Enhanced Photocatalytic Coupling of Benzylamine to N-Benzylidene Benzylamine over the Organic–Inorganic Composites F70-TiO2 Based on Fullerenes Derivatives and TiO2
Abstract
1. Introduction
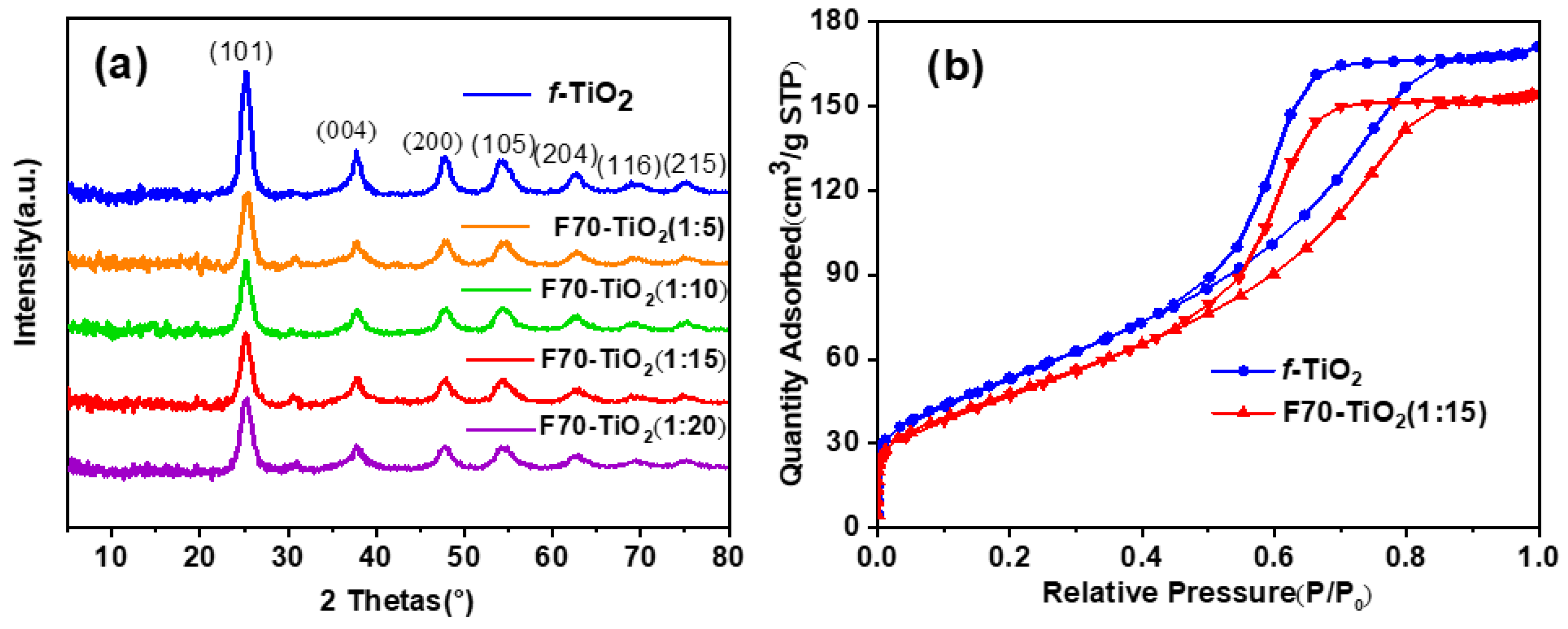
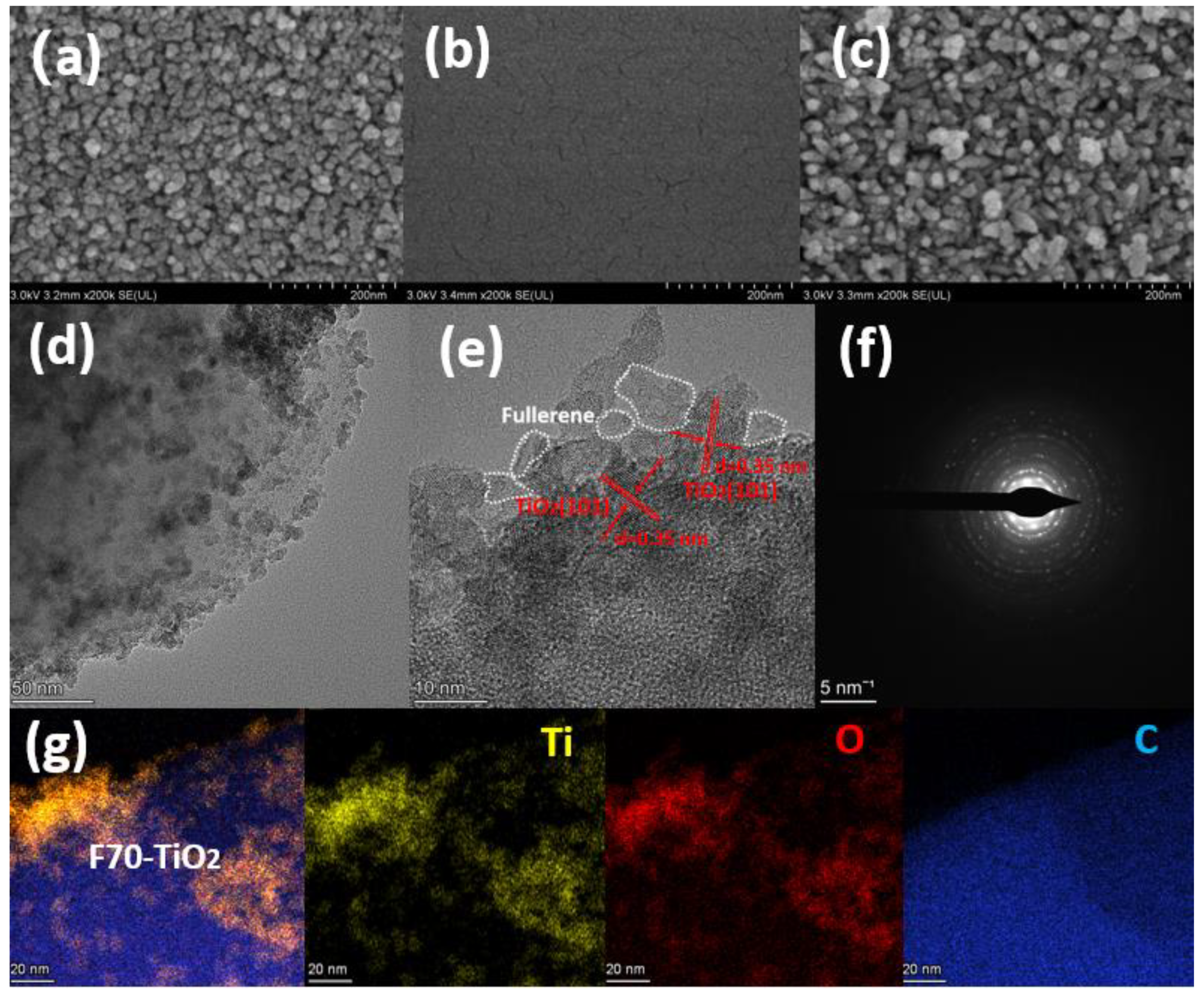
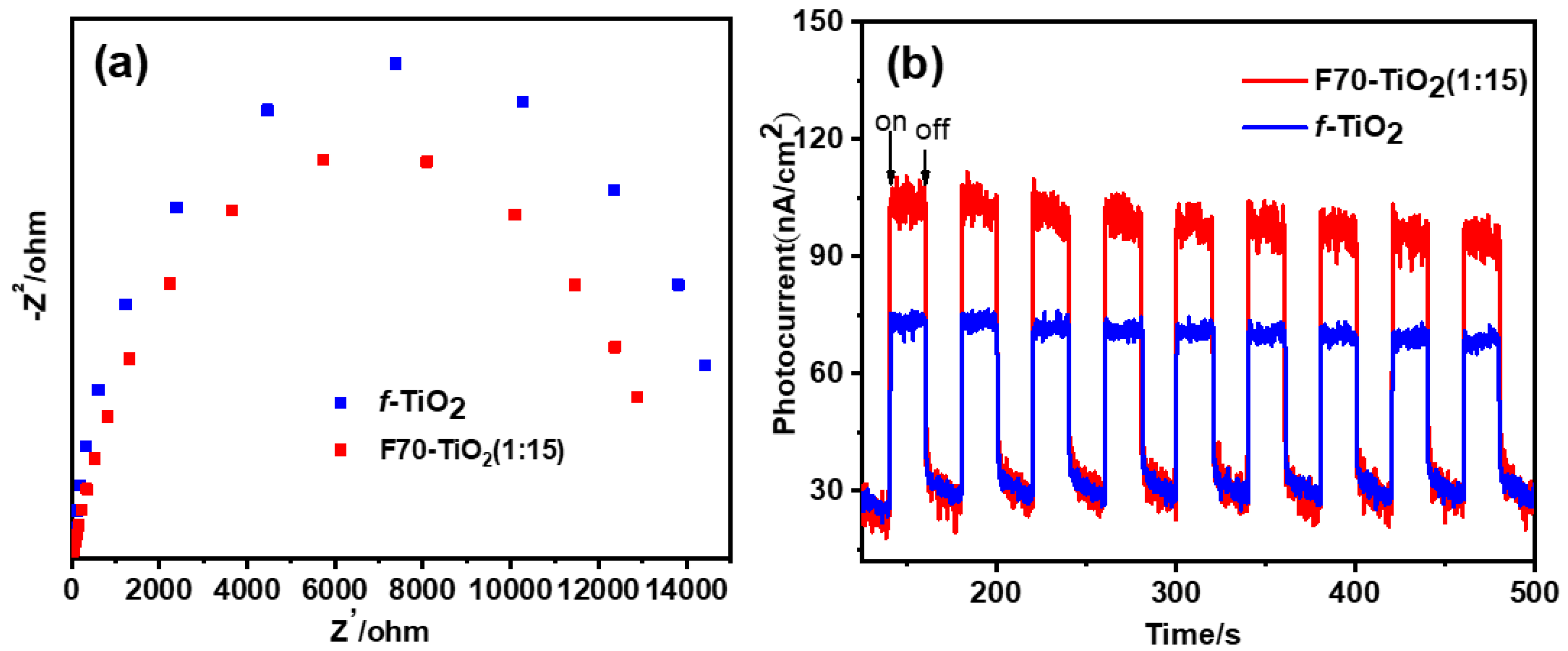
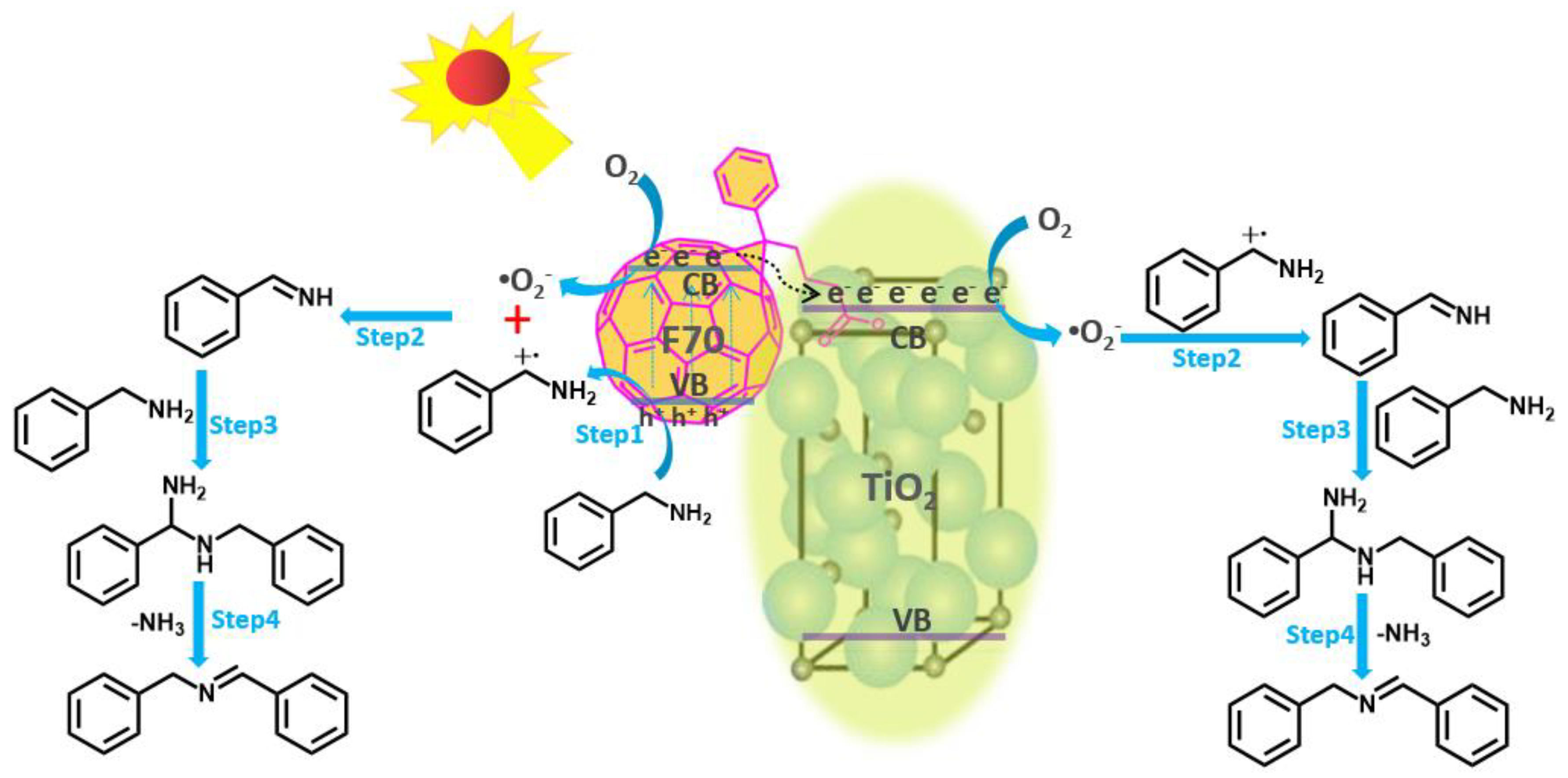
2. Results and Discussion
3. Experiments
3.1. Materials
3.2. Synthesis of F70-TiO2 Materials
3.3. Photocatalytic Studies
3.4. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Murahashi, S.I. Synthetic aspects of metal-catalyzed oxidations of amines and related reactions. Angew. Chem. Int. Ed. 1995, 34, 2443–2465. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishitani, H. Catalytic enantioselective addition to lmines. Chem. Rev. 1999, 99, 1069–1094. [Google Scholar] [CrossRef]
- Patil, R.D.; Adimurthy, S. Catalytic methods for imine synthesis. Asian J. Org. Chem. 2013, 2, 726–744. [Google Scholar] [CrossRef]
- Dutta, B.; March, S.; Achola, L.; Sahoo, S.; He, J.; Amin, A.S.; Wu, Y.; Poges, S.; Alpay, P.; Suib, S. Mesoporous cobalt/manganese oxide: A highly selective bifunctional catalyst for amine-imine transformations. Green Chem. 2018, 20, 1–30. [Google Scholar] [CrossRef]
- Lang, X.J.; Zhao, J.C.; Xiaodong, C. Visible-light-induced photoredox catalysis of dye-sensitized titanium dioxide: Selective aerobic oxidation of organic sulfides. Angew. Chem. Int. Ed. 2016, 55, 4697–4700. [Google Scholar] [CrossRef]
- Lang, X.J.; Chen, X.D.; Zhao, J.C. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Li, G.S.; Jiang, B.; Li, X.; Lian, Z.C.; Xiao, S.N.; Zhu, J.; Zhang, D.Q.; Li, H.X. C60/Bi2TiO4F2 Heterojunction photocatalysts with Enhanced visible-light activity for environmental remediation. ACS Appl. Mater. Interfaces 2013, 5, 7190–7197. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1994, 95, 69–96. [Google Scholar] [CrossRef]
