Dextran Methacrylate Reactions with Hydroxyl Radicals and Hydrated Electrons in Water: A Kinetic Study Using Pulse Radiolysis
Abstract
1. Introduction
2. Results
2.1. Generation of Hydroxyl Radicals and Hydrated Electrons and Their Reactivity towards Polysaccharides in Dilute Aqueous Solutions
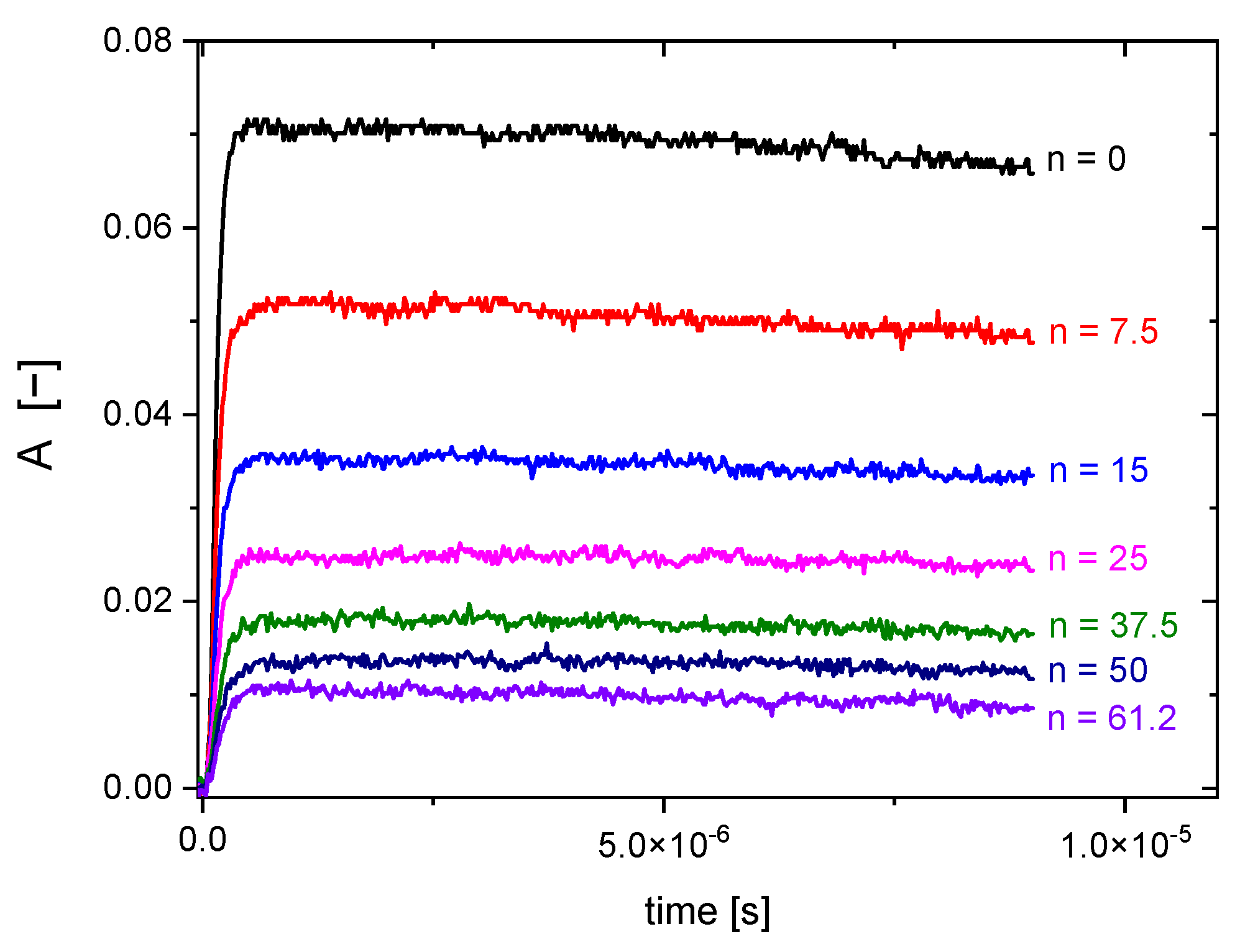
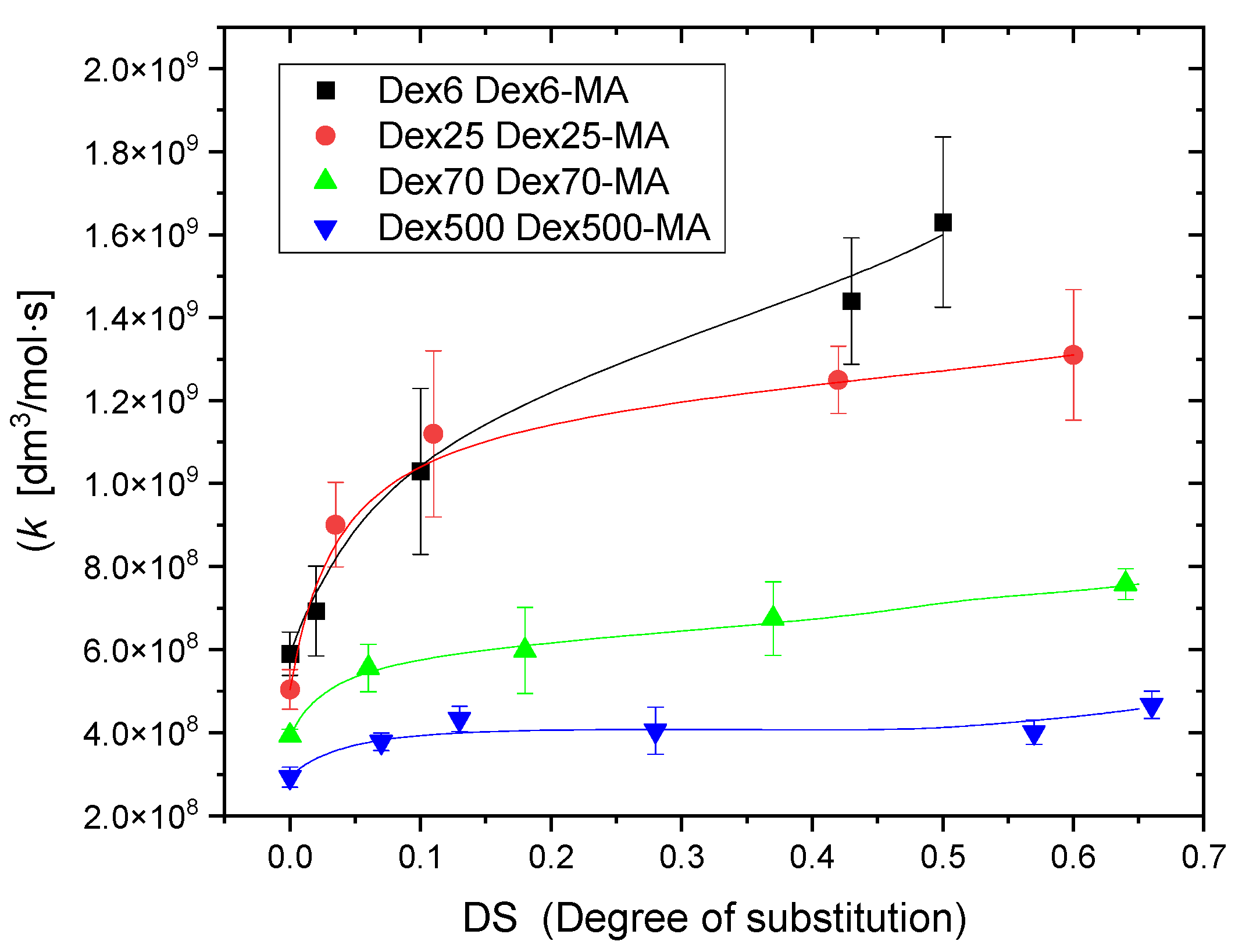
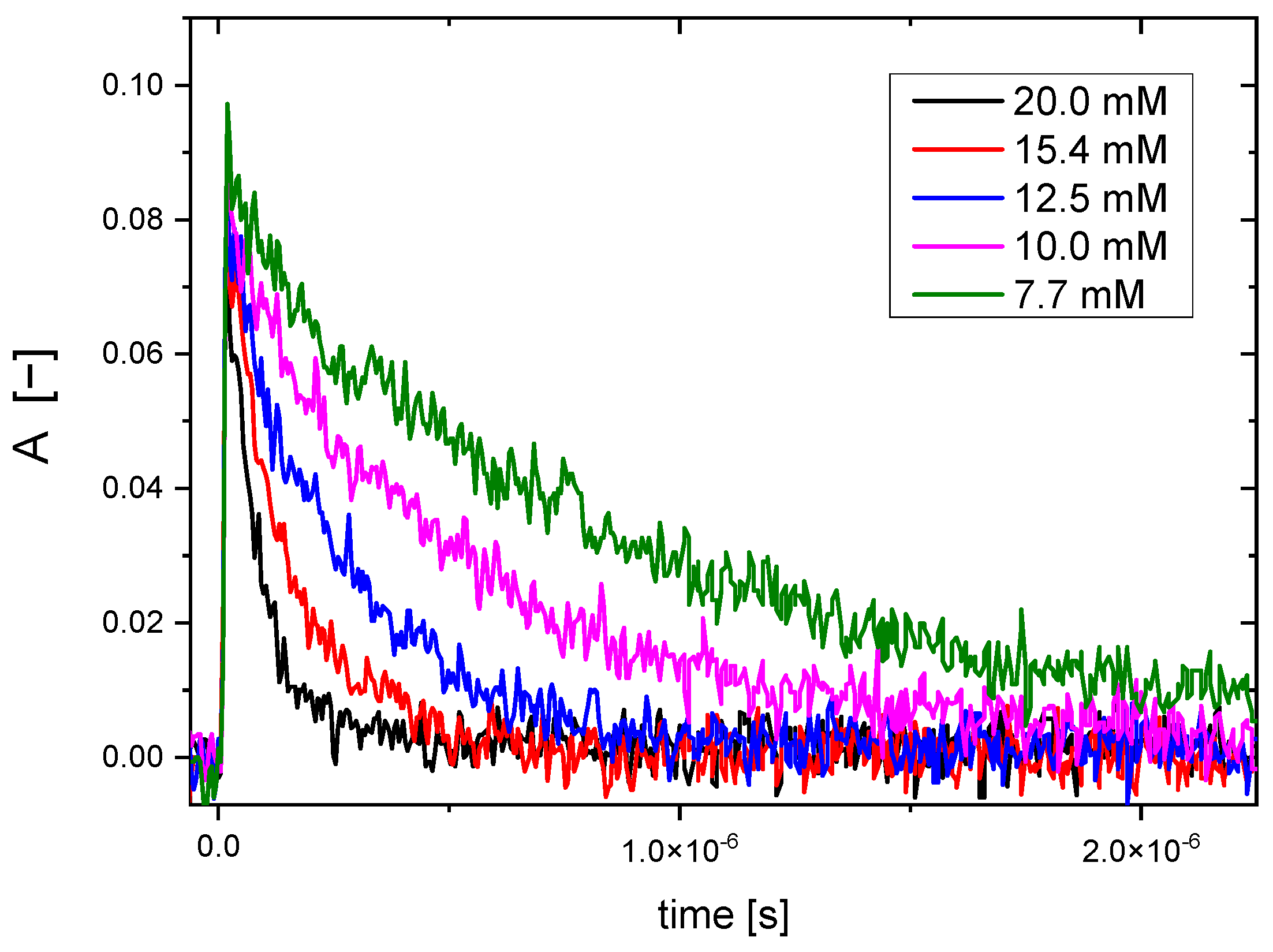
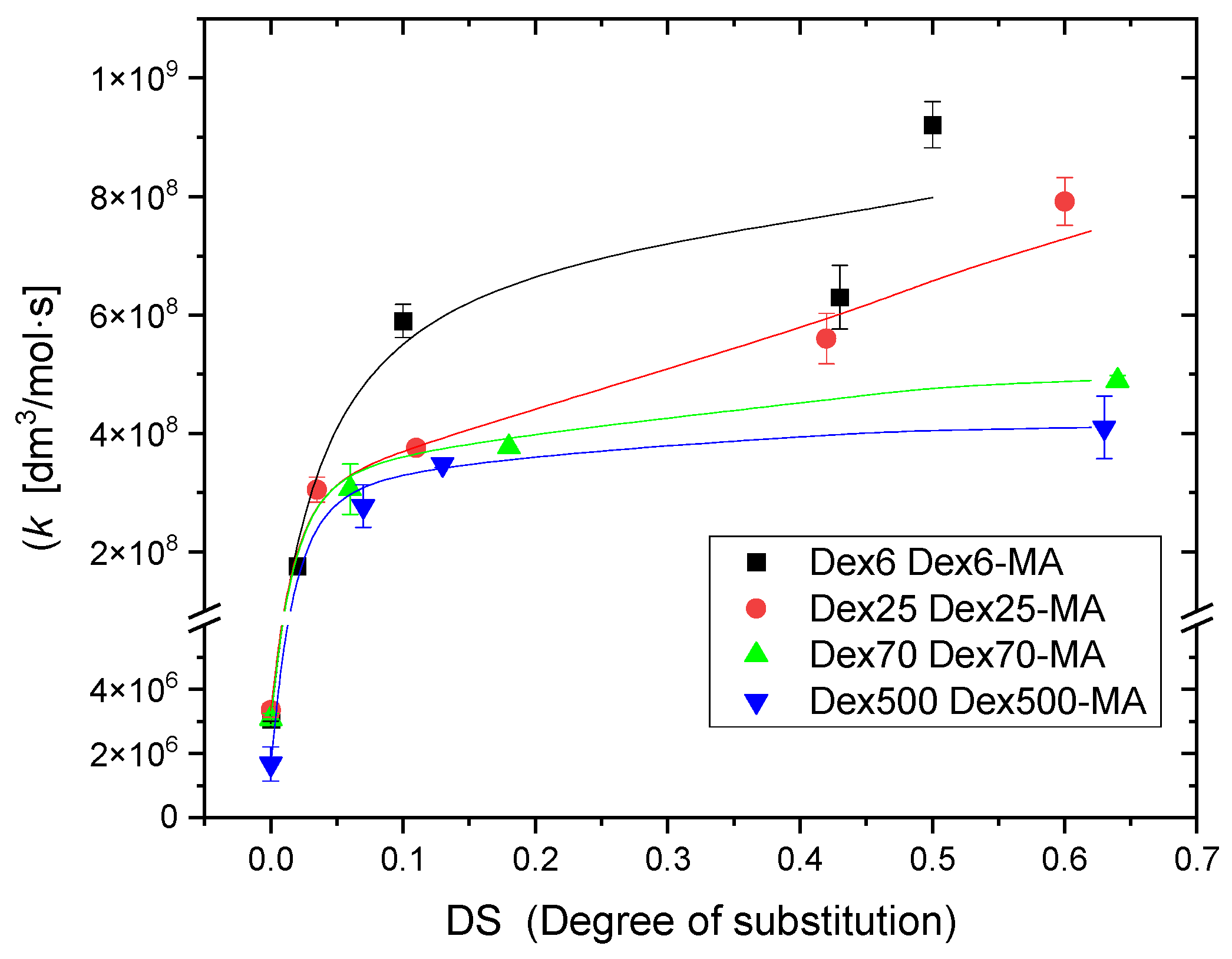
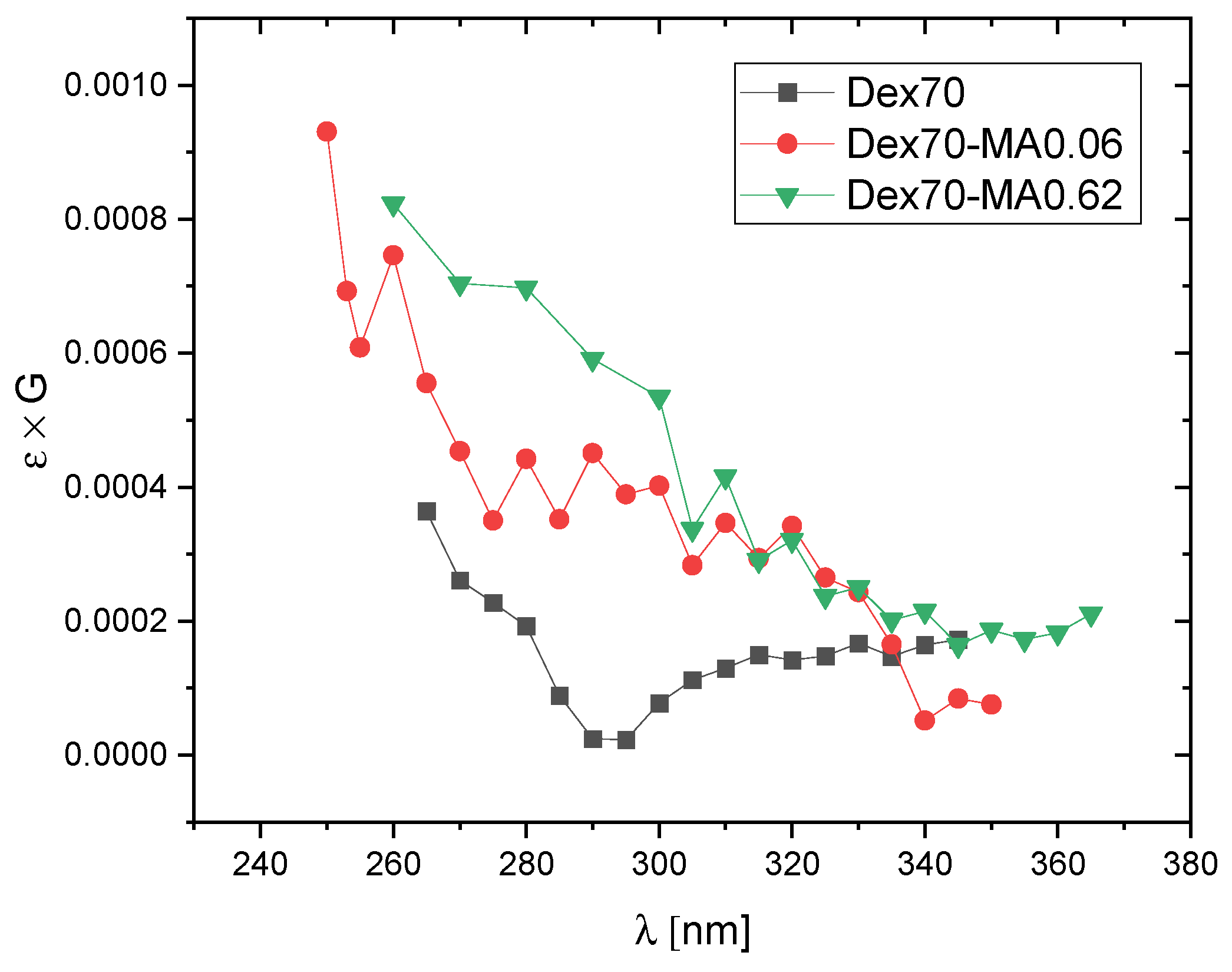
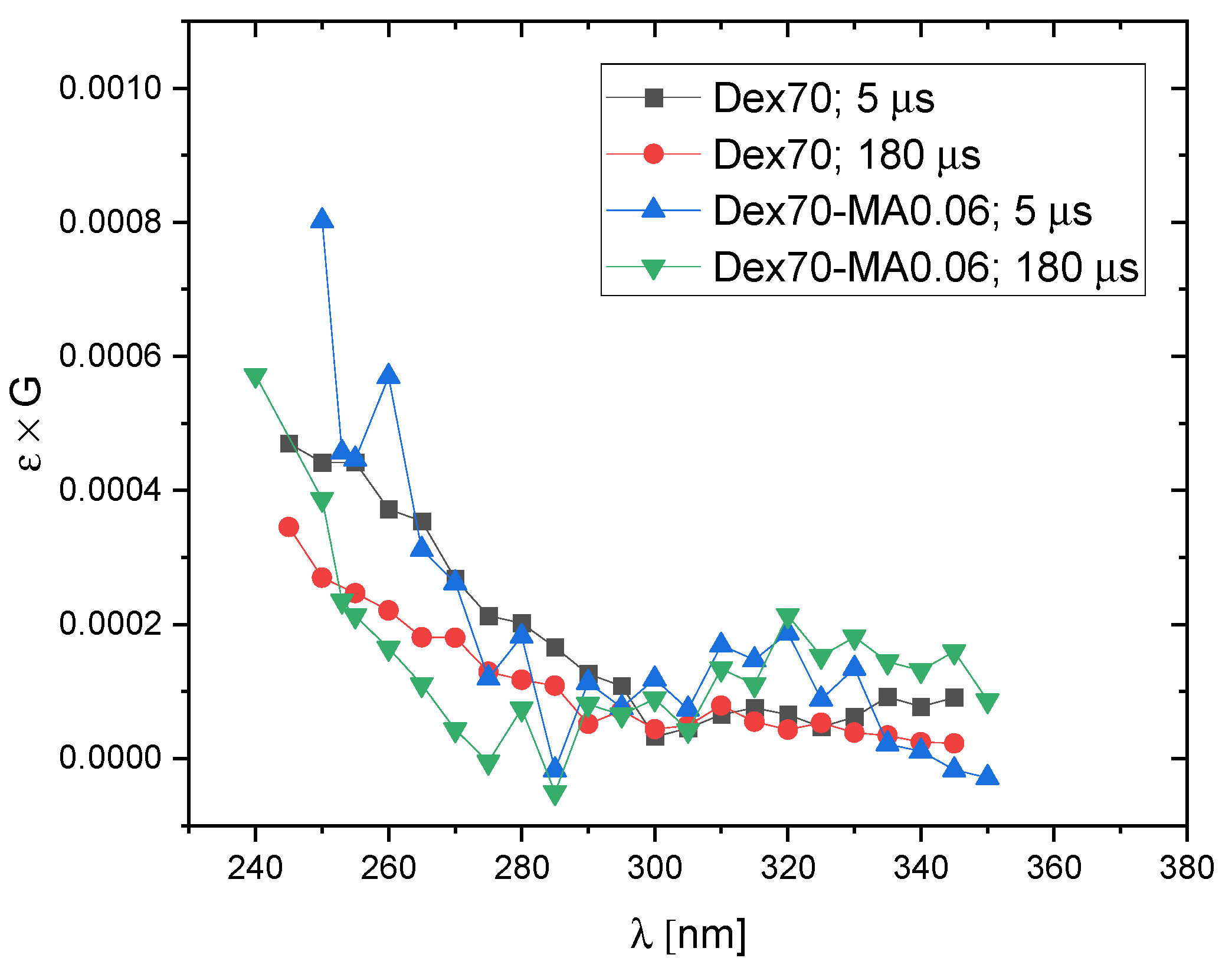
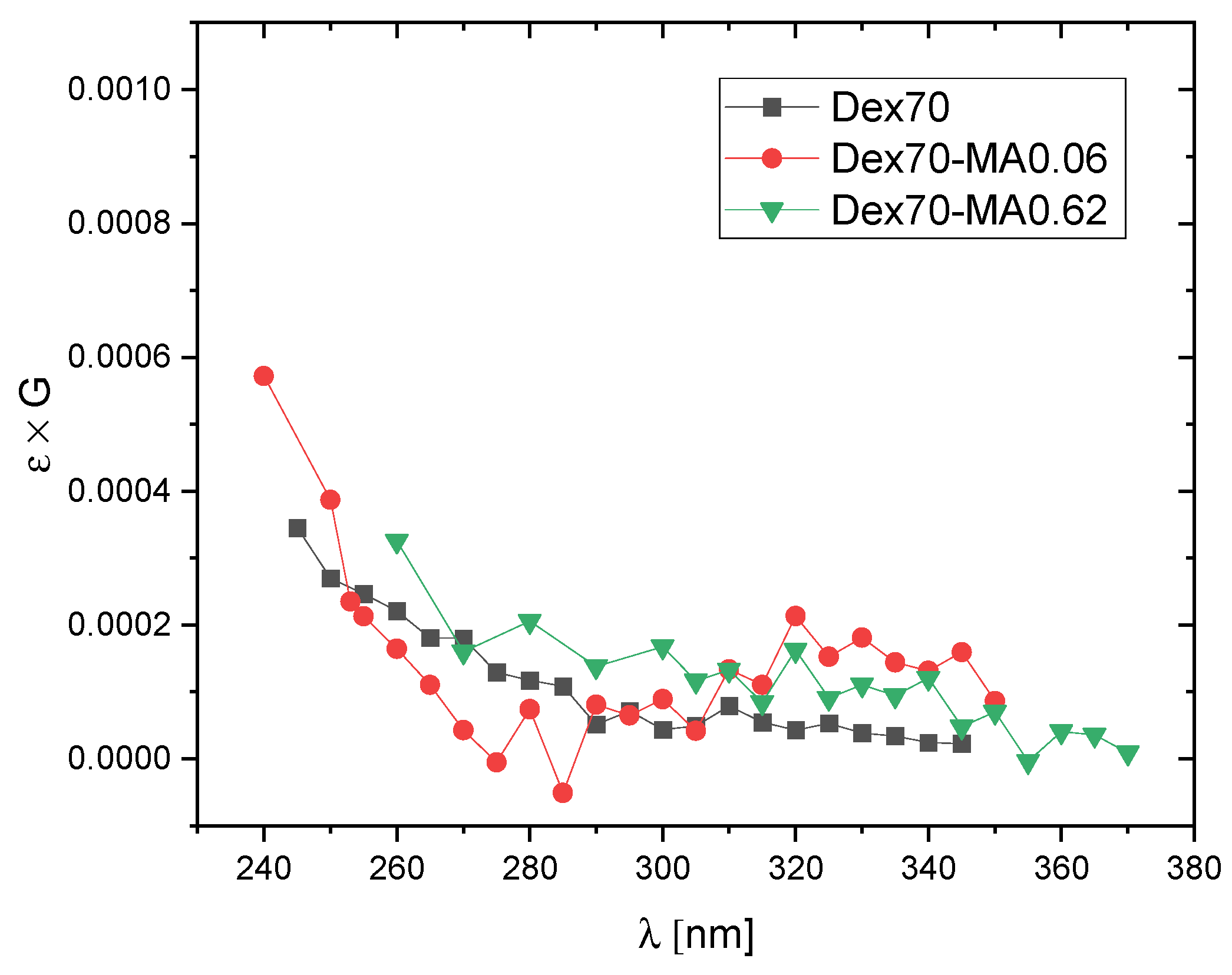
2.2. Reactivity of Hydroxyl Radicals with Dextran and Dextran Methacrylate
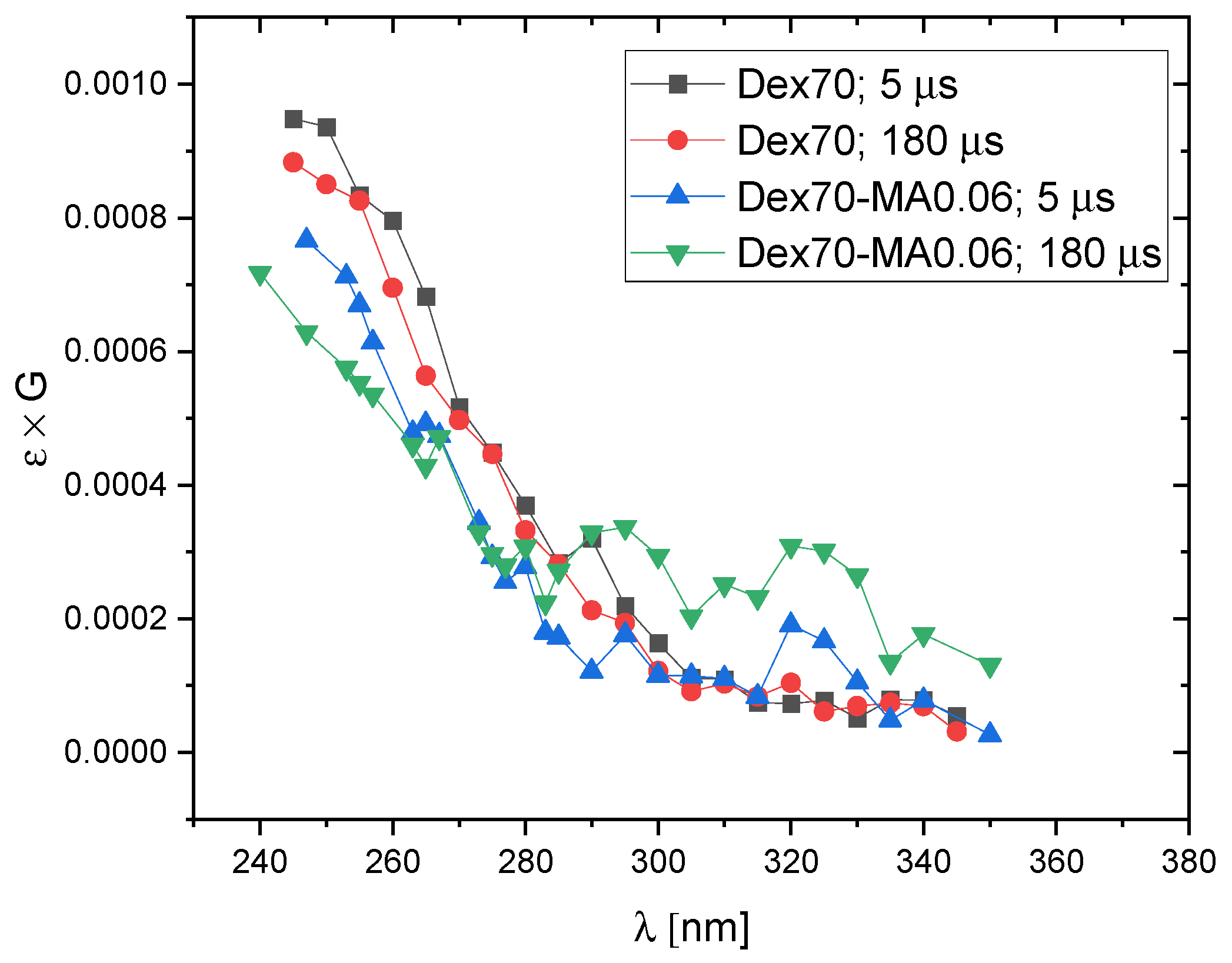
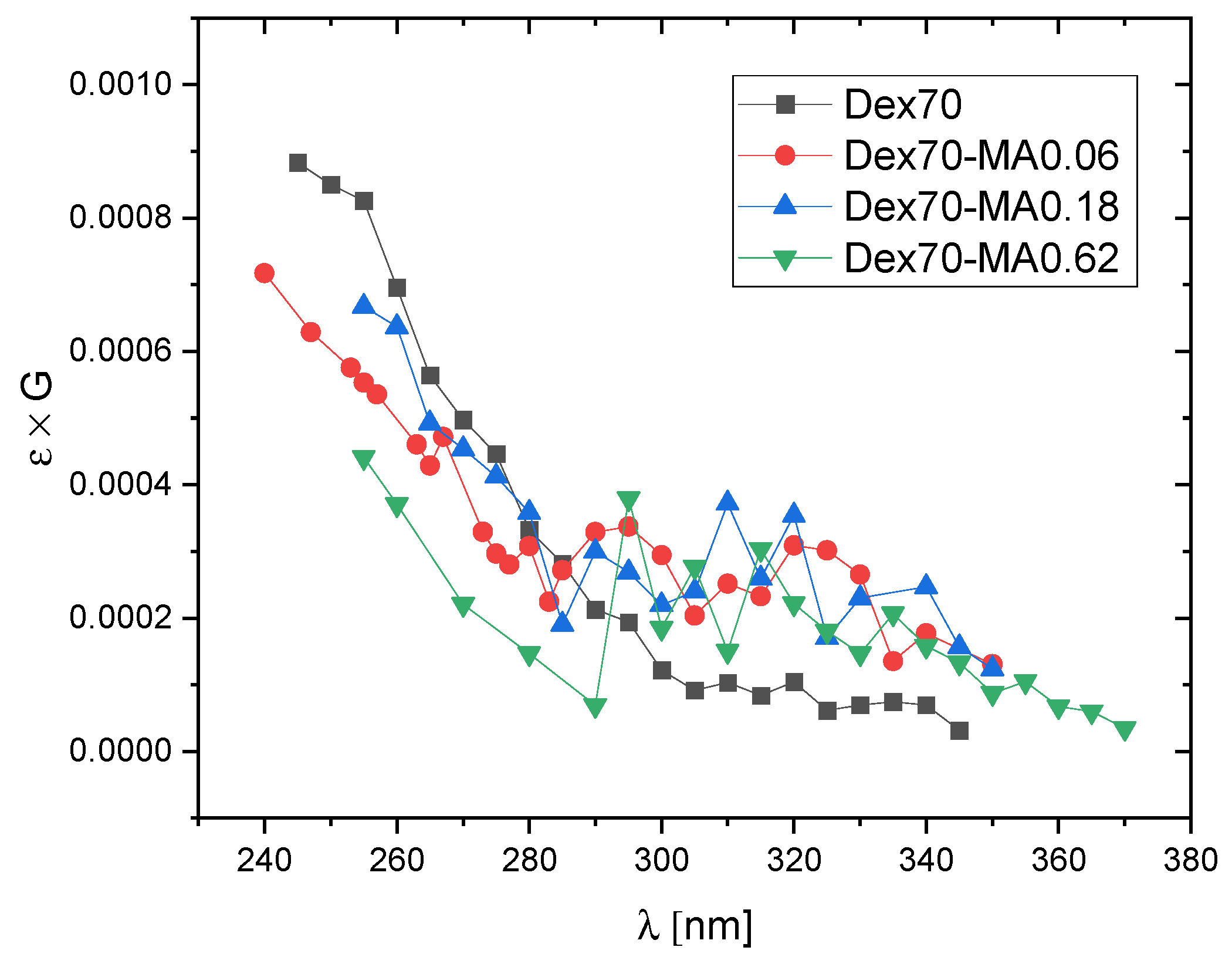
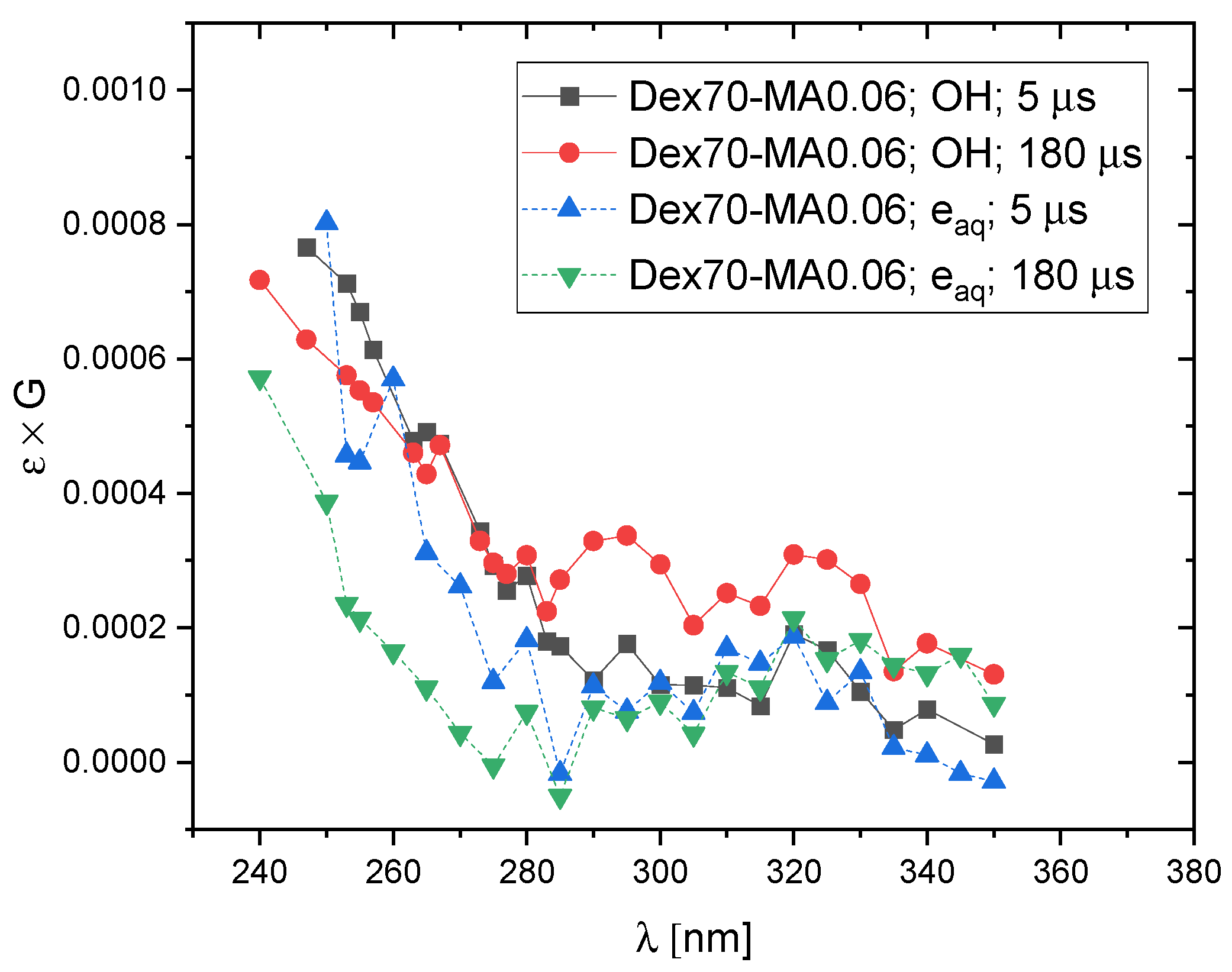
2.3. Reactivity of Hydrated Electrons with Dextran and Dextran Methacrylate
3. Discussion
3.1. General Remarks
3.2. Reactivity of Hydroxyl Radicals with Dextran and Dextran Methacrylate
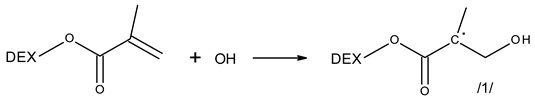
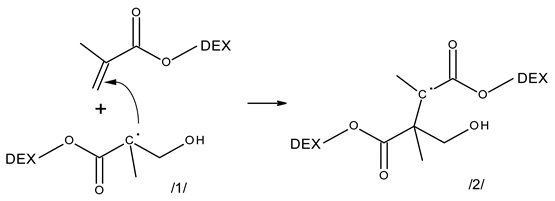
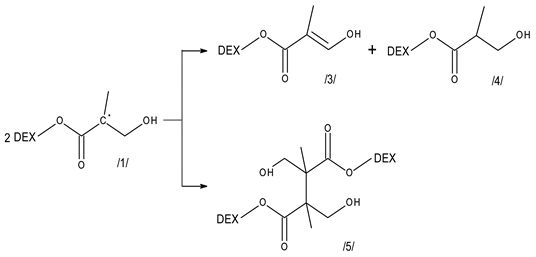
3.3. Reactivity of Hydrated Electrons with Dextran and Dextran Methacrylate
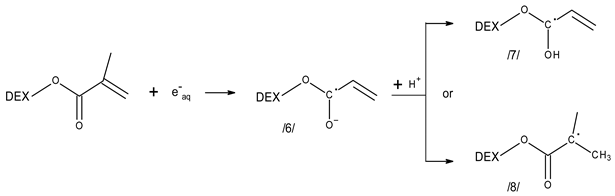
4. Materials and Methods
4.1. Materials
4.2. Dextran Purification and Synthesis of Dextran Methacrylate
4.3. Pulse Radiolysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: Oxford, UK, 1960. [Google Scholar]
- Chapiro, A. Radiation Chemistry of Polymeric Systems; John Wiley & Sons: New York, NY, USA, 1962. [Google Scholar]
- Dole, M. The Radiation Chemistry of Macromolecules; Academic Press: New York, NY, USA; London, UK, 1972; ISBN 978-0-12-219801-4. [Google Scholar]
- Coqueret, X. Obtaining High-Performance Polymeric materials by Radiation. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 131–150. [Google Scholar]
