Optimization and Validation of a Method to Determine Enolones and Vanillin Derivatives in Wines—Occurrence in Spanish Red Wines and Mistelles
Abstract
1. Introduction
2. Results and Discussion
- The first difference is the number of compounds analyzed, as this new methodology quantifies enolones and vanillin derivatives.
- Ethyl maltol and 3′,4′-(methylenedioxy)acetophenone addition to the initial sample. These compounds have similar chemical formulations as the analytes and, therefore, should perfectly imitate their behavior, particularly regarding the intermolecular interactions that they exert towards different matrix components. Furthermore, their addition before starting the extraction process allows the internal standards (IS) to undergo the same analytical process as the analytes, so it can make the signal of the analytes independent of the injected volume, in addition to small matrix differences, thus reducing matrix effects.
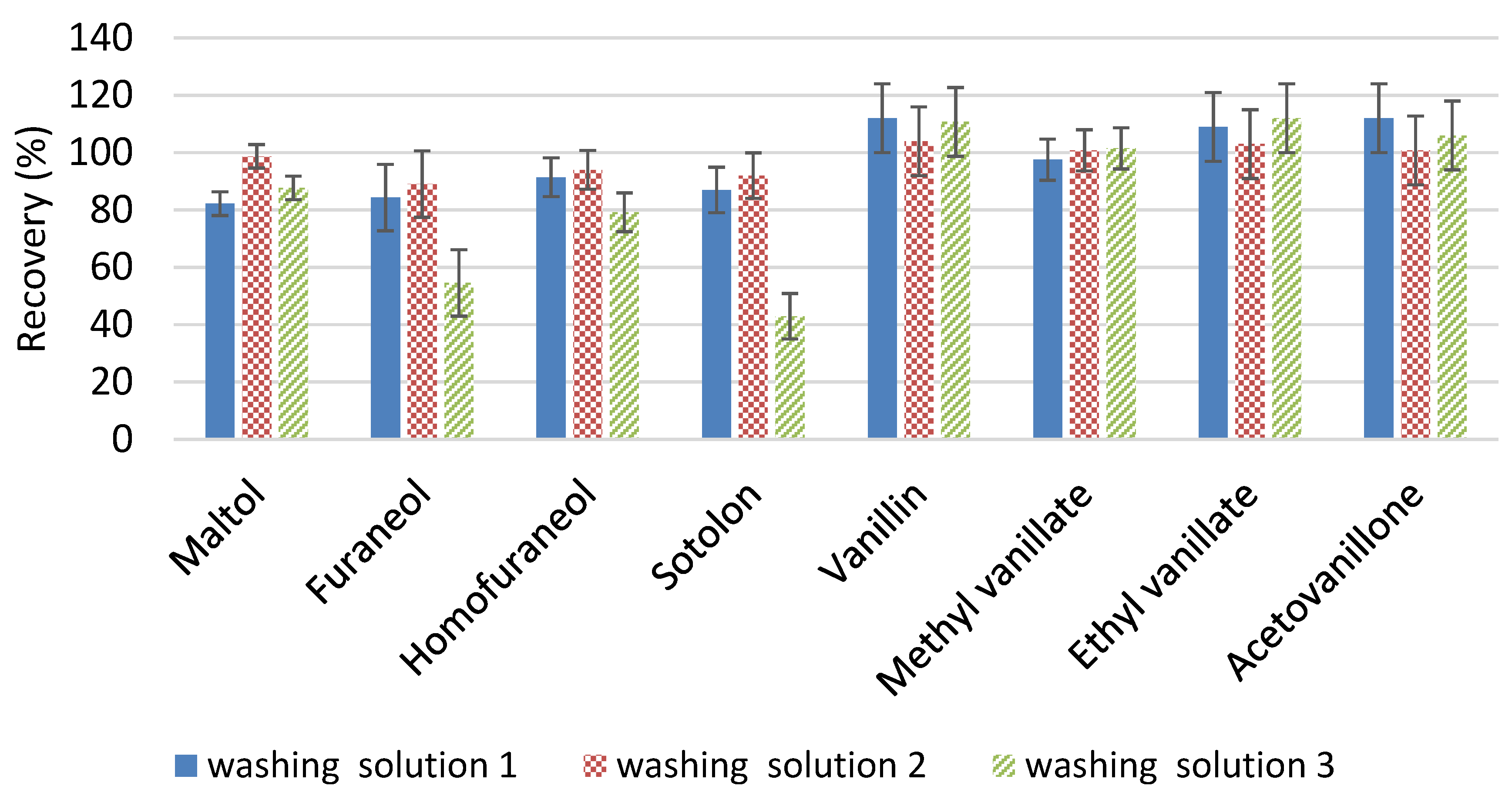
- Optimization of some stages of the extraction process such as the first washing step, the resin drying process or the lack of a need to concentrate the extract, with the time savings that this implies. The study of the washing stage deals with obtaining cleaner samples in order to improve the resolution of the chromatographic peaks and decrease the detection (LDs) and quantification limits (LQs). On the other hand, the introduction of drying stages under nitrogen stream instead of under vacuum helps to prevent the oxidation of the analytes.