- Long, Y.Z.; Lu, Y.; Huang, Y.; Peng, Y.C.; Lu, Y.J.; Kang, S.Z.; Mu, J. Effect of C60 on the photocatalytic activity of TiO2 nanorods. J. Phys. Chem. C 2009, 113, 13899–13905. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.K.; Yu, J.G.; Yip, H.Y.; Wong, P.K.; Zhao, J.C. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ. Sci. Technol. 2005, 39, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Han, C.; Xu, Y.J.; Foley IV, J.J.; Zhang, D.T.; Codrington, J.; Gray, S.K.; Sun, Y.G. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat. Photonics 2016, 10, 473–482. [Google Scholar] [CrossRef]
- Paul, A.D.; Jeremy, J.P.; Devyn, E.D. Plasmonic enhancement of visible-light water splitting with Au-TiO2 composite aerogels. Nanoscale 2013, 5, 8073–8083. [Google Scholar]
- Zhang, J.; Liao, W.Q.; Zheng, H.; Zhang, Y.S.; Xia, L.B.; Teng, B.T.; Lu, J.Q.; Huang, W.X.; Zhang, Z.H. Morphology-engineered highly active and stable Pd/TiO2 catalysts for CO2 hydrogenation into formate. J. Catal. 2022, 405, 152–163. [Google Scholar] [CrossRef]
- Mukurala, N.; Suman, S.; Bhardwaj, A.; Mokurala, K.; Jin, S.H.; Kushwaha, A.K. Cu2FeSnS4 decorated Ni-TiO2 nanorods heterostructured photoanode for enhancing water splitting performance. Appl. Surf. Sci. 2012, 551, 149377. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Nitrogen and sulfur co-doped TiO2nanosheets with exposed {001} facets: Synthesis, characterization and visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2011, 13, 4853–4861. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Wang, W.G.