- Rosiak, J.; Rucinska-Rybus, A.; Pekala, W. Method of Manufacturing Hydrogel Dressings. U.S. Patent 4,871,490, 3 October 1989. [Google Scholar]
- Rosiak, J.M. Hydrogel dressings HDR. In Radiation Effects on Polymers; ACS Symposium Series 475; Clough, R.C., Shalaby, S.W., Eds.; American Chemical Society: Washington, DC, USA, 1991; pp. 271–299. [Google Scholar]
- Rosiak, J.M.; Ulański, P.; Pajewski, L.A.; Yoshii, F.; Makuuchi, K. Radiation formation of hydrogels for biomedical purposes. Some remarks and comments. Radiat. Phys. Chem. 1995, 46, 161–168. [Google Scholar] [CrossRef]
- Varca, G.H.C.; Perossi, G.G.; Grasselli, M.; Lugão, A.B. Radiation synthesized protein-based nanoparticles: A technique overview. Radiat. Phys. Chem. 2014, 105, 48–52. [Google Scholar] [CrossRef]
- Charlesby, A. The degradation of cellulose by ionizing radiation. J. Polym. Sci. 1955, 15, 263–270. [Google Scholar] [CrossRef]
- Phillips, G.O.; Moody, G.J. Radiation chemistry of carbohydrates. Part II. Irradiation of aqueous solutions of dextran with gamma radiation. J. Chem. Soc. 1958, 3534–3539. [Google Scholar] [CrossRef]
- Phillips, G.O. Radiation chemistry of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1961, 16, 13–58. [Google Scholar]
- von Sonntag, C. Free radical reactions of carbohydrates as studied by radiation techniques. Adv. Carbohydr. Chem. Biochem. 1980, 37, 7–77. [Google Scholar]
- von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor and Francis: London, UK, 1987. [Google Scholar]
- Ershov, B.G. Radiation-chemical degradation of cellulose and other polysaccharides. Russ. Chem. Rev. 1998, 67, 315–334. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Coqueret, X.; Zaman, K.; Sen, M.; Ulanski, P. (Eds.) The Radiation Chemistry of Polysaccharides; International Atomic Energy Agency: Vienna, Austria, 2016. [Google Scholar]
