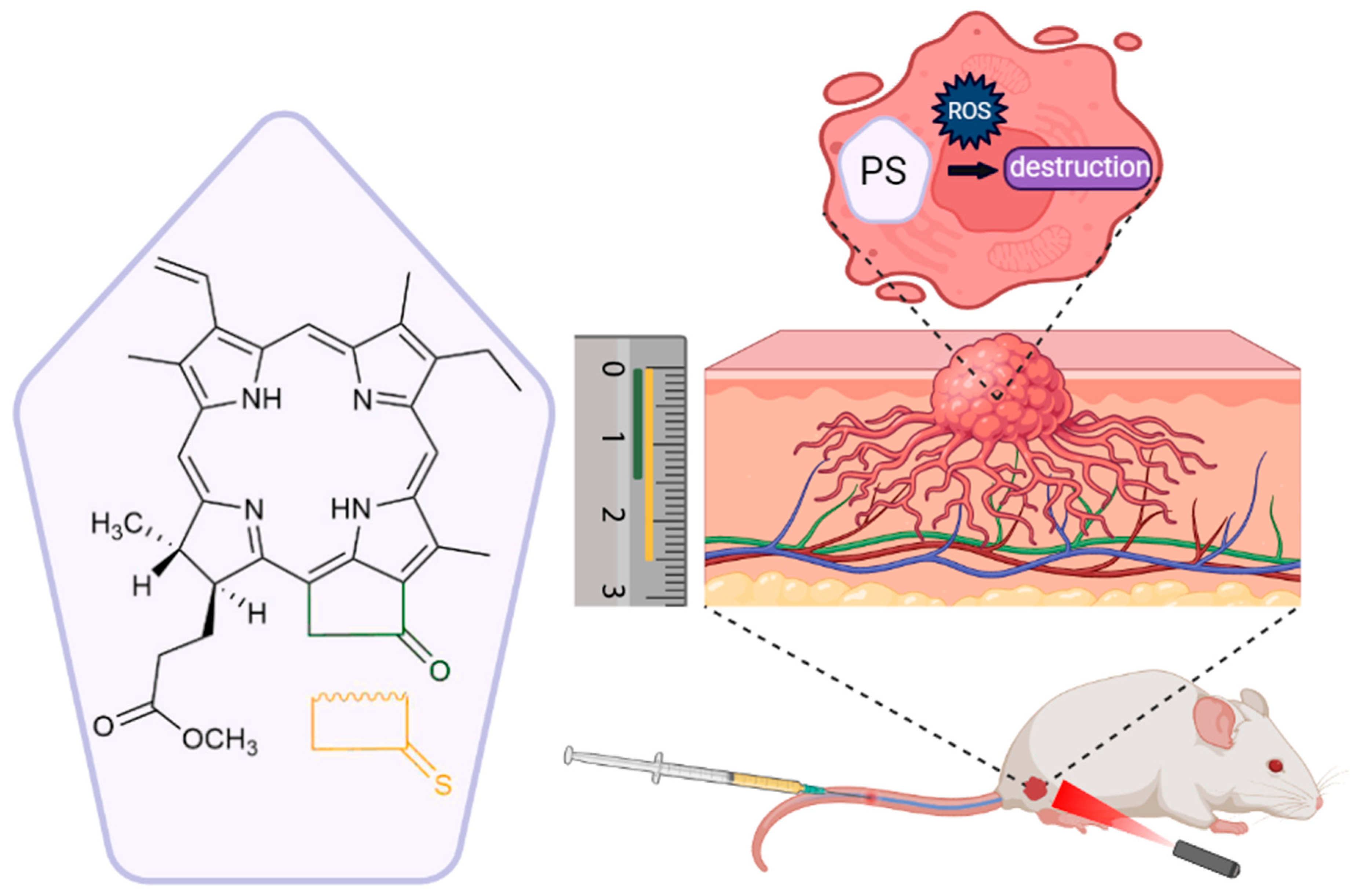
Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties
Abstract
1. Introduction
2. Results and Discussion
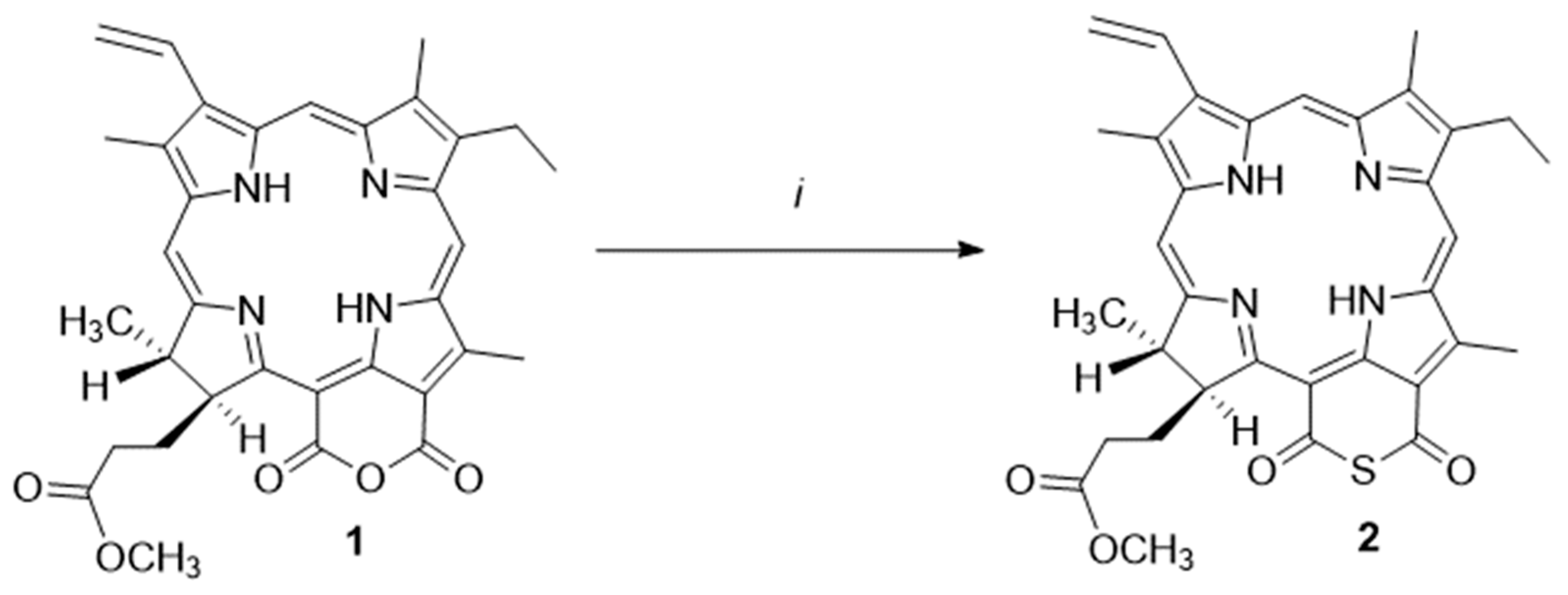
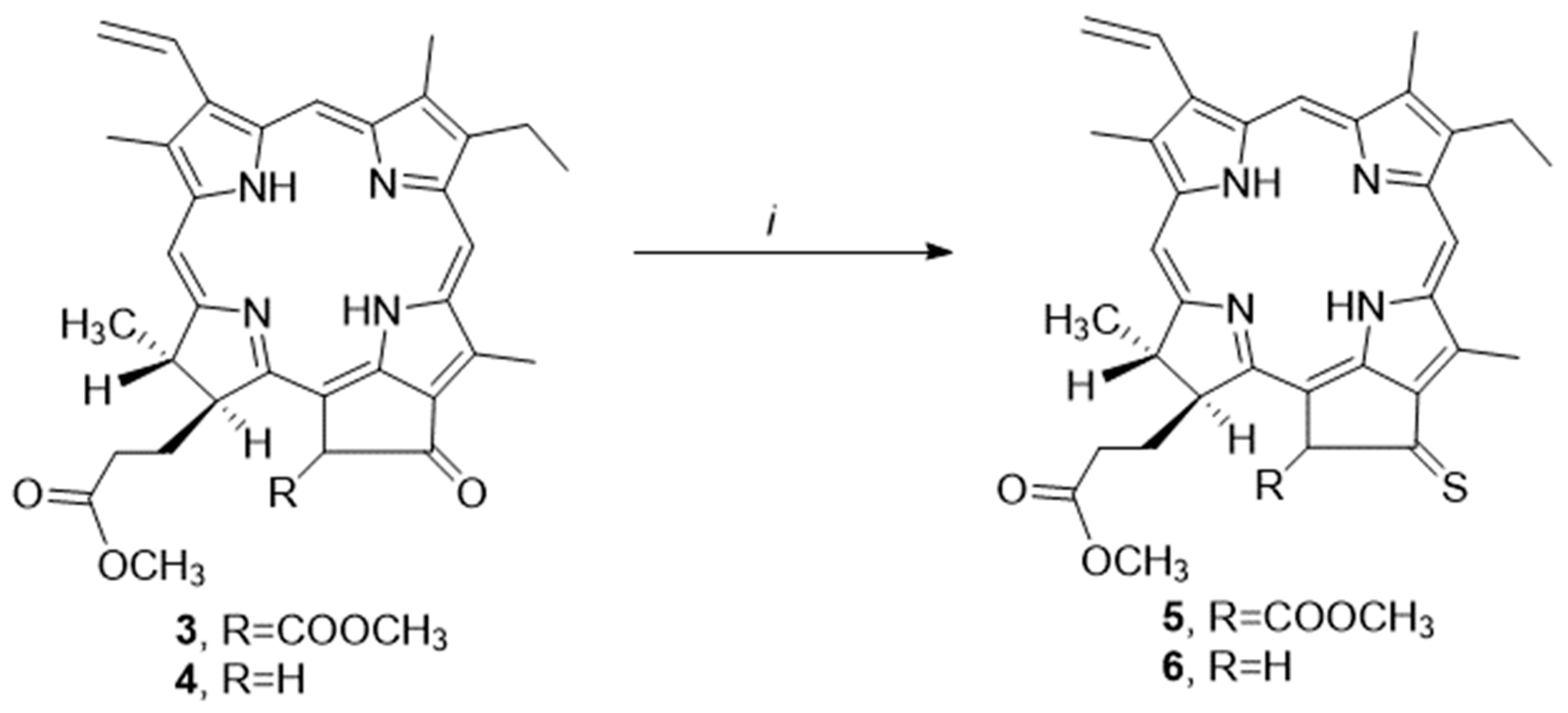
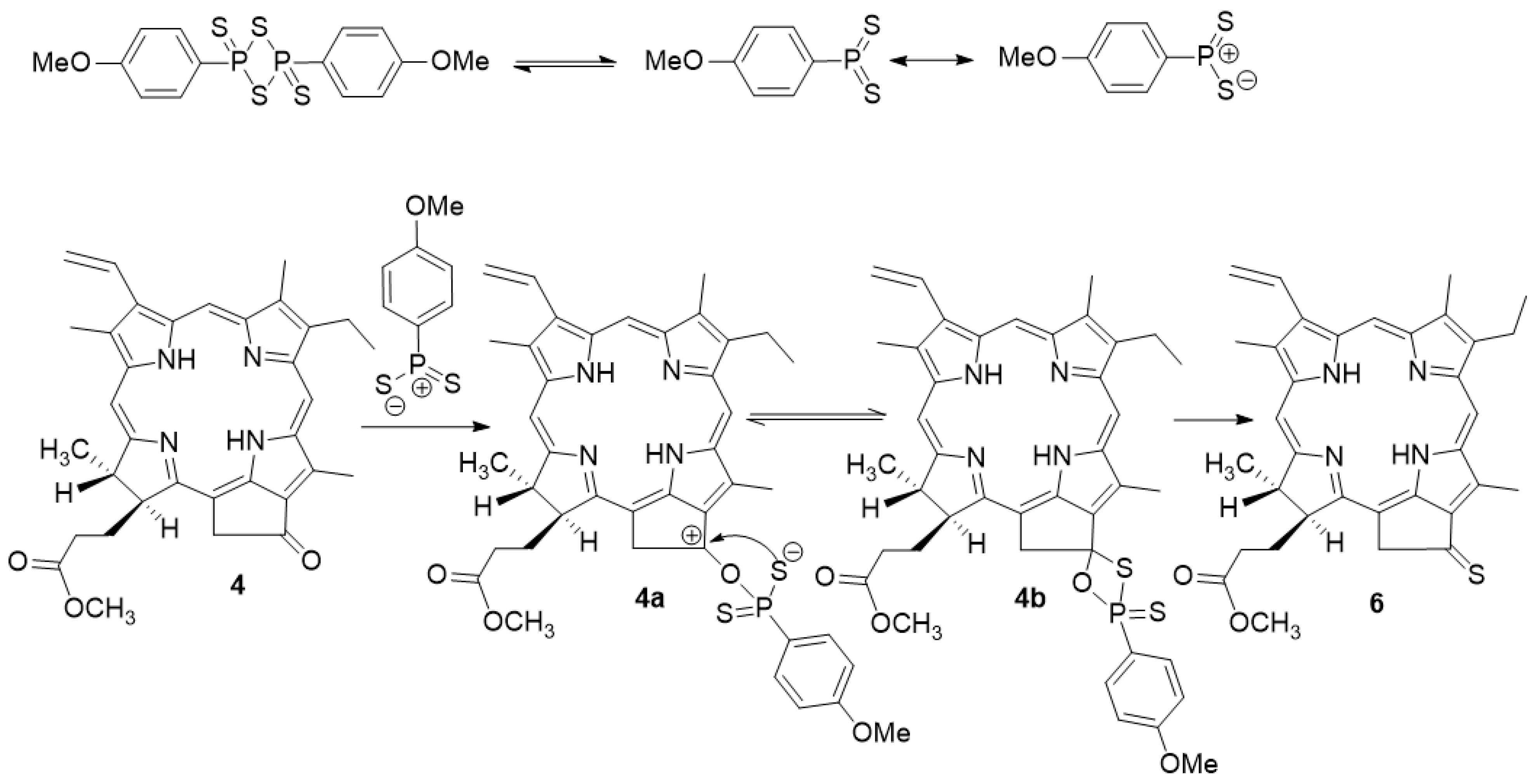
2.1. Synthesis Thiocarbonyl Derivatives
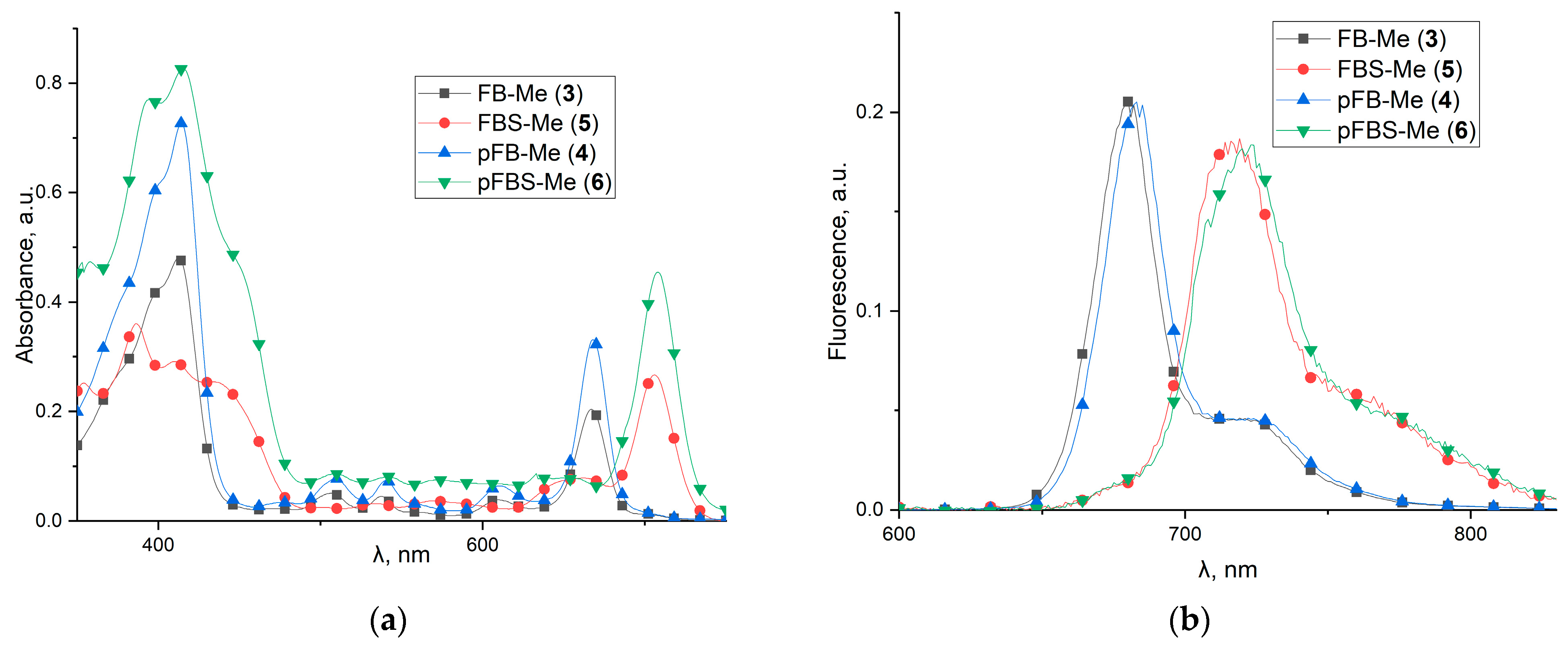
2.2. UV-Vis Spectroscopy, Fluorescence, and Stability
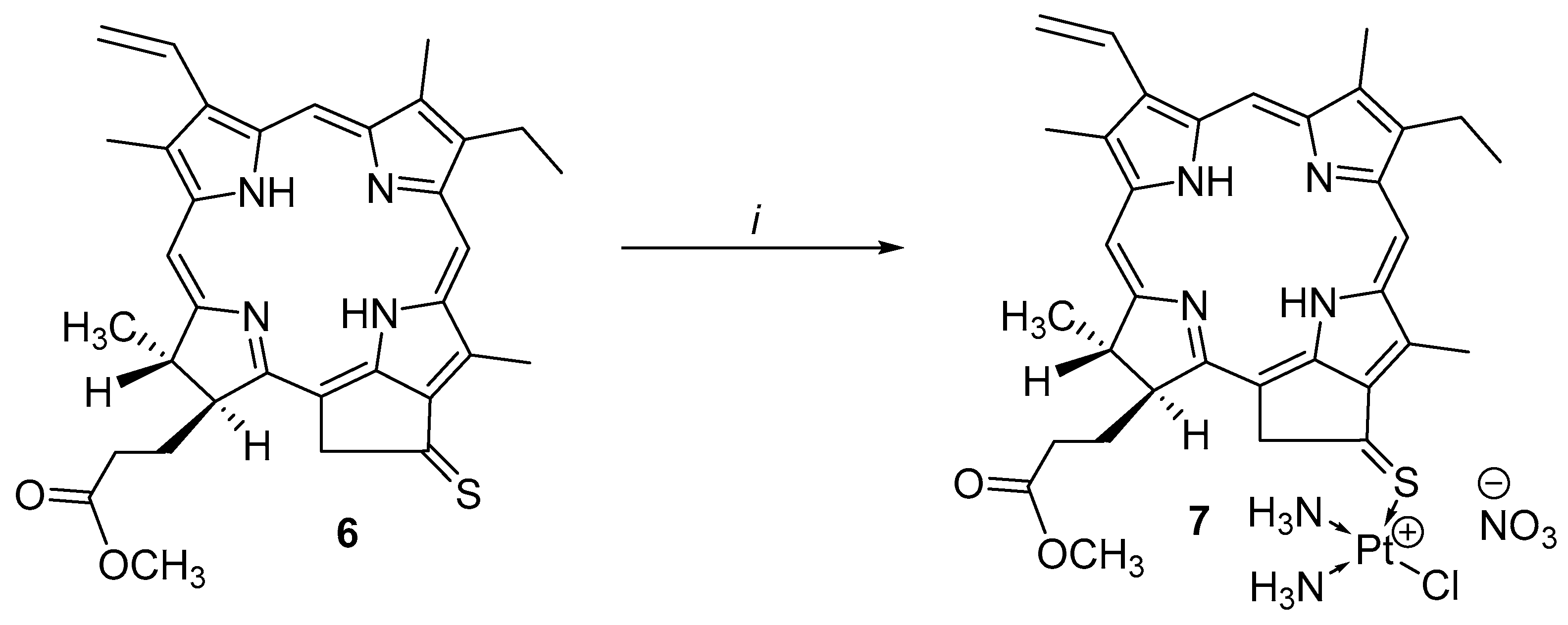
2.3. Synthesis Platinum Complex (II)
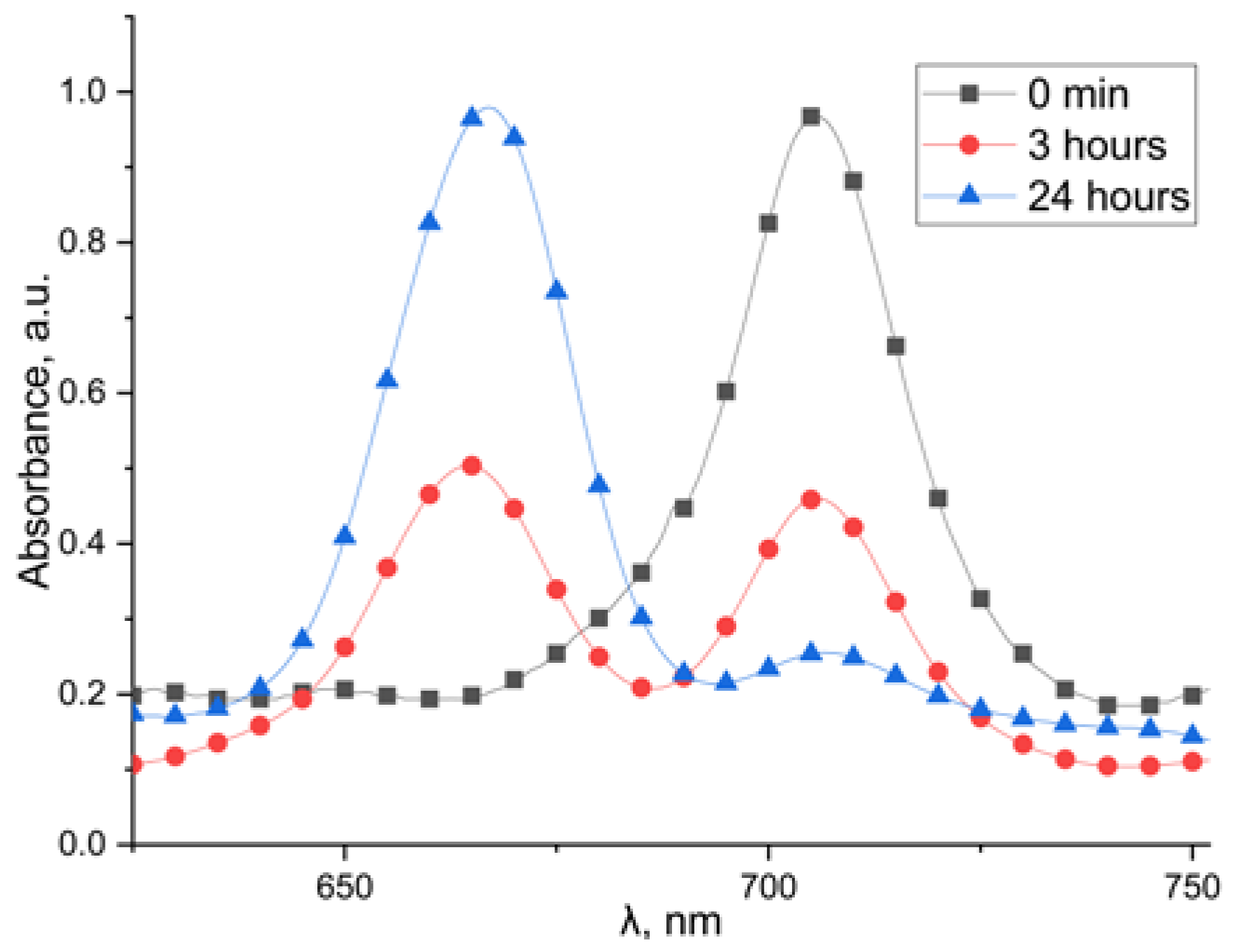
2.4. Stability of Platinum Complexes (II)
2.5. Biological Studies In Vitro
3. Materials and Methods
3.1. Synthesis
3.1.1. Cyclic Thioanhydride of Chlorin p6 (2)
3.1.2. Pheophorbide a 131-Thioketone (5)
3.1.3. Pyropheophorbide a 131-Thioketone (6)
3.1.4. Platinum Complex of Pyropheophorbide a 131-Thioketone (7)
3.2. Preparation of Micellar Solutions of Compounds (4) and (7)
3.3. Biological Studies In Vitro
3.3.1. Characterization of Test Systems In Vitro
3.3.2. Preparation of the Substances to Be Studied
3.3.3. Procedure for Studying Photoinduced and Dark Activity In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagn. Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, E.V. Clinical implementation and scientific development of photodynamic therapy in Russia in 2010–2020. Biomed. Photonics 2021, 10, 4–22. [Google Scholar] [CrossRef]
- Yi, M.; Xiong, B.; Li, Y.; Guo, W.; Huang, Y.; Lu, B. Manipulate tumor hypoxia for improved photodynamic therapy using nanomaterials. Eur. J. Med. Chem. 2022, 247, 115084. [Google Scholar] [CrossRef]