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Comm. 2001, 47, 6906–6908. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.Q.; Sun, Y.G.; Xu, Y.J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.Z.; Xu, Y.J. TiO2 graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.H.; Xu, Y.J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Kalinowski, J.; Giro, G.; Camaioni, N.; Fattori, V.; Marco, P.D. Photoconduction in solid films of C60. Synth. Met. 1996, 77, 181–188. [Google Scholar] [CrossRef]
- Yang, M.Q.; Zhang, N.; Xu, Y.J. Synthesis of fullerene, carbon nanotube, and graphene TiO2 nanocomposite photocatalysts for selective oxidation: A comparative study. ACS Appl. Mater. Interfaces 2013, 5, 1156–1164. [Google Scholar] [CrossRef]
- Nierengarten, J.F.; Gu, T.; Aernouts, T.; Geens, W.; Poortmans, J.; Hadziioannou, G.; Tsamouras, D. Fullerene oligophenyleneethynylene conjugates: Relationships between charge-carrier mobility, photovoltaic characteristics and chemical structure. Appl. Phys. A 2004, 79, 47–49. [Google Scholar] [CrossRef]
- Zhu, S.B.; Xu, T.G.; Fu, H.B.; Zhao, J.C.; Zhu, Y.F. Synergetic effect of Bi2WO6 photocatalyst with C60 and enhanced photoactivity under visible irradiation. Environ. Sci. Technol. 2007, 41, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudi, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Dong, G.F.; Zheng, H.Y.; Duan, L.; Wang, L.D.; Qiu, Y. High-performance Organic optocouplers based on a photosensitive interfacial C60/NPB heterojunction. Adv. Mater. 2009, 21, 2501–2504. [Google Scholar] [CrossRef]
- Katsumata, K.I.; Matsushita, N.; Okada, K. Preparation of TiO2-fullerene composites and their photocatalytic activity under visible light. Int. J. Photoenergy 2012, 2012, 256096. [Google Scholar] [CrossRef]