- Ramnani, S.P.; Chaudhari, C.V.; Patil, N.D.; Sabharwal, S. Synthesis and characterization of crosslinked chitosan formed by gamma irradiation in the presence of carbontetrachloride as a sensitizer. J. Polym. Sci. A Polym. Chem. 2004, 42, 3897–3909. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Phillips, G.O.; Williams, P.A.; du Plessis, T.A. Application of ionizing radiations to produce new polysaccharides and proteins with enhanced functionality. Nucl. Instr. Meth. B 2007, 265, 37–43. [Google Scholar] [CrossRef]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of radiation-induced cross-linked hydroxypropylcellulose. Macromol. Mater. Eng. 2002, 287, 285–295. [Google Scholar] [CrossRef]
- Wach, R.A.; Rokita, B.; Bartoszek, N.; Katsumura, Y.; Ulanski, P.; Rosiak, J.M. Hydroxyl radical-induced crosslinking and radiation-initiated hydrogel formation in dilute aqueous solutions of carboxymethylcellulose. Carbohydr. Polym. 2014, 112, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mitomo, H.; Nagasawa, N.; Yoshii, F.; Kume, T. Radiation synthesis and characteristic of the hydrogels based on carboxymethylated chitin derivatives. Carbohydr. Polym. 2003, 51, 169–175. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Wach, R.A.; Stojek, P.; Kamińska, M.; Rosiak, J.M.; Ulański, P. Synthesis of chitosan and carboxymethyl chitosan hydrogels by electron beam irradiation. Prog. Chem. Appl. Chitin Its Deriv. 2016, 21, 27–45. [Google Scholar] [CrossRef]
- Wach, R.A.; Adamus-Wlodarczyk, A.; Olejnik, A.K.; Matusiak, M.; Tranquilan-Aranilla, C.; Ulanski, P. Carboxymethylchitosan hydrogel manufactured by radiation-induced crosslinking as potential nerve regeneration guide scaffold. React. Funct. Polym. 2020, 152, 104588. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2023, 15, 144. [Google Scholar] [CrossRef]
- Wach, R.A.; Palmeri, G.; Adamus-Wlodarczyk, A.; Rokita, B.; Olejnik, A.K.; Dispenza, C.; Ulanski, P. Dual Stimuli-Responsive Polysaccharide Hydrogels Manufactured by Radiation Technique. Appl. Sci. 2022, 12, 11764. [Google Scholar] [CrossRef]
