Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome
Abstract
1. Introduction
2. Coriander Phenolics
3. Extractive Methods: Traditional and Innovative
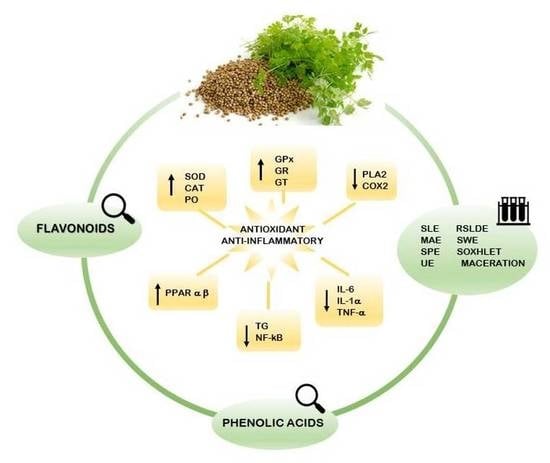
4. Antioxidant Activity of Coriander Extracts
5. Anti-Inflammatory Activity of Coriander Polyphenols
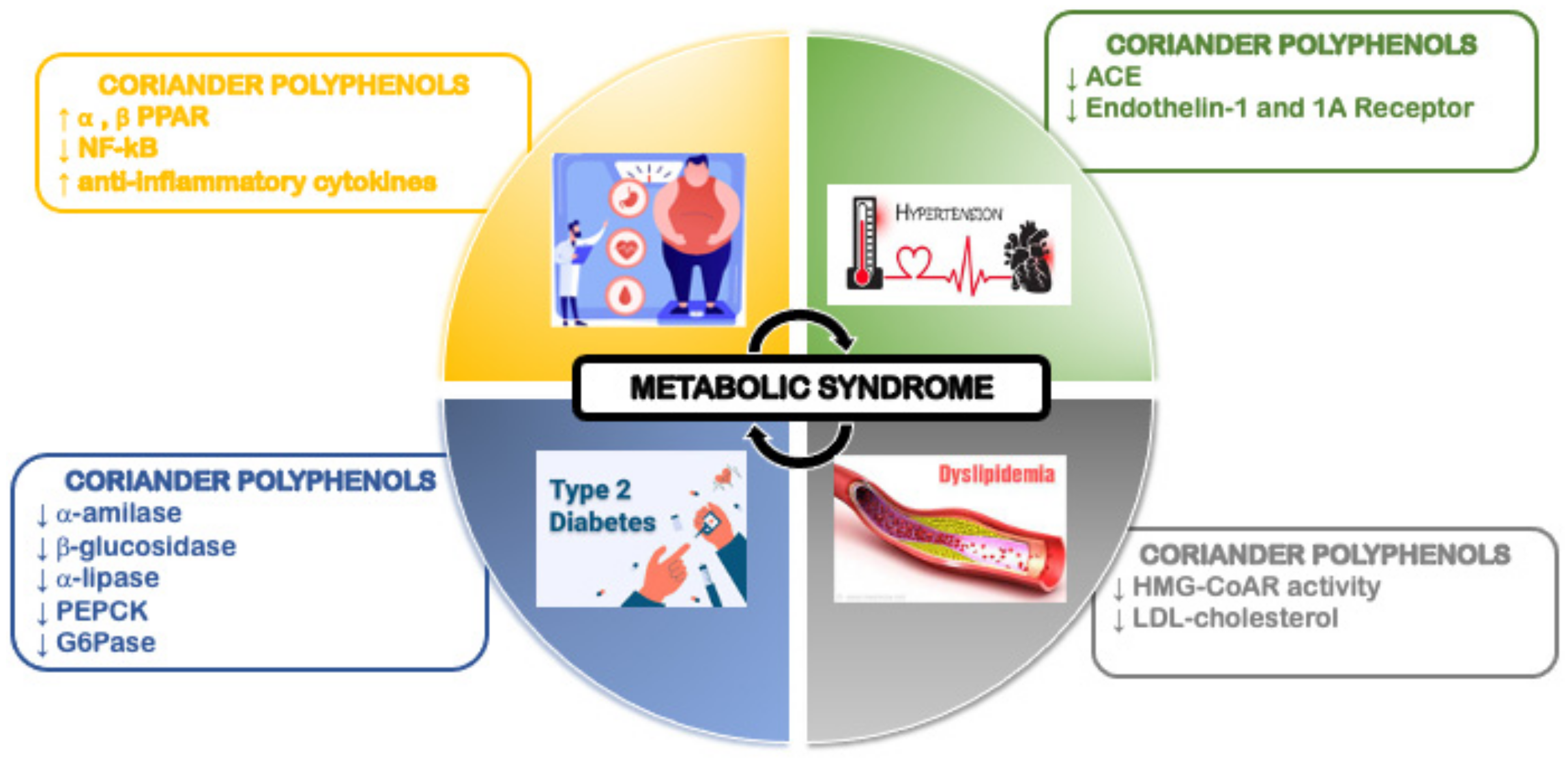
6. Coriander and Obesity
7. Coriander and Diabetes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahib, N.G.; Anwar, F.; Gilani, A.-H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals—A Review. Phytoth. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Mhemdi, H.; Rodier, E.; Kechaou, N.; Fages, J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J. Food. Eng. 2011, 105, 609–616. [Google Scholar] [CrossRef]
- Omidbaigi, R. Approaches to Production and Processing of Medicinal Plants; Tarrahane-Nashr Publication: Tehran, Iran, 1997; Volume 2, pp. 1–424. [Google Scholar]
- Sobhani, Z.; Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. Ethnobotanical and phytochemical aspects of the edible herb Coriandrum sativum L. J. Food Sci. 2022, 87, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Phaiphan, A.; Murugan, Y.; Baharin, B. Comparative study of bioactive compounds in curry and coriander leaves: An update. J. Chem. Pharm. Res. 2013, 5, 590–594. [Google Scholar]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules 2021, 27, 209. [Google Scholar] [CrossRef]
- Msaada, K.; Jemia, M.B.; Salem, N.; Bachrouch, O.; Sriti, J.; Tammar, S.; Bettaieb, I.; Jabri, I.; Kefi, S.; Limam, F. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arabian J. Chem. 2017, 10, S3176–S3183. [Google Scholar] [CrossRef]
- WFO. Plant List Coriandrum L. Available online: https://wfoplantlist.org/plant-list/taxon/wfo-4000009306-2022-12 (accessed on 27 February 2023).
- Bhat, S.; Kaushal, P.; Kaur, M.; Sharma, H. Coriander (Coriandrum sativum L.): Processing, nutritional and functional aspects. African J. Plant Sci. 2014, 8, 25–33. [Google Scholar]
- Uchibayashi, M. The coriander story. Yakushigaku Zasshi 2001, 36, 56–57. [Google Scholar]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef]
- Fukushima, S.; Cohen, S.M.; Eisenbrand, G.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L. FEMA GRAS assessment of natural flavor complexes: Lavender, Guaiac Coriander-derived and related flavoring ingredients. Food Chem. Toxicol. 2020, 145, 111584. [Google Scholar] [CrossRef]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Coriander. In Chemistry of Spices; CABI: London, UK, 2008; pp. 190–210. [Google Scholar]
- Önder, A. Coriander and Its Phytoconstituents for the Beneficial Effects. In Potential of Essential Oils; Hany, A.E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 165–185. [Google Scholar]
- Usmanghani, K.; Saeed, A.; Alam, M.T. Indusyunic Medicine: Traditional Medicine of Herbal Animal and Mineral Origin in Pakistan; Department of Pharmacognosy, Faculty of Pharmacy, University of Karachi: Karachi, Pakistan, 1997. [Google Scholar]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, H.; Ayyildiz, H.F.; Ismail, U.; Kara, H. Antimicrobial Activity of Coriander. In Handbook of Coriander (Coriandrum sativum), 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 123–144. [Google Scholar]