- Hotta, H.; Kang, S.; Umeyama, T.; Matano, Y.; Yoshida, K.; Isoda, S.; Imahori, H. Effects of fullerene substituents on structure and photoelectrochemical properties of fullerene nanoclusters electrophoretically deposited on nanostructured SnO2 electrodes. J. Phys. Chem. B 2005, 109, 5700–5706. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.D.; Zhang, F.Z.; Zhu, L.; Park, C.Y.; Ghosh, T.; Choi, J.G.; Oh, W.C. Synthesis and characterization of M-fullerene/TiO2 photocatalysts designed for degradation azo dye. Mat. Sci. Eng. C 2012, 32, 2175–2182. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, J.F.; Shen, M.H.; Liu, J.M.; Huang, L.B.; Zhong, Y.H.; Qin, S.; Guo, J.; Su, C.Y. A porous hybrid material based on calixarene dye and TiO2 demonstrating high and stable photocatalytic performance. J. Mater. Chem. A 2019, 7, 19852–19861. [Google Scholar] [CrossRef]
- Wu, H.M.; Wang, M.Y.; Jing, F.; Kong, D.R.; Chen, Y.F.; Jia, C.M.; Li, J.W. Enhanced photocatalytic hydrogen production performance of pillararene-doped mesoporous TiO2 with extended visible-light response. Chin. Chem. Lett. 2022, 33, 1983–1987. [Google Scholar] [CrossRef]
- Jing, F.; Guo, Y.M.; Li, B.; Chen, Y.F.; Jia, C.M.; Li, J.W. Enhanced photocatalytic hydrogen production under visible light of an organic-inorganic hybrid material based on enzo [1,2-b:4,5-b’] dithiophene polymer and TiO2. Chin. Chem. Lett. 2022, 33, 1303–1307. [Google Scholar] [CrossRef]
- Huang, J.F.; Liu, J.M.; Xiao, L.M.; Zhong, Y.H.; Liu, L.; Qin, S.; Guo, J.; Su, C.Y. Facile synthesis of porous hybrid materials based on Calix-3 dye and TiO2 for high photocatalytic water splitting performance with excellent stability. J. Mater. Chem. A 2019, 7, 2993. [Google Scholar] [CrossRef]