- Nawrotek, K.; Kubicka, M.; Gatkowska, J.; Wieczorek, M.; Michlewska, S.; Bekier, A.; Wach, R.; Rudnicka, K. Controlling the Spatiotemporal Release of Nerve Growth Factor by Chitosan/Polycaprolactone Conduits for Use in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2022, 23, 2852. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sathiyasree, M.; Rahim, S.B.A.; Renitta, R.E.; Kasipandian, K.; Krithika Shree, S.; Rajalakshmi, D.; Shobana, N.; Dhiva, S.; Abirami, S.; et al. Scaffold Using Chitosan, Agarose, Cellulose, Dextran and Protein for Tissue Engineering—A Review. Polymers 2023, 15, 1525. [Google Scholar] [CrossRef]
- Luanda, A.; Badalamoole, V. Past, present and future of biomedical applications of dextran-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 228, 794–807. [Google Scholar] [CrossRef]
- Szafulera, K.; Wach, R.A.; Olejnik, A.K.; Rosiak, J.M.; Ulański, P. Radiation synthesis of biocompatible hydrogels of dextran methacrylate. Radiat. Phys. Chem. 2018, 142, 115–120. [Google Scholar] [CrossRef]
- Reichelt, S.; Naumov, S.; Knolle, W.; Prager, A.; Decker, U.; Becher, J.; Weisser, J.; Schnabelrauch, M. Studies on the formation and characterization of macroporous electron-beam generated hyaluronan cryogels. Radiat. Phys. Chem. 2014, 105, 69–77. [Google Scholar] [CrossRef]
- Naumov, S.; Knolle, W.; Becher, J.; Schnabelrauch, M.; Reichelt, S. Electron-beam generated porous dextran gels: Experimental and quantum chemical studies. Int. J. Radiat. Biol. 2014, 90, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, S.; Becher, J.; Weisser, J.; Prager, A.; Decker, U.; Möller, S.; Berg, A.; Schnabelrauch, M. Biocompatible polysaccharide-based cryogels. Mater. Sci. Eng. C 2014, 35, 164–170. [Google Scholar] [CrossRef]