- Otvagin, V.F.; Kuzmina, N.S.; Kudriashova, E.S.; Nyuchev, A.V.; Gavryushin, A.E.; Fedorov, A.Y. Conjugates of porphyrinoid-based photosensitizers with cytotoxic drugs: Current progress and future directions toward selective photodynamic therapy. J. Med. Chem. 2022, 65, 1695–1734. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazonova, A.; Filonenko, E. Advantages of combined photodynamic therapy in the treatment of oncological diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef]
- Krishnaswami, V.; Natarajan, B.; Sethuraman, V.; Natesan, S.; RajSelvaraj, B. Nano based photodynamic therapy to target tumor microenvironment. Nano Trends 2023, 1, 100003. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef]
- Xu, B.; He, P.; Wang, Y.; Wang, H.; Zhang, J.; Zhu, J.; Pu, W.; Chen, H. PDT for Gastric Cancer—The View from China. Photodiagn. Photodyn. Ther. 2023, 42, 103366. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H.; Downie, G.H.; Cuenca, R.E. A clinical review of PDT for cutaneous malignancies. Photodiagn. Photodyn. Ther. 2006, 3, 214–226. [Google Scholar] [CrossRef]
- He, P.; Zhang, F.; Xu, B.; Wang, Y.; Pu, W.; Wang, H.; Wang, B.; Zhang, J.; Chen, H.; Li, Y. Research progress of potential factors influencing photodynamic therapy for Gastrointestinal Cancer. Photodiagn. Photodyn. Ther. 2023, 41, 103271. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2006, 473, 347–364. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Kuzmin, I.S.; Toporkov, G.A.; Yuriev, D.Y.; Kalistratova, A.V.; Kovalenko, L.V. Synthesis and biological activity of N-phosphonacetyl-L-aspartate’s structural analogs N-(α-dietoxyphosphorylcyclopropylcarbonyl)-amino acids. Fine Chem. Technol. 2020, 15, 26–35. [Google Scholar]
- Koifman, O.I.; Ageeva, T.A.; Kuzmina, N.S.; Otvagin, V.F.; Nyuchev, A.V.; Fedorov, A.Y.; Belykh, D.V.; Lebedeva, N.S.; Yurina, E.S.; Syrbu, S.A.; et al. Synthesis Strategy of Tetrapyrrolic Photosensitizers for Their Practical Application in Photodynamic Therapy. Macroheterocycles 2022, 15, 207–302. [Google Scholar] [CrossRef]