- Kenji, K.; Masaki, Y.; Emi, F.; Toru, K.; Masafumi, U.; Minoru, H. High performance of Si-O-Ti bonds for anchoring sensitizing dyes on TiO2 electrodes in dye-sensitized solar cells evidenced by using alkoxysilylazobenzenes. Chem. Lett. 2010, 39, 260–262. [Google Scholar]
- Zhang, L.; Cole, J.M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol-gel derived organic-inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-gel. Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Wen, J.Y.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Trofymchuk, I.M.; Roik, N.; Belyakova, L. Sol-gel synthesis of ordered β-cyclodextrin-containing silicas. Nanoscale Res. Lett. 2016, 11, 174. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, C.; Duan, Y.; Wang, C.; Zhao, Z.; Wang, H.; Gao, Y. Phosphorus-doped TiO2 for visible light-driven oxidative coupling of benzylamines and photodegradation of phenol. Appl. Surf. Sci. 2020, 527, 146693. [Google Scholar] [CrossRef]
- Wang, H.T.; Yu, J.N.; Wei, S.; Lin, M.M.; Song, Y.J.; Wu, L. Surface coordination enhanced visible-light photocatalytic coupling of benzylamine to N-benzylidene benzylamine over the Pd/NH2-MIL-125 (Ti) nanosheets. Chem. Eng. J. 2022, 441, 1360600. [Google Scholar] [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterization and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Juntrapirom, S.; Tantraviwat, D.; Thongsook, O.; Oraphan, A.; Supanan, P.; Soraya, C. Natural sunlight driven photocatalytic coupling of primary amines over TiO2/BiOBr heterojunction. Appl. Surf. Sci. 2021, 545, 149015.1–149015.7. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Li, D.; Jiang, H.L. Photocatalytic hydrogen production coupled with selective benzylamine oxidation over MOF composites. Angew. Chem. Int. Ed. 2018, 57, 5379–5383. [Google Scholar] [CrossRef] [PubMed]