- Dima, C.; Ifrim, G.A.; Coman, G.; Alexe, P.; Dima, Ş. Supercritical CO2 Extraction and Characterization of Coriandrum sativum L. Essential Oil. J. Food Process Eng. 2016, 39, 204–211. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60 Pt 10, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Med. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool-A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, T.; Yasuzawa, T.; Ueshima, S. Effects of Coriandrum sativum on Migration and Invasion Abilities of Cancer Cells. J. Nutr. Sci. Vitaminol. 2020, 66, 468–477. [Google Scholar] [CrossRef]
- Ghazanfari, N.; Mortazavi, S.A.; Yazdi, F.T.; Mohammadi, M. Microwave-assisted hydrodistillation extraction of essential oil from coriander seeds and evaluation of their composition, antioxidant and antimicrobial activity. Heliyon 2020, 6, e04893. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, B. Changes on essential oil composition of coriander (Coriandrum sativum L.) fruits during three stages of maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Nurzynska-Wierdak, R. Essential oil composition of the coriander (Coriandrum sativum L.) herb depending on the development stage. Acta Agrobot. 2013, 66, 53–60. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M. Essential Oil from Coriandrum sativum: A Review on Its Phytochemistry and Biological Activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-N.; Liu, Z.-H.; Zhao, Y.-P.; Zhao, L.-L.; Xue, T.-K.; Lan, Q.-K. Phytochemical and bioactive profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Márquez, D.; Rodríguez, R.; Balagurusamy, N.; Carrillo, M.; Belmares, R.; Contreras, J.; Nevárez, G.; Aguilar, C. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CYTA—J. Food 2014, 12, 271–276. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Introduction: Plant phenolics—Secondary metabolites with diverse functions. In Recent Advances in Polyphenol Research; Daayf, F., Lattanzio, V., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 1, pp. 1–35. [Google Scholar]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; García-Fraile, P.; Escribano-Bailón, M.T.; Rivas, R. Increase in phenolic compounds of Coriandrum sativum L. after the application of a Bacillus halotolerans biofertilizer. J. Sci. Food Agric. 2020, 100, 2742–2749. [Google Scholar] [CrossRef]
- Deshpande, S.; Sathe, S.; Salunkhe, D. Chemistry and safety of plant polyphenols. In Nutritional and Toxicological Aspects of Food Safety; Springer: Boston, MA, USA, 1984; pp. 457–495. [Google Scholar] [CrossRef]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef]
- Sriti, J.; Wannes, W.A.; Talou, T.; Vilarem, G.; Marzouk, B. Chemical composition and antioxidant activities of tunisian and canadian coriander (Coriandrum sativum L.) Fruit. J. Essent. Oil Res. 2011, 23, 7–15. [Google Scholar] [CrossRef]