- Thönes, S.; Kutz, L.M.; Oehmichen, S.; Becher, J.; Heymann, K.; Saalbach, A.; Knolle, W.; Schnabelrauch, M.; Reichelt, S.; Anderegg, U. New E-beam-initiated hyaluronan acrylate cryogels support growth and matrix deposition by dermal fibroblasts. Int. J. Biol. Macromol. 2017, 94, 611–620. [Google Scholar] [CrossRef]
- Buxton, G.V. Radiation chemistry of the liquid state. (1) Water and homogeneous aqueous solutions. In Radiation Chemistry: Principles and Applications; Aziz, F., Rodgers, M.A.J., Eds.; Verlag Chemie: Weinheim, Germany, 1987; pp. 321–349. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (.OH/.O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry. From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- Coqueret, X.; Sabharwal, S.; Khairul Zaman, H.M.D.; Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulanski, P. Introduction to the Radiation Chemistry of Polymers. In The Radiation Chemistry of Polysaccharides; Al-Assaf, S., Coqueret, X., Khairul Zaman, H.M.D., Sen, M., Ulanski, P., Eds.; International Atomic Energy Agency: Vienna, Austria, 2016; pp. 25–75. [Google Scholar]
- Al-Assaf, S.; Gulrez, S.K.H.; Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulanski, P. Radiation Modification of Polysaccharides. In The Radiation Chemistry of Polysaccharides; Al-Assaf, S., Coqueret, X., Khairul Zaman, H.M.D., Sen, M., Ulanski, P., Eds.; International Atomic Energy Agency: Vienna, Austria, 2016; pp. 77–115. [Google Scholar]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of carboxymethylcellulose of various degree of substitution at high concentration in aqueous solutions of natural pH. Radiat. Phys. Chem. 2003, 68, 771–779. [Google Scholar] [CrossRef]
- Wach, R.A.; Kudoh, H.; Zhai, M.; Nagasawa, N.; Muroya, Y.; Yoshii, F.; Katsumura, Y. Rate constants of reactions of carboxymethylcellulose with hydrated electron, hydroxyl radical and the decay of CMC macroradicals. A pulse radiolysis study. Polymer 2004, 45, 8165–8171. [Google Scholar] [CrossRef]