- Grin, M.A.; Tikhonov, S.I.; Petrova, A.S.; Pogorilyy, V.A.; Noev, A.N.; Tatarskiy, V.V.; Shpakovsky, D.B.; Milaeva, E.V.; Kalinina, E.V.; Chernov, A.A.; et al. New derivatives of bacteriopurpurin with thiolated Au (I) complexes: Dual darkand light activated antitumor potency. Anti-Cancer Agents Med. Chem. 2020, 20, 49–58. [Google Scholar] [CrossRef]
- Grin, M.A.; Pogorilyy, V.A.; Noev, A.N.; Tikhonov, S.I.; Majouga, A.G.; Mironov, A.F. Bacteriochlorophyll a derivatives with sulfur-containing amino acids as promising photosensitizers for cancer PDT. Macroheterocycles 2018, 11, 89–94. [Google Scholar] [CrossRef]
- Pantiushenko, I.V.; Rudakovskaya, P.G.; Starovoytova, A.V.; Mikhaylovskaya, A.A.; Abakumov, M.A.; Kaplan, M.A.; Tsygankov, A.A.; Majouga, A.G.; Grin, M.A.; Mironov, A.F. Development of bacteriochlorophyll a-based near-infrared photosensitizers conjugated to gold nanoparticles for photodynamic therapy of cancer. Biochemistry 2015, 80, 752–762. [Google Scholar] [CrossRef]
- Mironov, A.F.; Grin, M.A.; Pantushenko, I.V.; Ostroverkhov, P.V.; Ivanenkov, Y.A.; Filkov, G.I.; Plotnikova, E.A.; Karmakova, T.A.; Starovoitova, A.V.; Burmistrova, N.A.; et al. Synthesis and Investigation of photophysical and biological properties of novel s-containing bacteriopurpurinimides. J. Med. Chem. 2017, 60, 10220–10230. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Le, T.N.; Hansen, P.E.; Duus, F. Preparation and structural characterization of a new class of stable thioketones: Ortho-hydroxythioacetophenones. Tetrahedron Lett. 2006, 47, 8433–8435. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Kaushik, M.P. Recent advances in thionating reagents for the synthesis of organosulfur compounds. J. Sulfur Chem. 2006, 27, 353–386. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdulmalek, E. A focused review of synthetic applications of Lawesson’s reagent in organic synthesis. Molecules 2021, 26, 6937. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N. Lawesson’s Reagent in Heterocycle Synthesis; Springer: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Yu, Y.; Czepukojc, B.; Jacob, B.; Jiang, Y.; Zeller, M.; Brueckner, C.; Zhang, J.L. Porphothionolactones: Synthesis, structure, physical, and chemical properties of a chemodosimeter for hypochlorite. Org. Biomol. Chem. 2013, 11, 4613. [Google Scholar] [CrossRef] [PubMed]
- Richeter, S.; Jeandon, C.; Gisselbrecht, J.P.; Graff, R.; Ruppert, R.; Callot, H.J. Synthesis of New Porphyrins with Peripheral Conjugated Chelates and Their Use for the Preparation of Porphyrin Dimers Linked by Metal Ions. Inorg. Chem. 2004, 43, 251–263. [Google Scholar] [CrossRef]
- Tamiaki, H.; Xu, M.; Kinoshita, Y. Synthesis of oxo-, thioxo- and methylene-substituted bacteriochlorins by modifying chlorophyll-a and their electronic absorption in visible and near-infrared regions. J. Photochem. Photobiol. A Chem. 2013, 252, 60–68. [Google Scholar] [CrossRef]
- Pandey, R.K.; Shiau, F.Y.; Sumlin, A.B.; Dougherty, T.J.; Smith, K.M. Structure/activity relationships among photosensitizers related to pheophorbides and bacteriopheophorbides. Bioorg. Med. Chem. Lett. 1992, 2, 491–496. [Google Scholar] [CrossRef]
- Bruns, R.F.; Watson, I.A. Rules for Identifying Potentially Reactive or Promiscuous Compounds. J. Med. Chem. 2012, 55, 9763–9772. [Google Scholar] [CrossRef]
- Nhat, P.V.; Si, N.T.; Nguyen, T.; Duonh, L.; Nguyen, M.T. Elucidating the binding mechanism of thione-containing mercaptopurine and thioguanine drugs to small gold clusters. J. Comput. Chem. 2020, 41, 1748–1758. [Google Scholar] [CrossRef]