- Sriti, J.; Aidi Wannes, W.; Talou, T.; Ben Jemia, M.; Kchouk, M.; Marzouk, B. Antioxidant properties and polyphenol contents of different parts of coriander (Coriandrum sativum L.) fruit. Riv. Ital. Sostanze Grasse 2012, 89, 253–262. [Google Scholar]
- Nambiar, V.; Daniel, M.; Guin, P. Characterization of polyphenols from coriander leaves (Coriandrum sativum), red amaranthus (A. paniculatus) and green amaranthus (A. frumentaceus) using paper chromatography and their health implications. J. Herb. Med. Toxicol. 2010, 4, 173–177. [Google Scholar]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, F.; Amin Azarm, D.; Fatemi, F.; Pour-Aboughadareh, A.; Poczai, P. Methyl jasmonate induces genes involved in linalool accumulation and increases the content of phenolics in two Iranian coriander (Coriandrum sativum L.) Ecotypes. Genes 2022, 13, 1717. [Google Scholar] [CrossRef]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 2022, 11, 2656. [Google Scholar] [CrossRef]
- Bommakanti, V.; Puthenparambil Ajikumar, A.; Sivi, C.M.; Prakash, G.; Mundanat, A.S.; Ahmad, F.; Haque, S.; Prieto, M.A.; Rana, S.S. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations 2023, 10, 177. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food. Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci 2012, 1, 8–26. [Google Scholar]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. [Google Scholar]
- Demir, S.; Korukluoglu, M. A comparative study about antioxidant activity and phenolic composition of cumin (Cuminum cyminum L.) and coriander (Coriandrum sativum L.). Indian J. Tradit. Knowl. 2020, 19, 383–393. [Google Scholar]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Zeković, Z.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cvejin, A.; Elgndi, M.A.; Pavlić, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J. Supercrit. Fluids 2014, 95, 560–566. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Zeković, Z.; Bušić, A.; Komes, D.; Vladić, J.; Adamović, D.; Pavlić, B. Coriander seeds processing: Sequential extraction of non-polar and polar fractions using supercritical carbon dioxide extraction and ultrasound-assisted extraction. Food Bioprod. Process. 2015, 95, 218–227. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food. Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Trifan, A.; Bostănaru, A.-C.; Luca, S.V.; Grădinaru, A.C.; Jităreanu, A.; Aprotosoaie, A.C.; Miron, A.; Cioancă, O.; Hăncianu, M.; Ochiuz, L. Antifungal potential of Pimpinella anisum, Carum carvi and Coriandrum sativum extracts. A comparative study with focus on the phenolic composition. Farmacia 2020, 68, 22–27. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Costa de Oliveira, R.; Es-Safi, I.; Vasconcelos Mourao, E.M.; Bouhrim, M.; Kyrylchuk, A.; Soares Pontes, G.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Palatzidi, A.; Perez-Jimenez, J.; Mateos-Aparicio, I.; Garcia-Alonso, A. Phenolic compounds and antioxidant activity are differentially affected by drying processes in celery, coriander and parsley leaves. Int. J. Food Sci. Technol. 