- Czapski, G.; Bielski, B.H.J. Absorption spectra of the.·OH and O.·-radicals in aqueous solutions. Radiat. Phys. Chem. 1993, 41, 503–505. [Google Scholar] [CrossRef]
- Ulanski, P.; von Sonntag, C. OH-Radical-induced chain scission of chitosan in the absence and presence of dioxygen. J. Chem. Soc. Perkin Trans. 2 2000, 2000, 2022. [Google Scholar] [CrossRef]
- Baxendale, J.H.; Bevan, P.L.T.; Stott, D.A. Pulse radiolysis of aqueous thiocyanate and iodide solutions. Trans. Faraday Soc. 1968, 64, 2389–2397. [Google Scholar] [CrossRef]
- Buxton, G.V.; Stuart, C.R. Re-evaluation of the thiocyanate dosimeter for pulse radiolysis. J. Chem. Soc. Faraday Trans. 1995, 91, 279–281. [Google Scholar] [CrossRef]
- Henglein, A.; Schnabel, W.; Wendenburg, J. Einfhrung in Die Strahlenchemie; Verlag Chemie: Weinheim, Germany, 1969. [Google Scholar]
- Bartoszek, N.; Ulański, P.; Rosiak, J.M. Reaction of a low-molecular-weight free radical with a flexible polymer chain: Kinetic studies on the OH + poly(N-vinylpyrrolidone) model. Int. J. Chem. Kinet. 2011, 43, 474–481. [Google Scholar] [CrossRef]
- Dahlgren, B.; Dispenza, C.; Jonsson, M. Numerical simulation of the kinetics of radical decay in single-pulse high-energy electron-irradiated polymer aqueous solutions. J. Phys. Chem. A 2019, 123, 5043–5050. [Google Scholar] [CrossRef]
- Dahlgren, B.; Sabatino, M.A.; Dispenza, C.; Jonsson, M. Numerical simulations of nanogel synthesis using pulsed electron beam. Macromol. Theory Simul. 2020, 29, 1900046. [Google Scholar] [CrossRef]
- Kujawa, P.; Mohid, N.; Zaman, K.; Manshol, W.; Ulanski, P.; Rosiak, J.M. Pulse radiolysis of butyl acrylate in aqueous solution. Radiat. Phys. Chem. 1998, 53, 403–409. [Google Scholar] [CrossRef]
- Kozicki, M.; Kujawa, P.; Rosiak, J.M. Pulse radiolysis study of diacrylate macromonomer in aqueous solution. Radiat. Phys. Chem. 2002, 65, 133–139. [Google Scholar] [CrossRef]
