef]



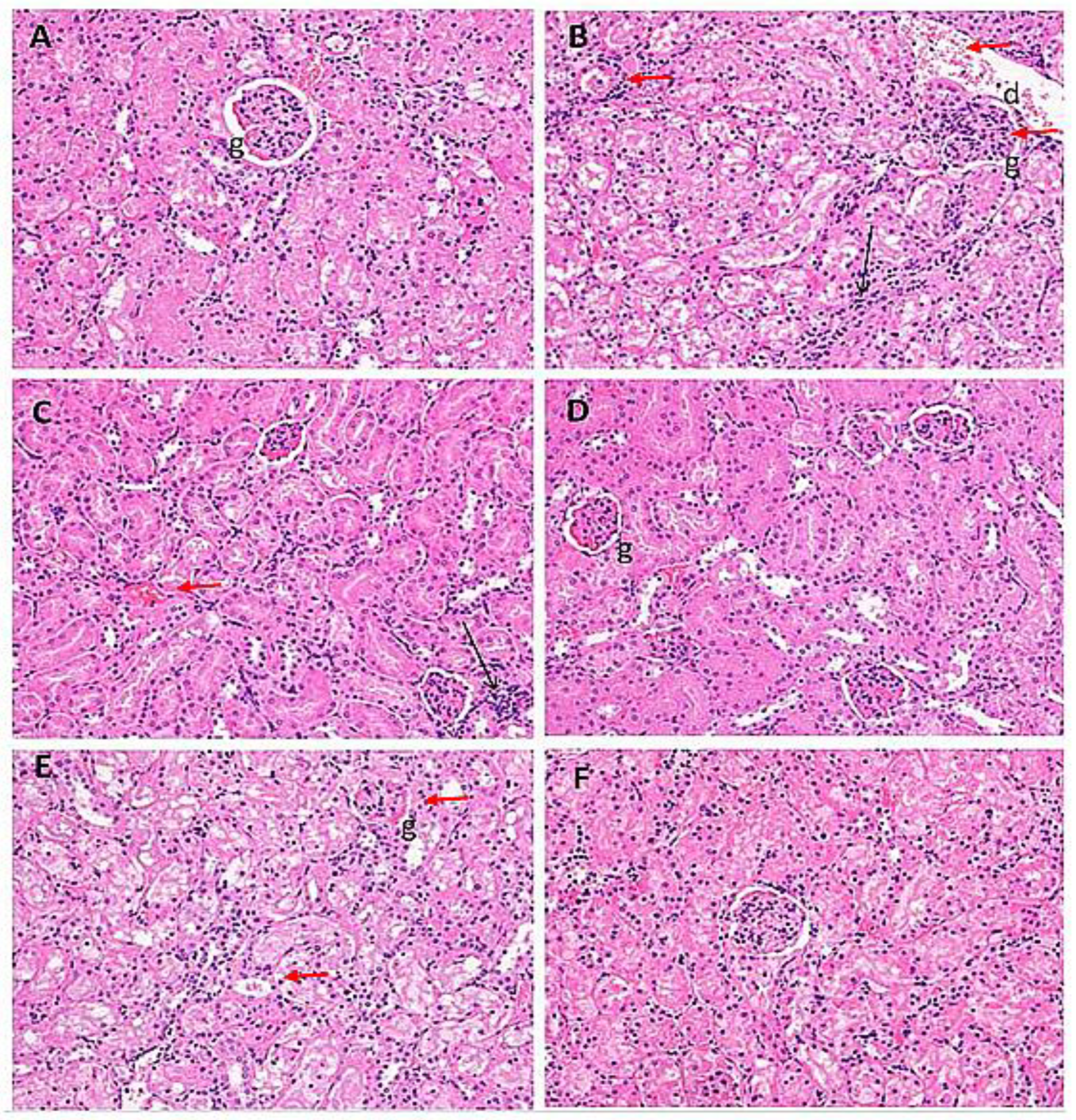
| Treatment Groups | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Oedematous glomeruli | − | +++ | ++ | − | +++ | − |
| Hemorrhage | − | ++ | ++ | − | − | − |
| Infiltration of inflammatory cells | − | +++ | ++ | + | ++ | + |
| Hyaline casts | − | ++ | ++ | − | ++ | + |
| Disintegrated nucleus | − | + | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazza, I.M.; Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Hasan, Z. Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System. Molecules 2023, 28, 5084. https://doi.org/10.3390/molecules28135084
Alhazza IM, Hassan I, Ebaid H, Al-Tamimi J, Hasan Z. Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System. Molecules. 2023; 28(13):5084. https://doi.org/10.3390/molecules28135084
Chicago/Turabian StyleAlhazza, Ibrahim M., Iftekhar Hassan, Hossam Ebaid, Jameel Al-Tamimi, and Zafrul Hasan. 2023. "Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System" Molecules 28, no. 13: 5084. https://doi.org/10.3390/molecules28135084
APA StyleAlhazza, I. M., Hassan, I., Ebaid, H., Al-Tamimi, J., & Hasan, Z. (2023). Zinc Oxide Nanoparticles Blunt Potassium-Bromate-Induced Renal Toxicity by Reinforcing the Redox System. Molecules, 28(13), 5084. https://doi.org/10.3390/molecules28135084