 | ||||
| Entry | Catalysts | hv | Conv. (%) | Select. (%) |
| 1 | f-TiO2 | + | 56.3 | 89.7 |
| 2 | F70-TiO2(1:5) | + | 94.4 | 77.1 |
| 3 | F70-TiO2(1:10) | + | 98.1 | 88.1 |
| 4 | F70-TiO2(1:15) | + | 98.5 | 93.9 |
| 5 | F70-TiO2(1:15) | − | 18.4 | 6.36 |
| 6 | F70-TiO2(1:20) | + | 97.6 | 83.1 |
| 7 | F70 | + | 83.8 | 86.0 |
| 8 | 1%Pd/F70-TiO2(1:15) | + | 96.6 | 88.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, H.; Li, B.; Su, S.; Zhong, X.; Kong, D.; Chen, Y.; Song, Y. Enhanced Photocatalytic Coupling of Benzylamine to N-Benzylidene Benzylamine over the Organic–Inorganic Composites F70-TiO2 Based on Fullerenes Derivatives and TiO2. Molecules 2023, 28, 4301. https://doi.org/10.3390/molecules28114301
Guo Y, Li H, Li B, Su S, Zhong X, Kong D, Chen Y, Song Y. Enhanced Photocatalytic Coupling of Benzylamine to N-Benzylidene Benzylamine over the Organic–Inorganic Composites F70-TiO2 Based on Fullerenes Derivatives and TiO2. Molecules. 2023; 28(11):4301. https://doi.org/10.3390/molecules28114301
Chicago/Turabian StyleGuo, Yanmeng, Hang Li, Bo Li, Shizhuo Su, Xin Zhong, Derui Kong, Yifan Chen, and Yujie Song. 2023. "Enhanced Photocatalytic Coupling of Benzylamine to N-Benzylidene Benzylamine over the Organic–Inorganic Composites F70-TiO2 Based on Fullerenes Derivatives and TiO2" Molecules 28, no. 11: 4301. https://doi.org/10.3390/molecules28114301
APA StyleGuo, Y., Li, H., Li, B., Su, S., Zhong, X., Kong, D., Chen, Y., & Song, Y. (2023). Enhanced Photocatalytic Coupling of Benzylamine to N-Benzylidene Benzylamine over the Organic–Inorganic Composites F70-TiO2 Based on Fullerenes Derivatives and TiO2. Molecules, 28(11), 4301. https://doi.org/10.3390/molecules28114301