- Behzadi, A.; Borgwardt, U.; Henglein, A.; Schamberg, E.; Schnabel, W. Pulsradiolytische Untersuchung der Kinetik diffusionkontrollierter Reaktionen des OH-Radikals mit Polymeren und Oligomeren in wässriger Lösung. Ber. Bunsenges. Phys. Chem. 1970, 74, 649–653. [Google Scholar]
- Matheson, M.S.; Mamou, A.; Silverman, J.; Rabani, J. Reaction of hydroxyl radicals with polyehylene oxide in aqueous solution. J. Phys. Chem. 1973, 77, 2420–2424. [Google Scholar] [CrossRef]
- Zainuddin, P.U.; Rosiak, J.M. M. Pulse radiolysis of poly(ethylene oxide) in aqueous solution. I. Formation of macroradicals. Radiat. Phys. Chem. 1995, 46, 913–916. [Google Scholar] [CrossRef]
- Safrany, A.; Biro, A.; Wojnarovits, L. Pulse radiolysis of aqueous solutions of ethyl acrylate and hydroxy ethyl acrylate. Radiat. Phys. Chem. 1993, 42, 1027–1030. [Google Scholar] [CrossRef]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Sabharwal, S.; Mohan, H. Structure-reactivity studies on the polymerization and crosslinking behavior of tri(propylene glycol) diacrylate in aqueous solutions. React. Funct. Polym. 2007, 67, 282–293. [Google Scholar] [CrossRef]
- Mehnert, R.; Naumov, S.; Knolle, W.; Janovsky, I. Radical formation in electron-irradiated acrylates studied by pulse radiolysis and electron paramagnetic resonance. Macromol. Phys. Chem. 2000, 201, 2447–2454. [Google Scholar] [CrossRef]
- Bartoszek, N.; Sawicki, P.; Kadłubowski, S.; Ulański, P.; Rosiak, J.M. Determination of Propagation Rate Coefficient for the Polymerization of N-Vinylpyrrolidone in Aqueous Solution by Pulsed Electron Polymerization and Size Exclusion Chromatography. ACS Macro Lett. 2014, 3, 639–642. [Google Scholar] [CrossRef]