- Dekkiche, H.; Carvalho, M.-A.; Jeandon, C.; Karmazin, L.; Boundon, C.; Ruhlmann, L.; Ruppert, R. Synthesis and electrochemistry of nickel(II)porphyrins bearing external palladium(II) or platinum(II) complexes. J. Porphyr. Phthalocyanines 2021, 25(10n12), 1133–1142. [Google Scholar] [CrossRef]
- Carvalho, M.-A.; Dekkiche, H.; Nagasaki, M.; Kikkawa, Y.; Ruppert, R. Coordination-Driven Construction of Porphyrin Nano-Ribbons at a HOPG/Liquid Interface. J. Am. Chem. Soc. 2019, 141, 10137–10141. [Google Scholar] [CrossRef]
- Pogorilyy, V.; Kirin, N.; Mironov, A.; Grin, M. A new cyclic thioanhydride derived from chlorophyll a and its aurophilic properties. Dyes Pigments 2021, 184, 108858. [Google Scholar] [CrossRef]
- Pogorilyy, V.; Plyutinskaya, A.; Suvorov, N.; Diachkova, E.; Vasil’ev, Y.; Pankratov, A.; Mironov, A.; Grin, M. The First Selenoanhydride in the Series of Chlorophyll a Derivatives, Its Stability and Photoinduced Cytotoxicity. Molecules 2021, 26, 7298. [Google Scholar] [CrossRef]
- van Asselt, R.; Vanderzande, D.; Gelan, J.; Froehling, P.E.; Aagaard, O. New synthetic routes to poly (isothianaphthene). II. Mechanistic aspects of the reactions of phthalic anhydride and phthalide with phosphorus pentasulfide. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1553–1560. [Google Scholar] [CrossRef]
- Huang, T.B.; Qian, X.; Tao, Z.F.; Wang, K.; Song, G.H.; Liu, L.F. The improved synthesis, Diels-Alder reactions, and desulfuration of trithio-1, 8-naphthalic anhydride. Heteroat. Chem. Int. J. Main Group Elem. 1999, 10, 141–146. [Google Scholar] [CrossRef]