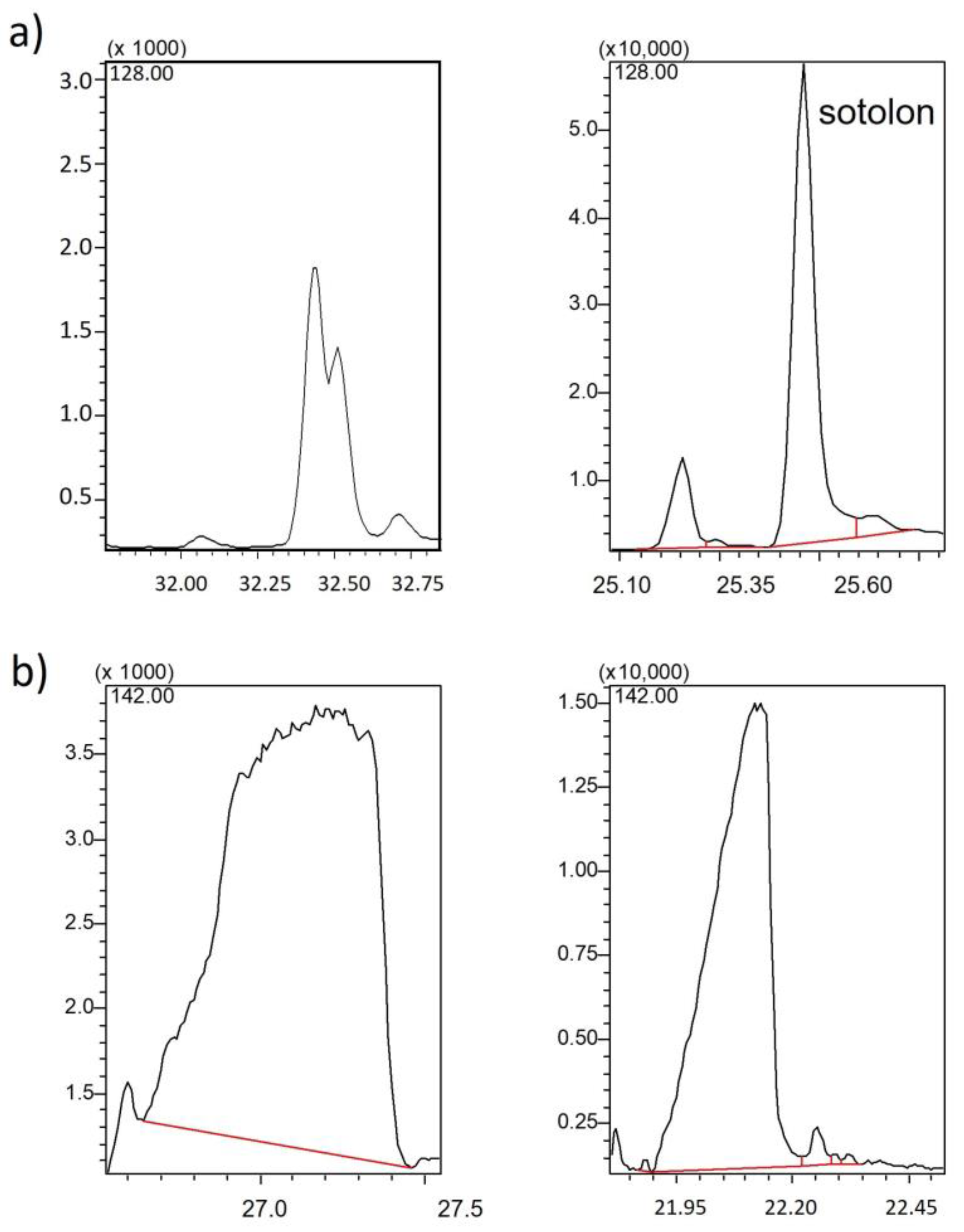
- Large volume injection, the use of acquisition windows, and acquisition in single ion monitoring (SIM) mode (Table 1) to gain sensitivity.
- Taking into consideration the societal needs towards more efficient, fast, and green analytical methods, an automatization and re-escalation of the extraction process were carried out. This implies a faster and more user-friendly method than in previous versions, with a reduction of organic solvents and resins.