- Sawicki, P.; Łapienis, G.; Kadłubowski, S.; Ulański, P.; Rosiak, J.M. Determination of kinetic parameters of N-vinylpyrrolidone radical polymerization in water by Pulsed Electron Polymerization-Size Exclusion Chromatography (PEP-SEC). Radiat. Phys. Chem. 2023, 202, 110543. [Google Scholar] [CrossRef]
- van Dijk-Wotthuis, W.N.E.; Franssen, O.; Talsma, H.; van Steenbergen, M.J.; Kettenes-van den Bosch, J.J.; Hennink, W.E. Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar] [CrossRef]
- Karolczak, S.; Hodyr, K.; Polowinski, M. Pulse radiolysis system based on ELU-6E LINAC. II. Development and upgrading the system. Radiat. Phys. Chem. 1992, 39, 1–5. [Google Scholar] [CrossRef]

| Initial Molecular Weight of Dextran Substrates (kDa) | Series of Dex-MA | Determined DS 1 |
|---|---|---|
| 6 | Dex6-MA | 0.02, 0.11, 0.43, 0.50 |
| 25 | Dex25-MA | 0.04, 0.11, 0.42, 0.60 |
| 70 | Dex70-MA | 0.06, 0.18, 0.37, 0.64 |
| 500 | Dex500-MA | 0.07, 0.13, 0.28, 0.57, 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafulera, K.J.; Wach, R.A.; Ulański, P. Dextran Methacrylate Reactions with Hydroxyl Radicals and Hydrated Electrons in Water: A Kinetic Study Using Pulse Radiolysis. Molecules 2023, 28, 4231. https://doi.org/10.3390/molecules28104231
Szafulera KJ, Wach RA, Ulański P. Dextran Methacrylate Reactions with Hydroxyl Radicals and Hydrated Electrons in Water: A Kinetic Study Using Pulse Radiolysis. Molecules. 2023; 28(10):4231. https://doi.org/10.3390/molecules28104231
Chicago/Turabian StyleSzafulera, Kamila J., Radosław A. Wach, and Piotr Ulański. 2023. "Dextran Methacrylate Reactions with Hydroxyl Radicals and Hydrated Electrons in Water: A Kinetic Study Using Pulse Radiolysis" Molecules 28, no. 10: 4231. https://doi.org/10.3390/molecules28104231
APA StyleSzafulera, K. J., Wach, R. A., & Ulański, P. (2023). Dextran Methacrylate Reactions with Hydroxyl Radicals and Hydrated Electrons in Water: A Kinetic Study Using Pulse Radiolysis. Molecules, 28(10), 4231. https://doi.org/10.3390/molecules28104231