- Lakshmikantham, M.V.; Chen, W.; Cava, M.P. Thioanhydrides. 3. Synthesis, properties and Diels-Alder reactions of sulfur analogs of 1, 8-naphthalic anhydride. J. Org. Chem. 1989, 54, 4746–4750. [Google Scholar] [CrossRef]
- Kuchin, A.V.; Karmanova, L.P.; Belykh, D.V. Method of Preparing Pheophorbid Methyl Ester. RU Patent No. 2180342C2, 24 March 1999. [Google Scholar]
- Rajee, R.; Ramamurthy, V. Oxidation of thiones by singlet and triplet oxygen. Tetrahedron Lett. 1978, 19, 5127–5130. [Google Scholar] [CrossRef]
- Sánchez-Arroyo, A.J.; Pardo, Z.; Moreno, F.; Herrera, A.; Martín, N.; García-Fresnadillo, D. Photochemical Oxidation of Thioketones by Singlet Molecular Oxygen Revisited: Insights into Photoproducts, Kinetics, and Reaction Mechanism. J. Org. Chem. 2015, 80, 10575–10584. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, N.; Wang, Y.; Shi, Q.; Yu, R.; Gu, B.; Maswikiti, E.P.; Chen, H. Photodynamic therapy combined with systemic chemotherapy for unresectable extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2023, 41, 103318. [Google Scholar] [CrossRef]
- Grin, M.; Ostroverkhov, P.; Suvorov, N.; Tikhonov, S.; Popov, A.; Shelyagina, A.; Kirin, N.; Nichugovskiy, A.; Usachev, M.; Bragina, N. Potential agents for combined photodynamic and chemotherapy in oncology based on Pt (II) complexes and pyridine-containing natural chlorins. J. Porphyr. Phthalocyan. 2023, 27, 728–740. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharm. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel tetrapyrrole structure Photosensitizers for cancer Photodynamic therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Brandis, A.; Kozyrev, A.; Kurnygina, V.; Mironov, A.; Nekrasova, V.; Nikitina, T.; Fragina, A.I. The Method of Producing Purpurin-18. RU Patent No. 2054944, 27 February 1996. [Google Scholar]