| Acquisition Window | Peak Number | Compound | RT (min) | m/z | Added (μg/L) |
|---|---|---|---|---|---|
| 10.00–14.00 | 1 | 2-octanol (IS1) | 9.897 | 97 | |
| 20.70–21.65 | 2 | Maltol | 19.004 | 126 | 206 |
| 21.66–23.10 | 3 | Ethyl maltol (IS2) | 20.010 | 140 | |
| 4 | Furaneol | 20.684 | 128, 85 | 108 | |
| 23.12–25.49 | 5 | Homofuraneol | 22.124 | 142 | 131 |
| 25.51–28.49 | 6 | Sotolon | 25.533 | 128, 83 | 209 |
| 28.51–31.49 | 7 | 3′,4′-(methylenedioxy)acetophenone (IS3) | 29.010 | 149, 164 | |
| 31.51–35.99 | 8 | Vanillin | 31.630 | 151, 152 | 160 |
| 9 | Methyl vanillate | 32.069 | 151, 182 | 126 | |
| 10 | Ethyl vanillate | 32.378 | 196, 151 | 145 | |
| 11 | Acetovanillone | 32.493 | 166, 151 | 131 |
2.1. Method Optimization
2.1.1. First Washing Step
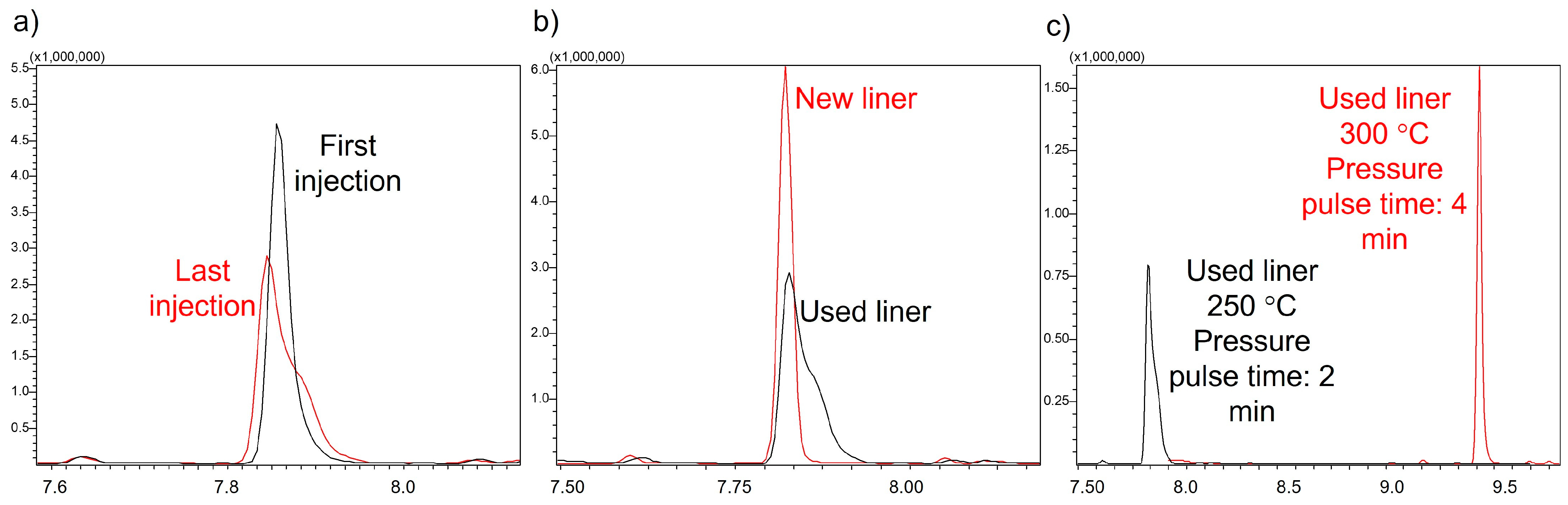
2.1.2. Injection Parameters
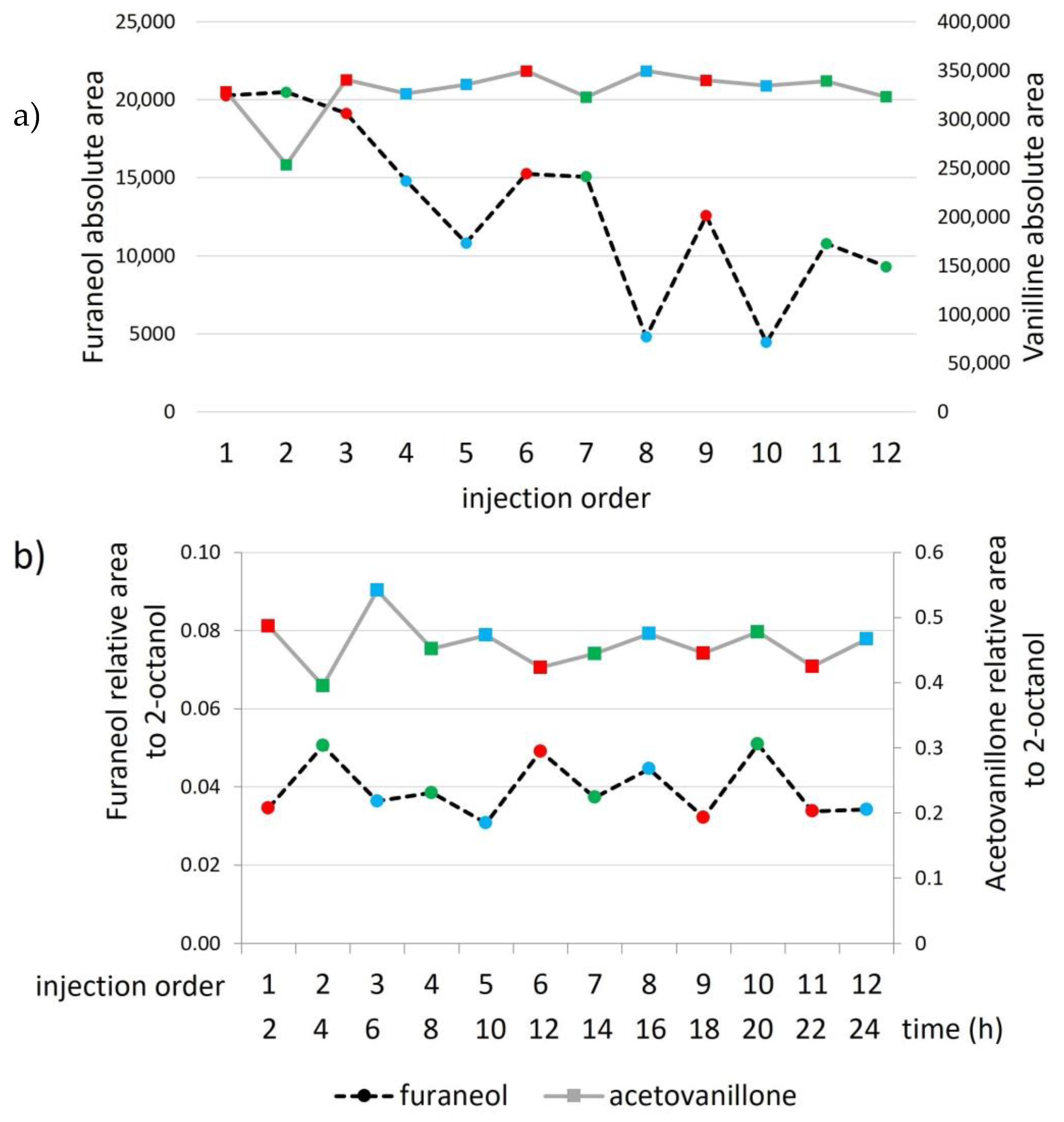
2.1.3. Extracts’ Stability
2.1.4. Automated SPE
2.2. Method Validation
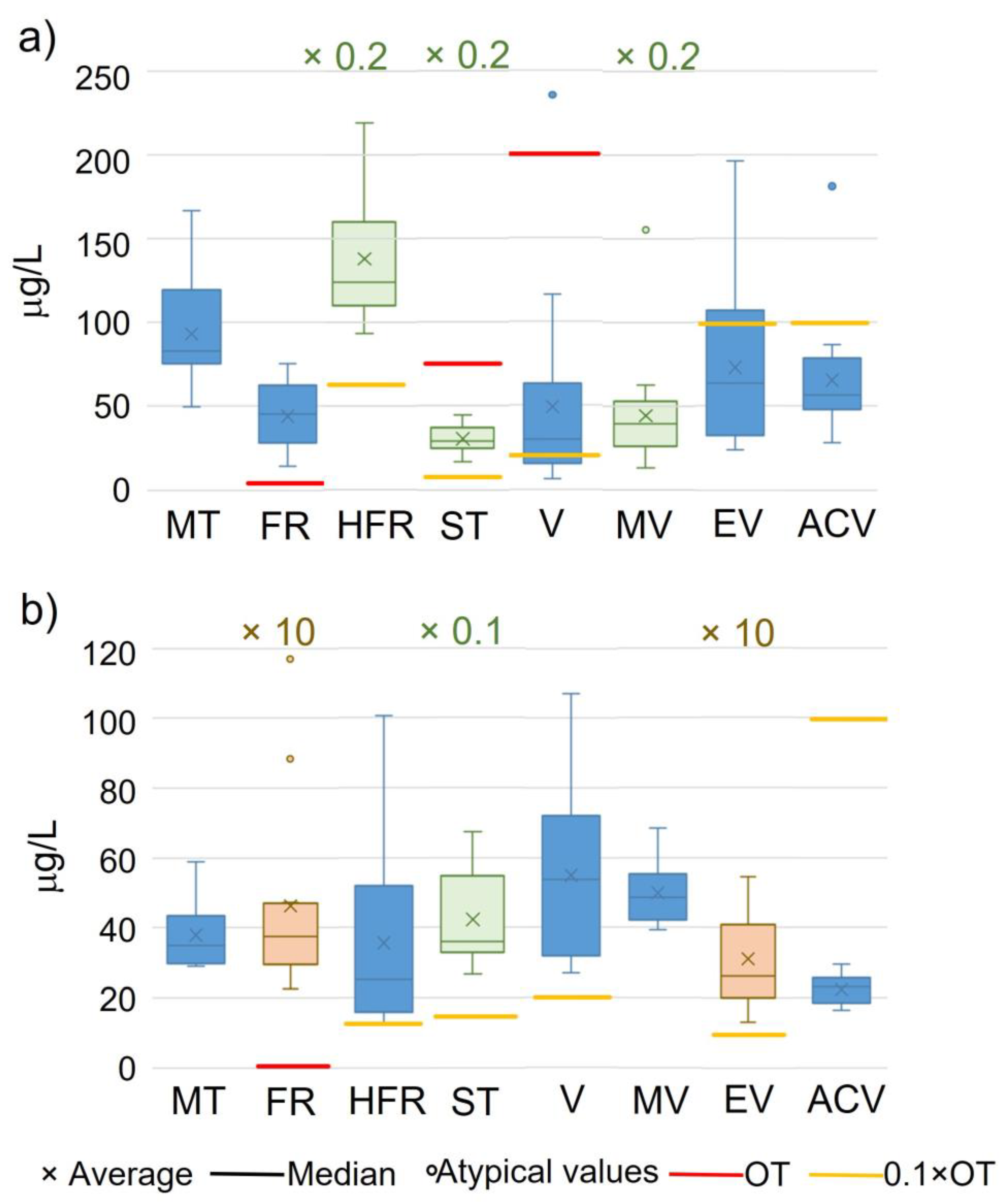
2.3. Levels of Enolones and Vanillin Derivatives in Spanish Red Wines and Mitelles
3. Materials and Methods
3.1. Reagents, Standards, Materials, and Samples
3.1.1. Solvents and Reagents
3.1.2. Solid Phase Extraction (SPE)
3.1.3. Chemical Standards
3.1.4. Samples
3.2. Method Optimization
3.2.1. First Washing-Up Step Optimization
3.2.2. Injection and GC-MS Parameter Optimization
3.2.3. Extract Stability
3.2.4. Automated SPE
3.3. New Proposed Method
3.3.1. Extraction Procedure
3.3.2. GC-MS Conditions
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- de-la-Fuente-Blanco, A.; Ferreira, V. Gas chromatography olfactometry (GC-O) for the (semi)quantitative screening of wine aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Barbe, J.-C.; Bertrand, A. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone: A key odorant of the typical aroma of oxidative aged Port wine. J. Agric. Food Chem. 2003, 51, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Camara, J.S.; Marques, J.C.; Alves, M.A.; Ferreira, A.C.S. 3-hydroxy-4,5-dimethyl-2(5H)-furanone levels in fortified Madeira wines: Relationship to sugar content. J. Agric. Food Chem. 2004, 52, 6765–6769. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.; Perestrelo, R.; Cassaca, R.; Castillo, M.; Santos, M.; Pereira, J.; Camara, J.S. A fast and environment-friendly MEPSPEP/UHPLC-PDA methodology to assess 3-hydroxy-4,5-dimethyl-2(5H)-furanone in fortified wines. Food Chem. 2017, 214, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and identification of odorants generated in wine during its oxidation: A gas chromatography-olfactometric study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]