2022, 57, 3467–3476. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M.; Saari, N. Effect of drying techniques on the total phenolic. J. Med. Plants Res. 2012, 6, 161–167. [Google Scholar]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Fahmy, H.; Shreif, N.; Gharib, O. The protective effect of Coriandium sativum extract on hepato-renal toxicity induced in irradiated rats. Eur. J. Med. Plants 2014, 4, 196. [Google Scholar] [CrossRef]
- de Almeida Melo, E.; Bion, F.M.; Filho, J.M.; Guerra, N.B. In Vivo antioxidant effect of aqueous and etheric coriander (Coriandrum sativum L.) extracts. Eur. J. Lipid Sci. Technol. 2003, 105, 483–487. [Google Scholar] [CrossRef]
- Hashim, M.S.; Lincy, S.; Remya, V.; Teena, M.; Anila, L. Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. 2005, 92, 653–660. [Google Scholar] [CrossRef]
- Velaga, M.K.; Yallapragada, P.R.; Williams, D.; Rajanna, S.; Bettaiya, R. Hydroalcoholic seed extract of Coriandrum sativum (Coriander) alleviates lead-induced oxidative stress in different regions of rat brain. Biol. Trace Elem. Res. 2014, 159, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Samojlik, I.; Lakic, N.; Mimica-Dukic, N.; Đaković-Švajcer, K.; Bozin, B. Antioxidant and hepatoprotective potential of essential oils of coriander (Coriandrum sativum L.) and caraway (Carum carvi L.) (Apiaceae). J. Agric. Food Chem. 2010, 58, 8848–8853. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P.R.; Umadevi, M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food. Chem. Toxicol. 2009, 47, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Mechchate, H.; Es-safi, I.; Amaghnouje, A.; Boukhira, S.; A. Alotaibi, A.; Al-zharani, M.; A. Nasr, F.; M. Noman, O.; Conte, R.; Amal, E.H.E.Y.; et al. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules 2021, 26, 487. [Google Scholar] [CrossRef]
- Zanusso-Junior, G.; Melo, J.; Romero, A.; Dantas, J.; Caparroz-Assef, S.; Bersani-Amado, C.; Cuman, R. Evaluation of the anti-inflammatory activity of coriander (Coriandrum sativum L.) in rodents. Rev. Bras. Pl. Med. 2011, 13, 17–23. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of total polyphenols, flavonoids and anti-inflammatory potential of three Apiaceae species grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Abd El-Salam, H.; Hassan, A. Phyto-chemicals boost anti inflammatory effect against gamma radiation: Activities of ginger and coriander extracts. Arab. J. Nuclear Sci. Appl. 2017, 50, 278–291. [Google Scholar]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediat. Inflamm. 2013, 2013, 136584–136594. [Google Scholar] [CrossRef] [PubMed]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef]
- Dhanapakiam, P.; Joseph, J.M.; Ramaswamy, V.; Moorthi, M.; Kumar, A.S. The cholesterol lowering property of coriander seeds (Coriandrum sativum): Mechanism of action. J. Environ. Biol. 2007, 29, 53–56. [Google Scholar]
