Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature
Abstract
1. Introduction
2. Results and Discussion
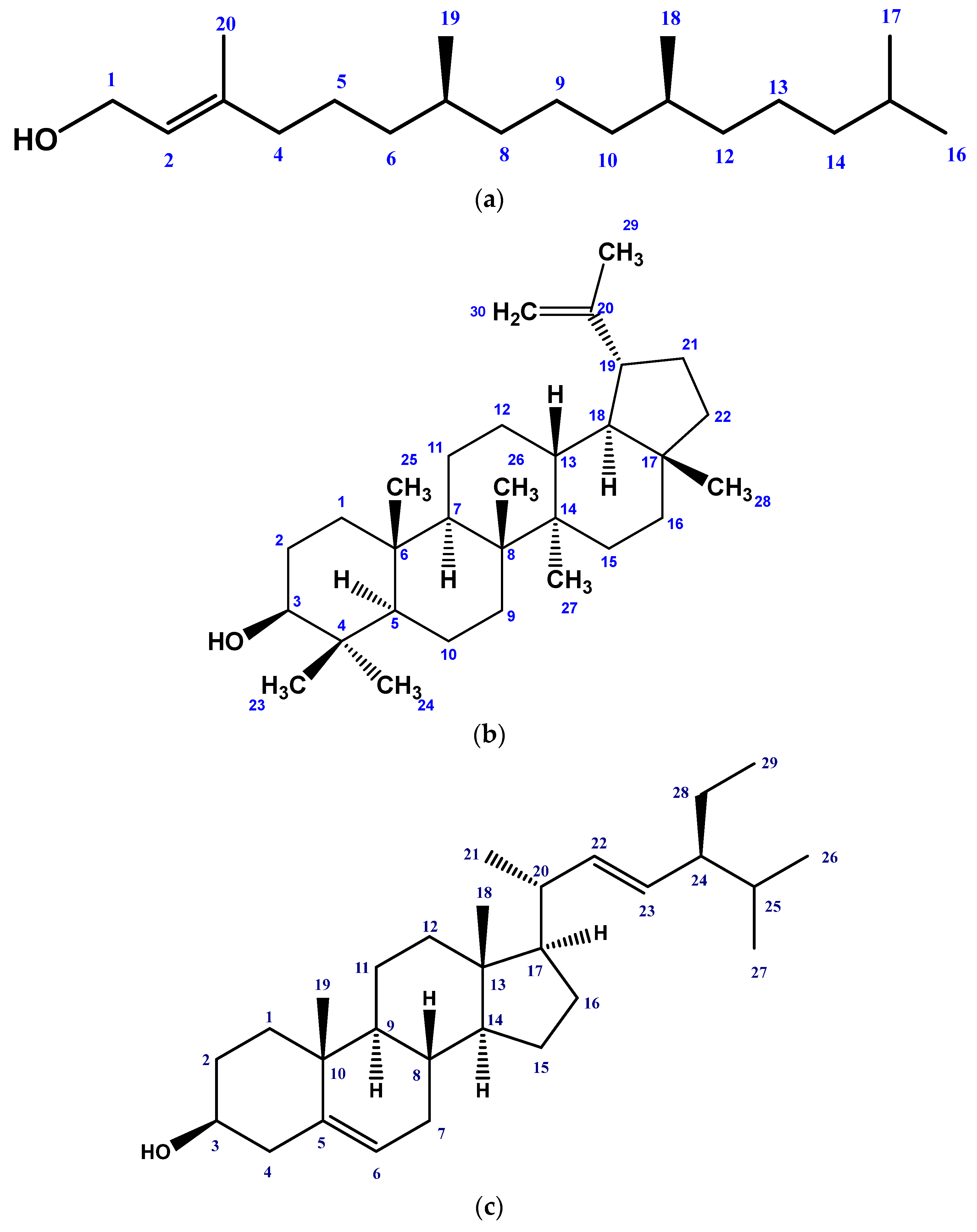
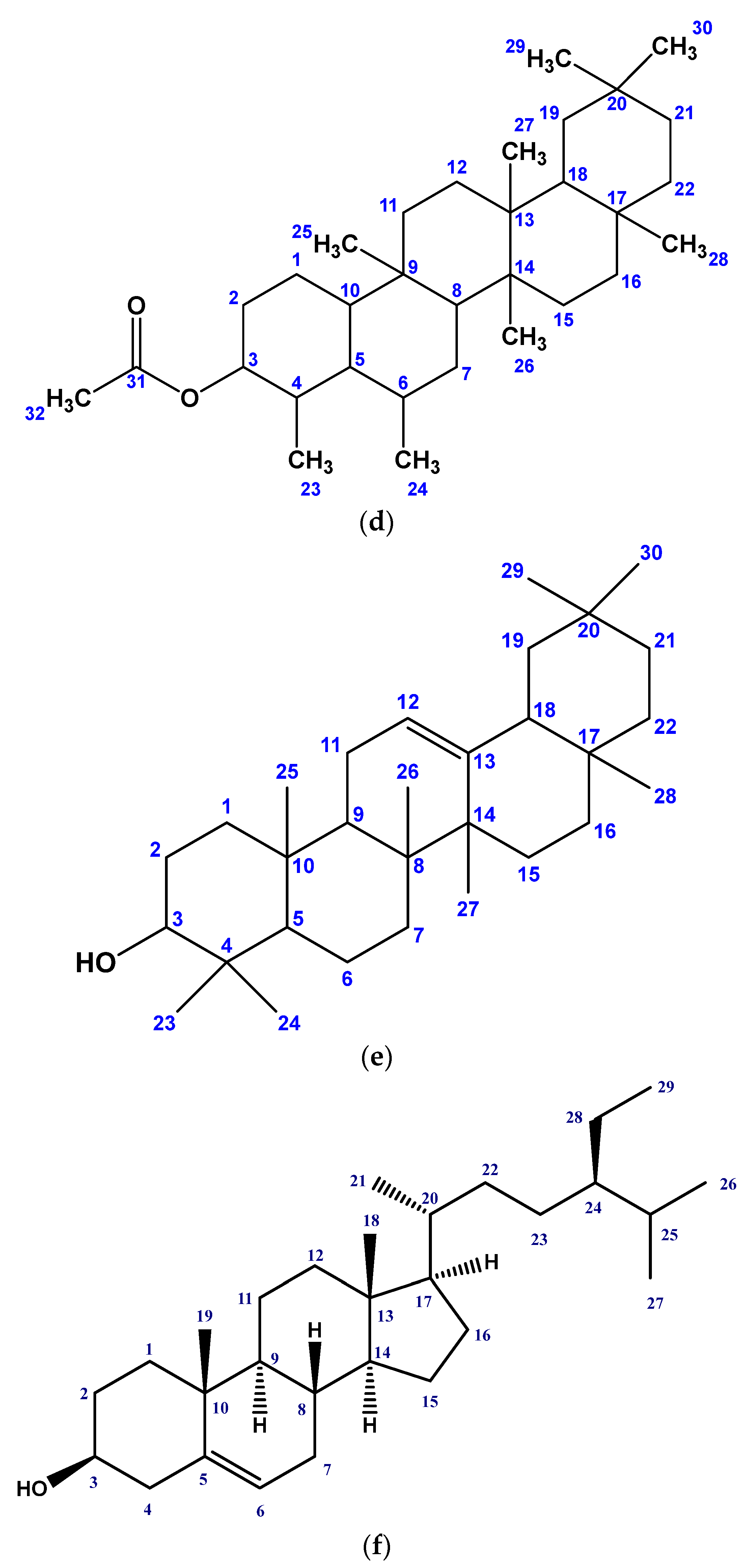
2.1. Isolated Phytochemicals from G. procumbens
2.2. Effect of G. procumbens Extracts on DPPH Free Radical Scavenging Activity
2.3. Effect of G. procumbens Extracts on Brine Shrimp Lethality Bioassay
2.4. Effect of G. procumbens Extracts on the Thrombolytic Activity
2.5. Effect of G. procumbens Extracts on the Anti-Diabetic Activity
2.6. Analysis of the Biological Investigation
3. Materials and Methods
3.1. Collection and Preparation of the Plant Material
3.2. Instrumentations, Drugs, and Chemicals
3.3. Experimental Design
3.3.1. Extraction of Plant Material
3.3.2. Isolation of Compounds
3.3.3. Preparation of Different Partitions for Biological Tests
3.3.4. Structural Identification of the Compounds
3.3.5. Test Animal Model
3.3.6. Acute Toxicity Study
3.4. Antioxidant Assay
DPPH Free Radical Scavenging Assay
3.5. Cytotoxicity Assay
Brine Shrimp Lethality Bioassay
3.6. In Vitro Thrombolytic Assay
3.7. Antidiabetic Assay
Alloxan-Induced Diabetic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Thomford, N.E.; Senthebane, A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Grace, O.M. Global Medicinal Uses of Euphorbia L.(Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Gozubuyuk, G.; Aktas, E.; Yigit, N. An Ancient Plant Lawsonia Inermis (Henna): Determination of in Vitro Antifungal Activity against Dermatophytes Species. J. Mycol. Med. 2014, 24, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hotwani, K.; Baliga, S.; Sharma, K. Phytodentistry: Use of Medicinal Plants. J. Complement. Integr. Med. 2014, 11, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lawrence, A.; Liang, J. Traditional Chinese Medicine for Treatment of Alcoholism: From Ancient to Modern. Am. J. Chin. Med. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- McGovern, P.E.; Mirzoian, A.; Hall, G.R. Ancient Egyptian Herbal Wines. Proc. Natl. Acad. Sci. USA 2009, 106, 7361–7366. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Harvey, A.L.; Clark, R.L.; Mackay, S.P.; Johnston, B.F. Current Strategies for Drug Discovery through Natural Products. Expert Opin. Drug Discov. 2010, 5, 559–568. [Google Scholar] [CrossRef]
- Harvey, A.; Edrada-Ebel, R.; Quinn, R. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Chang, J.; Kim, Y.; Kwon, H. Advances in Identification and Validation of Protein Targets of Natural Products without Chemical Modification. Nat. Prod. Rep. 2016, 33, 719–730. [Google Scholar] [CrossRef]
- Tansaz, M.; Tajadini, H. Comparison of Leiomyoma of Modern Medicine and Traditional Persian Medicine. J. Evid. Based. Complement. Altern. Med. 2016, 21, 160–163. [Google Scholar] [CrossRef]