- Lavigne, V.; Pons, A.; Darriet, P.; Dubourdieu, D. Changes in the sotolon content of dry white wines during barrel and bottle aging. J. Agric. Food Chem. 2008, 56, 2688–2693. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Hogg, T.; de Pinho, P.G. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J. Agric. Food Chem. 2003, 51, 1377–1381. [Google Scholar] [CrossRef]
- Collin, S.; Nizet, S.; Bouuaert, T.C.; Despatures, P.-M. Main odorants in Jura flor-sherry wines, relative contributions of sotolon, abhexon, and theaspirane-derived compounds. J. Agric. Food Chem. 2012, 60, 380–387. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef]
- Milheiro, J.; Vilamarim, R.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. An accurate single-step LLE method using keeper solvent for quantification of trace amounts of sotolon in Port and white table wines by HPLC-DAD. Food Chem. 2021, 350, 129268. [Google Scholar] [CrossRef]
- Pereira, V.; Leca, J.M.; Gaspar, J.M.; Pereira, A.C.; Marques, J.C. Rapid determination of sotolon in fortified wines using a miniaturized liquid-liquid extraction followed by LC-MS/MS Analysis. J. Anal. Methods Chem. 2018, 2018, 4393040. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Buica, A.; Fracassetti, D.; Stander, M.; Tirelli, A.; du Toit, W.J. Determination of sotolon content in South African white wines by two novel HPLC-UV and UPLC-MS methods. Food Chem. 2015, 169, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Bueno, M.; Franco-Luesma, E.; Cullere, L.; Fernandez-Zurbano, P. Key Changes in Wine Aroma Active Compounds during Bottle Storage of Spanish Red Wines under Different Oxygen Levels. J. Agric. Food Chem. 2014, 62, 10015–10027. [Google Scholar] [CrossRef] [PubMed]
- San Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and aromatic sensory descriptors (mainly fresh and dry fruit character) of Spanish red wines can be predicted from their aroma-active chemical composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alanon, M.E.; Calvo, E.; Cejudo, M.J.; Diaz-Maroto, M.C.; Perez-Coello, M.S. Volatile compounds as markers of ageing in Tempranillo red wines from La Mancha DO stored in oak wood barrels. J. Chromatogr. A 2011, 1218, 4910–4917. [Google Scholar] [CrossRef]
- Herrero, P.; Saenz-Navajas, M.P.; Avizcuri, J.M.; Cullere, L.; Balda, P.; Anton, E.C.; Ferreira, V.; Escudero, A. Study of Chardonnay and Sauvignon blanc wines from DOCa Rioja (Spain) aged in different French oak wood barrels: Chemical and aroma quality aspects. Food Res. Int. 2016, 89, 227–236. [Google Scholar] [CrossRef]