- Pandey, R.K.; Dougherty, T.J.; Pallenberg, A.J. Efficient Synthesis of Pyropheophorbide a and its Derivatives. Org. Process Res. Dev. 2004, 8, 287–290. [Google Scholar]


| Thioketone | Solvent | Temperature | Time | Yield |
|---|---|---|---|---|
| 5 | toluene | 0 °C | 48 h | 24% |
| toluene | 20 °C | 4 h | 36% | |
| toluene | 35 °C | 1 h | 56% | |
| toluene | 70 °C | 30 min | 43% | |
| toluene | 110 °C | 30 min | 19% | |
| pyridine | 35 °C | 30 min | 61% | |
| toluene, DIPEA | 35 °C | 45 min | 67% | |
| toluene, Et3N | 35 °C | 45 min | 73% | |
| K2CO3 in acetonitrile | 82 °C | 60 min | 32% | |
| 6 | toluene | 0 °C | 48 h | 27% |
| toluene | 20 °C | 4 h | 39% | |
| toluene | 35 °C | 1 h | 62% | |
| toluene | 70 °C | 30 min | 54% | |
| toluene | 110 °C | 30 min | 21% | |
| pyridine | 35 °C | 30 min | 69% | |
| toluene, DIPEA | 35 °C | 45 min | 77% | |
| toluene, Et3N | 35 °C | 45 min | 82% | |
| K2CO3 in acetonitrile | 82 °C | 60 min | 33% |
| Compound 7 | Compound 4 | |||||
|---|---|---|---|---|---|---|
| Time | Incubation Medium | pH | Intensity at 675 nm | Content, % | Intensity at 666 nm | Content, % |
| 0 min | Cell culture medium | 4 | 0.91299 | 100 | 0.95382 | 100 |
| 7 | 0.91300 | 0.95379 | ||||
| 9 | 0.91298 | 0.95381 | ||||
| Cell lysate S37 | - | 0.91302 | 0.95380 | |||
| 4 h | Cell culture medium | 4 | 0.22824 | 25 | 0.66767 | 70 |
| 7 | 0.88561 | 97 | 0.88702 | 93 | ||
| 9 | 0.79429 | 87 | 0.81073 | 85 | ||
| Cell lysate S37 | - | 0.81257 | 89 | 0.86795 | 91 | |
| 24 h | Cell culture medium | 4 | 0.15520 | 17 | 0.57229 | 60 |
| 7 | 0.85822 | 94 | 0.82980 | 87 | ||
| 9 | 0.68473 | 75 | 0.74397 | 78 | ||
| Cell lysate S37 | - | 0.76692 | 84 | 0.78212 | 82 | |
| 72 h | Cell culture medium | 4 | 0.10042 | 11 | 0.55321 | 58 |
| 7 | 0.83297 | 91 | 0.75349 | 79 | ||
| 9 | 0.52770 | 65 | 0.70582 | 73 | ||