- Xu, Q.; Bauer, R.; Hendry, B.M.; Fan, T.P.; Zhao, Z.; Duez, P.; Simmonds, M.S.J.; Witt, C.M.; Lu, A.; Robinson, N.; et al. The Quest for Modernisation of Traditional Chinese Medicine. BMC Complement. Altern. Med. 2013, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic Effects of Aronia Melanocarpa and Its Other Therapeutic Properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Iqbal Yatoo, M.; Khandia, R.; Iqbal Yatoo, M.; Dimri, U.; Gopalakrishnan, A.; Karthik, K.; Gopi, M.; Saminathan, M.; Saxena, A.; Alagawany, M.; et al. Beneficial Health Applications and Medicinal Values of Pedicularis Plants: A Review. Biomed. Pharmacother. 2017, 95, 1301–1313. [Google Scholar] [CrossRef]
- Thomford, N.E.; Awortwe, C.; Dzobo, K.; Adu, F.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Dandara, C. Inhibition of CYP2B6 by Medicinal Plant Extracts: Implication for Use of Efavirenz and Nevirapine-Based Highly Active Anti-Retroviral Therapy (HAART) in Resource-Limited. Molecules 2016, 21, 211. [Google Scholar] [CrossRef]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C.; Schmidt, T.J. In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia Laevis and Cassia Abbreviata: Implications For. Molecules 2016, 21, 891. [Google Scholar] [CrossRef]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Chirikure, S.; Dandara, C. Pharmacogenomics Implications of Using Herbal Medicinal Plants on African Populations in Health Transition. Pharmaceuticals 2015, 8, 637–663. [Google Scholar] [CrossRef]
- Thomford, N.E.; Mkhize, B.; Dzobo, K.; Mpye, K.; Rowe, A.; Parker, M.I.; Wonkam, A.; Skelton, M.; September, A.V.; Dandara, C. African Lettuce (Launaea taraxacifolia) Displays Possible Anticancer Effects and Herb–Drug Interaction Potential by CYP1A2, CYP2C9, and CYP2C19 Inhibition. Omi. A J. Integr. Biol. 2016, 20, 528–537. [Google Scholar] [CrossRef]
- Ji, S.; Fattahi, A.; Raffel, N.; Hoffmann, I.; Beckmann, M.W.; Dittrich, R.; Schrauder, M. Antioxidant Effect of Aqueous Extract of Four Plants with Therapeutic Potential on Gynecological Diseases; Semen Persicae, Leonurus Cardiaca, Hedyotis Diffusa, and Curcuma Zedoaria. Eur. J. Med. Res. 2017, 22, 50. [Google Scholar] [CrossRef]
- Ruhsam, M.; Hollingsworth, P. Authentication of Eleutherococcus and Rhodiola Herbal Supplement Products in the United Kingdom. J. Pharm. Biomed. Anal. 2018, 149, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bhore, S.; Ravichantar, N.; Loh, C. Screening of Endophytic Bacteria Isolated from Leaves of Sambung Nyawa [Gynura procumbens (Lour.) Merr.] for Cytokinin-like Compounds. Bioinformation 2010, 5, 191. [Google Scholar] [CrossRef]