- Matejicek, D.; Klejdus, B.; Mikes, O.; Sterbova, D.; Kuban, V. Application of solid-phase extraction for determination of phenolic compounds in barrique wines. Anal. Bioanal. Chem. 2003, 377, 340–345. [Google Scholar] [CrossRef]
- Stegarus, D.I.; Calugar, A.; Tanase, C.; Musca, A.; Botoran, O.R.; Manolache, M.; Babes, A.C.; Bunea, C.; Gal, E.; Bunea, A.; et al. Influence of oak chips and oak barrel ageing on volatile profile in Chardonnay wine of Romania. Appl. Sci. 2021, 11, 3691. [Google Scholar] [CrossRef]
- Arias-Perez, I.; Ontanon, I.; Ferreira, V.; Escudero, A. Maturation of Moristel in different vineyards: Amino acid and aroma composition of mistelles and wines with particular emphasis in Strecker aldehydes. Foods 2022, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Hevia, K.; Castro, R.; Natera, R.; Gonzalez-Garcia, J.A.; Barroso, C.G.; Duran-Guerrero, E. Optimization of head space sorptive extraction to determine volatile compounds from oak wood in fortified wines. Chromatographia 2016, 79, 763–771. [Google Scholar] [CrossRef]
- Karagiannis, S.; Economou, A.; Lanaridis, P. Phenolic and volatile composition of wines made from Vitis vinifera cv. muscat lefko grapes from the Island of Samos. J. Agric. Food Chem. 2000, 48, 5369–5375. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Jarauta, I.; Lopez, R.; Cacho, J. Quantitative determination of sotolon, maltol and free furaneol in wine by solid-phase extraction and gas chromatography-ion-trap mass spectrometry. J. Chromatogr. A 2003, 1010, 95–103. [Google Scholar] [CrossRef]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Gabrielli, M.; Fracassetti, D.; Tirelli, A. UHPLC quantification of sotolon in white wine. J. Agric. Food Chem. 2014, 62, 4878–4883. [Google Scholar] [CrossRef]
- Monde, K.; Nakahashi, A.; Miura, N.; Yaguchi, Y.; Sugimoto, D.; Emura, M. Stereochemical study of a novel tautomeric furanone, homofuraneol. Chirality 2009, 21, E110–E115. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortin, N.; Escudero, A.; Lopez, R.; Cacho, J. Chemical characterization of the aroma of Grenache rose wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Martin, B.; Etievant, P.X.; Lequere, J.L.; Schlich, P. More clues about sensory impact of sotolon in some flor Sherry wines. J. Agric. Food Chem. 1992, 40, 475–478. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Chen, S.; Wang, D.; Xu, Y. Characterization of odor-active compounds in sweet-type Chinese rice wine by aroma extract dilution analysis with special emphasis on sotolon. J. Agric. Food Chem. 2013, 61, 9712–9718. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Qian, M.C. Quantification of selected aroma-active compounds in pinot noir wines from different grape maturities. J. Agric. Food Chem. 2006, 54, 8567–8573. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Rodríguez-Bencomo, J.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Beyond the characterization of wine aroma compounds: Looking for analytical approaches in trying to understand aroma perception during wine consumption. Anal. Bioanal. Chem. 2011, 401, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.; Josephson, D.V.A. A method for determining significance of volatile compounds in foods. J. Food Sci. 1957, 22, 316–318. [Google Scholar] [CrossRef]
- Ferreira, V.; San Juan, F.; Escudero, A.; Culleré, L.; Fernández-Zurbano, P.; Saenz-Navajas, M.P.; Cacho, J. Modeling quality of premium Spanish red wines from gas chromatography-olfactometry data. J. Agric. Food Chem. 2009, 57, 7490–7498. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Ferreira, V.; De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P. Wine aroma vectors and sensory attributes. In Managing Wine Quality, Volume I: Viticulture and Wine Quality, 2nd ed.; Reynolds, A., Ed.; Elsevier (Woodhead Publishing): Cambridge, UK, 2021. [Google Scholar]
- Ferreira, V.; de-la-Fuente-Blanco, A.; Saenz-Navajas, M.-P. A new classification of perceptual interactions between odorants to interpret complex aroma systems. Application to model wine aroma. Foods 2021, 10, 1627. [Google Scholar] [CrossRef]
- Caille, S.; Salmon, J.M.; Bouvier, N.; Roland, A.; Samson, A. Modification of the olfactory sensory characteristics of Chardonnay wine through the increase in sotolon concentration. Food Qual. Prefer. 2017, 56, 225–230. [Google Scholar] [CrossRef]
- Ferreira, V.; Saenz-Navajas, M.-P.; Campo, E.; Herrero, P.; de la Fuente, A.; Fernandez-Zurbano, P. Sensory interactions between six common aroma vectors explain four main red wine aroma nuances. Food Chem. 2016, 199, 447–456. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef]
- Cullere, L.; Bueno, M.; Cacho, J.; Ferreira, V. Selectivity and efficiency of different reversed-phase and mixed-mode sorbents to preconcentrate and isolate aroma molecules. J. Chromatogr. A 2010, 1217, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
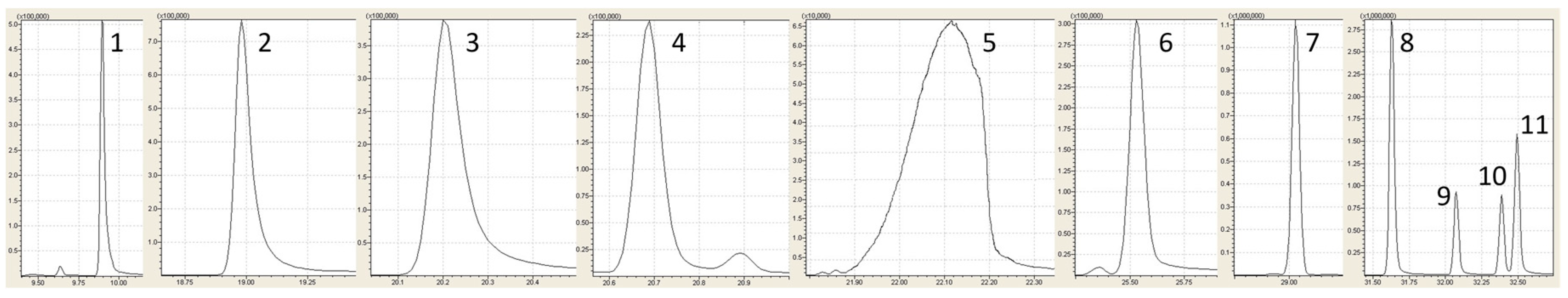
| Parameters | Ferreira et al., 2003 [24] | San Juan et al., 2011 [15] | Proposed Methodology |
|---|---|---|---|
| Adsorbent mass | 800 mg | 200 mg | 50 mg |
| Conditioning step | 8 mL MeOH 8 mL water/EtOH 13% v/v | 6 mL MeOH 6 mL water/EtOH 13% v/v | 1 mL DCM 1 mL MeOH 1 mL water/EtOH 13% v/v |
| Wine volume | 50 mL (+7.5 g (NH4)2 SO4) | 3 mL wine + 3 mL water (+0.9 g (NH4)2 SO4) | 3 mL wine + 3 mL water (+0.9 g (NH4)2 SO4) |
| Addition of IS to the sample | NO | NO | YES |