| Cell lysate S37 | - | 0.72127 | 79 | 0.72489 | 76 | |
| Compound | IC50, nM | |||
|---|---|---|---|---|
| Photoinduced cytotoxicity | Combined cytotoxicity | Dark cytotoxicity | ||
| 4 h PS incubation with cells Irradiation at 10 J/cm2, MTT after 24 h | 4 h PS incubation with cells Irradiation at 10 J/cm2, MTT after 48 h | 4 h PS incubation with cells Irradiation at 10 J/cm2, MTT after 72 h | 72 h incubation of compounds with cells, MTT | |
| (4) | 4198 ± 13 nM | 4076 ± 11 nM | 4023 ± 13 nM | >>36,400 nM |
| (7) | 478 ± 9 nM | 146 ± 7 nM | 98 ± 5 nM | 271 ± 7 nM |
| Cisplatin | - | - | - | 17,185 nM ± 15 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorilyy, V.; Ostroverkhov, P.; Efimova, V.; Plotnikova, E.; Bezborodova, O.; Diachkova, E.; Vasil’ev, Y.; Pankratov, A.; Grin, M. Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties. Molecules 2023, 28, 4215. https://doi.org/10.3390/molecules28104215
Pogorilyy V, Ostroverkhov P, Efimova V, Plotnikova E, Bezborodova O, Diachkova E, Vasil’ev Y, Pankratov A, Grin M. Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties. Molecules. 2023; 28(10):4215. https://doi.org/10.3390/molecules28104215
Chicago/Turabian StylePogorilyy, Viktor, Petr Ostroverkhov, Valeria Efimova, Ekaterina Plotnikova, Olga Bezborodova, Ekaterina Diachkova, Yuriy Vasil’ev, Andrei Pankratov, and Mikhail Grin. 2023. "Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties" Molecules 28, no. 10: 4215. https://doi.org/10.3390/molecules28104215
APA StylePogorilyy, V., Ostroverkhov, P., Efimova, V., Plotnikova, E., Bezborodova, O., Diachkova, E., Vasil’ev, Y., Pankratov, A., & Grin, M. (2023). Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties. Molecules, 28(10), 4215. https://doi.org/10.3390/molecules28104215