- Iskander, M.N.; Song, Y.; Coupar, I.M.; Jiratchariyakul, W. Antiinflammatory Screening of the Medicinal Plant Gynura procumbens. Plant Foods Hum. Nutr. 2002, 57, 233–244. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Asad, M. Chemical and Biological Investigations of the Leaves of Gynura procumbens. Int. J. Biosci. 2013, 3, 36–43. [Google Scholar] [CrossRef]
- Tan, H.L.; Chan, K.G.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Gynura procumbens: An Overview of the Biological Activities. Front. Pharmacol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Li, M.; Wang, H.; Ren, B.; Chen, J.; Li, W. Traditional Uses, Phytochemistry, Pharmacology and Toxicology of the Genus Gynura (Compositae): A Comprehensive Review. J. Ethnopharmacol. 2021, 276, 114145. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Shehu, S. Ethno-Botanical and Pharmaceutical Properties of Mollucan Spinach (Gynura procumbens Lour. Merr). J. Appl. Sci. Environ. Manag. 2022, 26, 1925. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Sutthikhum, V.; Siriamornpun, S. Potential of Gynura procumbens Leaves as Source of Flavonoid-Enriched Fractions with Enhanced Antioxidant Capacity. J. Funct. Foods 2015, 12, 120–128. [Google Scholar] [CrossRef]
- Mou, K.M.; Dash, P.R. A comprehensive review on Gynura procumbens leaves. Int. J. Pharmacogn. 2016, 3, 167–174. [Google Scholar] [CrossRef]
- Krishnan, V.; Ahmad, S.; Mahmood, M. Antioxidant Potential in Different Parts and Callus of Gynura procumbens and Different Parts of Gynura Bicolor. BioMed Res. Int. 2015, 2015, 147909. [Google Scholar] [CrossRef]
- Akowuah, G.; Amirin, S.; Mariam, A.; Aminah, I. Blood Sugar Lowering Activity of Gynura procumbens Leaf Extracts. Trop Med. Plants 2001, 2, 5–10. [Google Scholar]
- Zhang, X.; Tan, B. Effects of an Ethanolic Extract of Gynura procumbens on Serum Glucose, Cholesterol and Triglyceride Levels in Normal and Streptozotocin-Induced Diabetic Rats. Singap. Med. J. 2000, 41, 9–13. [Google Scholar]
- Yam, M.; Sadikun, A.; Ahmad, M.; Akowuah, G.; Asmawi, M. Toxicology Evaluation of Standardized Methanol Extract of Gynura procumbens. J. Ethnopharmacol. 2009, 123, 244–249. [Google Scholar]
- Akowuah, G.; Sadikun, A.; Mariam, A. Flavonoid Identification and Hypoglycaemic Studies of the Butanol Fraction from Gynura procumbens. Pharm. Biol. 2002, 40, 405–410. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Puangpronpitag, D.; Nakornriab, M. Evaluation of Phytochemical Composition and Antibacterial Property of Gynura procumbens Extract. Asian J. Plant Sci. 2012, 11, 77. [Google Scholar] [CrossRef]
- Rosidah, Y.M.; Sadikun, A.; Asmawi, M. Antioxidant Potential of Gynura procumbens. Pharm. Biol. 2008, 46, 616–625. [Google Scholar] [CrossRef]