| 1st washing step | 5 mL water | 1.5 mL water | 3 mL water containing 1% NaHCO3 pH 8 |
| 1st drying step | by applying vacuum 30 min | by applying vacuum 30 min | under nitrogen stream 30 min |
| 2nd washing step | PEN:DCM (95:5, v/v) 15 mL | PEN:DCM (95:5, v/v) 6 mL | PEN:DCM (95:5, v/v) 2 mL |
| 2nd drying step | NO | NO | under nitrogen stream 10 min |
| Elution | 6 mL DCM (+2-octanol) | 1.5 mL DCM:MeOH (95:5, v/v) (+2-octanol) | 600 µL DCM:MeOH (95:5, v/v) (+2-octanol) |
| Concentration | up to 0.1 mL | up to 0.5 mL | NO |
| SPE system | S.A. system | S.A. system | A. system |
| Repeatability RSD (%) a | Reproducibility RSD (%) b | LD (μg/L) | LQ (μg/L) | OT (μg/L) | |||
|---|---|---|---|---|---|---|---|
| IS1 | IS2 | IS3 | |||||
| Maltol | 2.79 | 4.11 | 2.77 | 7.74 | 0.48 | 1.58 | 5000 [9] |
| Furaneol | 3.79 | 11.6 | 10.3 | 16.6 | 0.27 | 0.89 | 5 [28] |
| Homofuraneol | 2.00 | 6.78 | 5.47 | 12.3 | 0.69 | 2.30 | 125 [28] |
| Sotolon | 3.02 | 7.94 | 5.02 | 9.88 | 0.42 | 1.41 | 15 [29] |
| Vanillin | 3.19 | 8.60 | 7.88 | 7.30 | 0.16 | 0.52 | 200 [30] |
| Methyl vanillate | 3.26 | 7.18 | 5.91 | 4.11 | 0.52 | 1.74 | 3000 [25] |
| Ethyl vanillate | 3.02 | 8.68 | 7.72 | 3.85 | 0.18 | 0.61 | 990 [25] |
| Acetovanillone | 2.27 | 6.01 | 5.08 | 5.87 | 0.34 | 1.13 | 1000 [25] |
| (a) | ||||||||||||||
| Linearity Range | YRW1 | ARW1 | MTL1 | p | Average Slope | RSD (%) | ||||||||
| (μg/L) | Slope a | s | r2 | Slopea | s | r2 | Slope a | s | r2 | |||||
| Related to IS1 | MT | 0.48–512 | 4.36 | 0.06 | 0.9994 | 5.43 | 0.27 | 0.9950 | 4.80 | 0.17 | 0.9995 | 0.001 | 4.87 | 11.1 |
| FR | 0.27–267 | 3.10 | 0.11 | 0.9965 | 3.51 | 0.09 | 0.9988 | 3.30 | 0.10 | 0.9975 | 0.006 | 3.30 | 6.16 | |
| HFR | 0.69–325 | 5.20 | 0.04 | 0.9998 | 5.22 | 0.10 | 0.9992 | 4.80 | 0.07 | 0.9990 | 0.001 | 5.08 | 4.72 | |
| ST | 0.42–517 | 3.19 | 0.18 | 0.9906 | 3.50 | 0.04 | 0.9998 | 3.45 | 0.11 | 0.9952 | 0.044 | 3.38 | 4.91 | |
| V | 0.16–395 | 12.6 | 0.23 | 0.9993 | 12.5 | 0.46 | 0.9973 | 12.0 | 0.35 | 0.9958 | 0.164 | 12.4 | 2.64 | |
| MV | 0.52–313 | 23.2 | 0.04 | 1.0000 | 17.4 | 0.33 | 0.9993 | 18.0 | 0.19 | 0.9990 | <0.001 | 19.5 | 16.3 | |
| EV | 0.18–359 | 6.52 | 0.91 | 0.9624 | 4.75 | 0.54 | 0.9748 | 4.50 | 0.73 | 0.9824 | 0.030 | 5.26 | 21.0 | |
| ACV | 0.34–325 | 9.55 | 0.03 | 1.0000 | 9.59 | 0.47 | 0.9929 | 9.20 | 0.05 | 0.9931 | 0.243 | 9.44 | 2.24 | |
| (b) | ||||||||||||||
| Linearity Range | YRW1 | ARW1 | MTL1 | p | Average Slope | RSD (%) | ||||||||
| (μg/L) | Slope a | s | r2 | Slope a | s | r2 | Slope a | s | r2 | |||||
| Related to IS2 | MT | 0.48–512 | 2.49 | 0.04 | 0.9993 | 2.43 | 0.08 | 0.9979 | 2.46 | 0.20 | 0.9995 | 0.873 | 2.46 | 1.09 |