- Saeid, J.; Al-Nasry, A. Effect of dietary Coriander seeds supplementation on growth performance carcass traits and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2010, 9, 867–870. [Google Scholar] [CrossRef]
- Cárdenas-García, M.; Ruiz, P.P.; González, P.P. Coriander (Coriandrum sativum) and Chia (Salvia hispanica) Intake Effect in Volunteers. Biochemistry 2016, 5, 602–603. [Google Scholar]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.; Qu, X. Comparison of cytotoxicity and the anti-adipogenic effect of green tea polyphenols with epigallocatechin-3-gallate in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef]
- Patel, D.K.; Desai, S.N.; Devkar, R.V.; Ramachandran, A.V. Coriandrum sativum L. aqueous extract mitigates high fat diet induced insulin resistance by controlling visceral adiposity in C57BL/6J Mice. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 127–135. [Google Scholar]
- Ngamdokmai, N.; Ingkaninan, K.; Scholfield, C.N.; Insumrong, K.; Neungchamnong, N.; Minale, G.; Warinhomhoun, S. A Thai Traditional Triple-Fruit Formulation “Phikud Tri-Phon” May Provide Fat Loss and Nutritional Benefits. Foods 2022, 11, 3067. [Google Scholar] [CrossRef]
- Nyakudya, T.; Makaula, S.; Mkumla, N.; Erlwanger, K. Dietary supplementation with coriander (Coriandrum sativum) seed: Effect on growth performance, circulating metabolic substrates, and lipid profile of the liver and visceral adipose tissue in healthy female rats. Int. J. Agric. Biol. 2014, 16, 125–131. [Google Scholar]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaware, S.; Narkar, N.; Tilak, A.; Raveendran, S.; Rane, P. Antidiabetic activity of Coriandrum sativum in streptozotocin induced diabetic rats. Int. J. Basic Clin. Pharmacol. 2019, 8, 925. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Kumar, P.; Kale, R.K.; Baquer, N.Z. Antihyperglycemic and protective effects of Trigonella foenum graecum seed powder on biochemical alterations in alloxan diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16 (Suppl. S3), 18–27. [Google Scholar]
- Yella, S.S.T.; Kumar, R.N.; Ayyanna, C.; Varghese, A.M.; Amaravathi, P.; Vangoori, Y. The combined effect of Trigonella foenum seeds and Coriandrum sativum leaf extracts in alloxan-induced diabetes mellitus wistar albino rats. Bioinformation 2019, 15, 716–722. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scandar, S.; Zadra, C.; Marcotullio, M.C. Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome. Molecules 2023, 28, 4187. https://doi.org/10.3390/molecules28104187
Scandar S, Zadra C, Marcotullio MC. Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome. Molecules. 2023; 28(10):4187. https://doi.org/10.3390/molecules28104187
Chicago/Turabian StyleScandar, Samir, Claudia Zadra, and Maria Carla Marcotullio. 2023. "Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome" Molecules 28, no. 10: 4187. https://doi.org/10.3390/molecules28104187
APA StyleScandar, S., Zadra, C., & Marcotullio, M. C. (2023). Coriander (Coriandrum sativum) Polyphenols and Their Nutraceutical Value against Obesity and Metabolic Syndrome. Molecules, 28(10), 4187. https://doi.org/10.3390/molecules28104187