- Bang, M.H.; Soo, Y.C.; Jang, T.O.; Sang, K.K.; Kwon, O.S.; Kang, T.C.; Moo, H.W.; Park, J.; Baek, N.I. Phytol, SSADH Inhibitory Diterpenoid of Lactuca Sativa. Arch. Pharm. Res. 2002, 25, 643–646. [Google Scholar] [CrossRef]
- Chaiyadej, K.; Wongthap, H.; Vadhanavikit, S.; Chantrapromma, K. Bioactive Constituents from the Twigs of Sonneratia Alba. Walailak J. Sci. Technol. 2004, 1, 15–22. [Google Scholar]
- Pateh, U.; Haruna, A.; Garba, M.; Iliya, I.; Sule, I.; Abubakar, M.; Ambi, A. Isolation of Stigmasterol, β-Sitosterol and 2-Hydroxyhexadecanoic Acid Methyl Ester from the Rhizomes of Stylochiton Lancifolius Pyer and Kotchy (Araceae). Niger. J. Pharm. Sci. 2009, 8, 19–25. [Google Scholar]
- Van Kiem, P.; Van Minh, C.; Huong, H.T.; Nam, N.H.; Lee, J.J.; Kim, Y.H. Pentacyclic Triterpenoids from Mallotus Apelta. Arch. Pharm. Res. 2004, 27, 1109–1113. [Google Scholar] [CrossRef]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. Β-Amyrin Synthase: Cloning of Oxidosqualene Cyclase That Catalyzes the Formation of the Most Popular Triterpene among Higher Plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.; Zotchev, S.; Dirsch, V. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.; Waltenberger, B.; Pferschy-Wenzig, E.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Feher, M.; Schmidt, J.M. Property Distributions: Differences between Drugs, Natural Products, and Molecules from Combinatorial Chemistry. J. Chem. Inf. Comput. Sci. 2003, 43, 218–227. [Google Scholar] [CrossRef]
- Barnes, E.; Kumar, R.; Davis, R. The Use of Isolated Natural Products as Scaffolds for the Generation of Chemically Diverse Screening Libraries for Drug Discovery. Nat. Prod. Rep. 2016, 33, 372–381. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Walsh, C. Lessons from Natural Molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.D.G.; Maccoss, M.; Heer, J.P. Importance of Rigidity in Designing Small Molecule Drugs to Tackle Protein-Protein Interactions (PPIs) through Stabilization of Desired Conformers. J. Med. Chem. 2018, 61, 4383–4389. [Google Scholar] [CrossRef]
- Hosaka, S.; Obuki, M.; Nakajima, J.; Suzuki, M. Comparative Study of Antioxidants as Quenchers or Scavengers of Reactive Oxygen Species Based on Quenching of MCLA-dependent Chemiluminescence. Lumin. J. Biol. Chem. Lumin. 2005, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Santiago, L.; Mayor, A. Lupeol: An Antioxidant Triterpene in Ficus Pseudopalma Blanco (Moraceae). Asian Pac. J. Trop. Biomed. 2014, 4, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant Effect of Phytol in a Pilocarpine Model in Mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rajab, M.S.; Cantrell, C.L.; Franzblau, S.G.; Fischer, N.H. Antimycobacterial Activity of (E)-Phytol and Derivatives: A Preliminary Structure-Activity Study. Planta Med. 1998, 64, 2–4. [Google Scholar] [CrossRef]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An Insight into the Cytotoxic Activity of Phytol at in Vitro Conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef]