| FR | 0.27–267 | 1.76 | 0.06 | 0.9961 | 1.64 | 0.10 | 0.9928 | 1.70 | 0.10 | 0.9975 | 0.300 | 1.70 | 3.63 | |
| HFR | 0.69–325 | 2.94 | 0.18 | 0.9997 | 2.45 | 0.20 | 0.9988 | 2.70 | 0.29 | 0.9990 | 0.097 | 2.70 | 9.10 | |
| ST | 0.42–517 | 1.80 | 0.10 | 0.9904 | 1.66 | 0.07 | 0.9964 | 1.75 | 0.13 | 0.9952 | 0.054 | 1.74 | 4.26 | |
| Related to IS3 | V | 0.16–395 | 5.85 | 0.31 | 0.9963 | 6.54 | 0.47 | 0.9899 | 6.21 | 0.37 | 0.9958 | 0.174 | 6.20 | 5.53 |
| MV | 0.52–313 | 7.58 | 0.19 | 0.9982 | 7.18 | 0.11 | 0.9996 | 7.32 | 0.24 | 0.9990 | 0.092 | 7.36 | 2.77 | |
| EV | 0.18–359 | 2.27 | 0.19 | 0.9861 | 2.21 | 0.11 | 0.9953 | 2.23 | 0.75 | 0.9824 | 0.988 | 2.24 | 1.27 | |
| ACV | 0.34–325 | 4.06 | 0.15 | 0.9959 | 4.28 | 0.03 | 0.9999 | 4.08 | 0.33 | 0.9931 | 0.437 | 4.14 | 2.88 | |
| YRW2 | ARW2 | MTL2 | %R Mean | s | ta100 | p | |
|---|---|---|---|---|---|---|---|
| Maltol | 99 | 98 | 102 | 100 | 1.96 | 0.13 | 0.91 |
| Furaneol | 82 | 103 | 83 | 89 | 12.0 | 0.51 | 0.66 |
| Homofuraneol | 103 | 93 | 92 | 96 | 6.08 | 0.40 | 0.73 |
| Sotolon | 91 | 92 | 102 | 95 | 6.05 | 0.47 | 0.69 |
| Vanillin | 108 | 108 | 95 | 104 | 7.27 | 0.28 | 0.80 |
| Methyl vanillate | 99 | 105 | 98 | 101 | 3.60 | 0.13 | 0.91 |
| Ethyl vanillate | 98 | 108 | 107 | 105 | 5.30 | 0.50 | 0.67 |
| Acetovanillone | 103 | 92 | 106 | 101 | 7.18 | 0.05 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, M.; Zapata, J.; Culleré, L.; Franco-Luesma, E.; de-la-Fuente-Blanco, A.; Ferreira, V. Optimization and Validation of a Method to Determine Enolones and Vanillin Derivatives in Wines—Occurrence in Spanish Red Wines and Mistelles. Molecules 2023, 28, 4228. https://doi.org/10.3390/molecules28104228
Bueno M, Zapata J, Culleré L, Franco-Luesma E, de-la-Fuente-Blanco A, Ferreira V. Optimization and Validation of a Method to Determine Enolones and Vanillin Derivatives in Wines—Occurrence in Spanish Red Wines and Mistelles. Molecules. 2023; 28(10):4228. https://doi.org/10.3390/molecules28104228
Chicago/Turabian StyleBueno, Mónica, Julián Zapata, Laura Culleré, Ernesto Franco-Luesma, Arancha de-la-Fuente-Blanco, and Vicente Ferreira. 2023. "Optimization and Validation of a Method to Determine Enolones and Vanillin Derivatives in Wines—Occurrence in Spanish Red Wines and Mistelles" Molecules 28, no. 10: 4228. https://doi.org/10.3390/molecules28104228
APA StyleBueno, M., Zapata, J., Culleré, L., Franco-Luesma, E., de-la-Fuente-Blanco, A., & Ferreira, V. (2023). Optimization and Validation of a Method to Determine Enolones and Vanillin Derivatives in Wines—Occurrence in Spanish Red Wines and Mistelles. Molecules, 28(10), 4228. https://doi.org/10.3390/molecules28104228