- Carolina De Menezes, C.; Santos, P.; Salvadori, M.S.; Gomes Mota, V.; Muratori Costa, L.; Cardoso De Almeida, A.A.; Lopes De Oliveira, G.A.; Pereira Costa, J.; Pergentino De Sousa, D.; Mendes De Freitas, R.; et al. Antinociceptive and Antioxidant Activities of Phytol in Vivo and in Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Shaker, M.; Hazem, S.; Ashamallah, S. Inhibition of the JAK/STAT Pathway by Ruxolitinib Ameliorates Thioacetamide-Induced Hepatotoxicity. Food Chem. Toxicol. 2016, 96, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Irudayaraj, S.; Duraipandiyan, V.; Al-Dhabi, N.; Agastian, P.; Ignacimuthu, S. Antioxidant and Free Radical Scavenging Effects of β-Amyrin Isolated from S. cochinchinensis Moore. Leaves. Ind. Crop. Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Sakthivel, R.; Malar, D.; Devi, K. Phytol Shows Anti-Angiogenic Activity and Induces Apoptosis in A549 Cells by Depolarizing the Mitochondrial Membrane Potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular Mechanism of β-Sitosterol and Its Derivatives in Tumor Progression. Front. Oncol. 2022, 12, 926975. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and Its Derivatives as Anticancer and Anti-Inflammatory Agents: Molecular Mechanisms and Therapeutic Efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Wen, S.; Gu, D.; Zeng, H. Antitumor Effects of Beta-Amyrin in Hep-G2 Liver Carcinoma Cells Are Mediated via Apoptosis Induction, Cell Cycle Disruption and Activation of JNK and P38. J. BUON 2018, 23, 965–970. [Google Scholar] [PubMed]
- Alam, S.; Dhar, A.; Hasan, M.; Richi, F.T.; Emon, N.U.; Aziz, M.A.; Mamun, A.A.; Chowdhury, M.N.R.; Hossain, M.J.; Kim, J.K.; et al. Antidiabetic Potential of Commonly Available Fruit Plants in Bangladesh: Updates on Prospective Phytochemicals and Their Reported MoAs. Molecules 2022, 27, 8709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Moller, D. New Approaches in the Treatment of Type 2 Diabetes. Curr. Opin. Chem. Biol. 2000, 4, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.; Williams, K.; Narayan, K.; Leibson, C.; Haffner, S.; Stern, M. A Population Perspective on Diabetes Prevention: Whom Should We Target for Preventing Weight Gain? Diabetes Care 2003, 26, 1999–2004. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Ben Salem, M.; Ben Abdallah Kolsi, R.; Dhouibi, R.; Ksouda, K.; Charfi, S.; Yaich, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Jamoussi, K.; et al. Protective Effects of Cynara scolymus Leaves Extract on Metabolic Disorders and Oxidative Stress in Alloxan-Diabetic Rats. BMC Complement. Altern. Med. 2017, 17, 328. [Google Scholar] [CrossRef]
- Pushparaj, P.; Tan, C.; Tan, B. Effects of Averrhoa bilimbi Leaf Extract on Blood Glucose and Lipids in Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 2000, 72, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Ali, E.; Uddin, S.; Shaw, S.; Islam, M.; Ahmed, M.; Shill, M.; Karmakar, U.; Yarla, N.; Khan, I.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Tsai, F.S.; Lin, L.W.; Wu, C.R. Lupeol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 145–175. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Chokshi, H.P.; Desai, H.K. Separation of Diterpenoid Alkaloid Mixtures Using Vacuum Liquid Chromatography. J. Nat. Prod. 1986, 49, 892–900. [Google Scholar] [CrossRef]
- VanWagenen, B.C.; Larsen, R.; Cardellina, J.H.; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a Potent Insecticide from the Sponge Ulosa Ruetzleri. J. Org. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Islam, A.; Emon, N.; Islam, Q.; Ahsan, M. Chemico-Biological Profiling of Blumea lacera (Burm. f.) DC.(Family: Asteraceae) Provides New Insights as a Potential Source of Antioxidant, Cytotoxic. Evid.-Based Complement. Altern. Med. 2022, 2022, 2293415. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Rashid, M.; Sarker, M.; Rahman, M.; Emon, N.U.; Arman, M.; Mohamed, I.N.; Haque, M.R. Antidiarrheal, Antimicrobial and Antioxidant Potentials of Methanol Extract of Colocasia gigantea Hook. f. Leaves: Evidenced from in Vivo and in Vitro Studies. BMC Complement. Med. Ther. 2021, 21, 119. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Emon, N.; Ahsan, M. Isolation, Characterization and Pharmacological Investigations of a New Phenolic Compound along with Four Others Firstly Reported Phytochemicals from Glycosmis cyanocarpa (Blume) Spreng. Molecules 2022, 27, 5972. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Singh, O. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Mini Rev. Med. Chem. 2017, 18, 9–25. [Google Scholar] [CrossRef]
- Süzen, S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. In Bioactive Heterocycles V; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–178. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef]
- Ahmed, F.; Rahman, M.S. Preliminary Assessment of Free Radical Scavenging, Thrombolytic and Membrane Stabilizing Capabilities of Organic Fractions of Callistemon citrinus (Curtis.) Skeels Leaves. BMC Complement. Altern. Med. 2016, 16, 8. [Google Scholar] [CrossRef]
- Ragavan, B.; Krishnakumari, S. Antidiabetic Effect of T. arjuna Bark Extract in Alloxan Induced Diabetic Rats. Indian J. Clin. Biochem. 2006, 21, 123–128. [Google Scholar] [CrossRef]
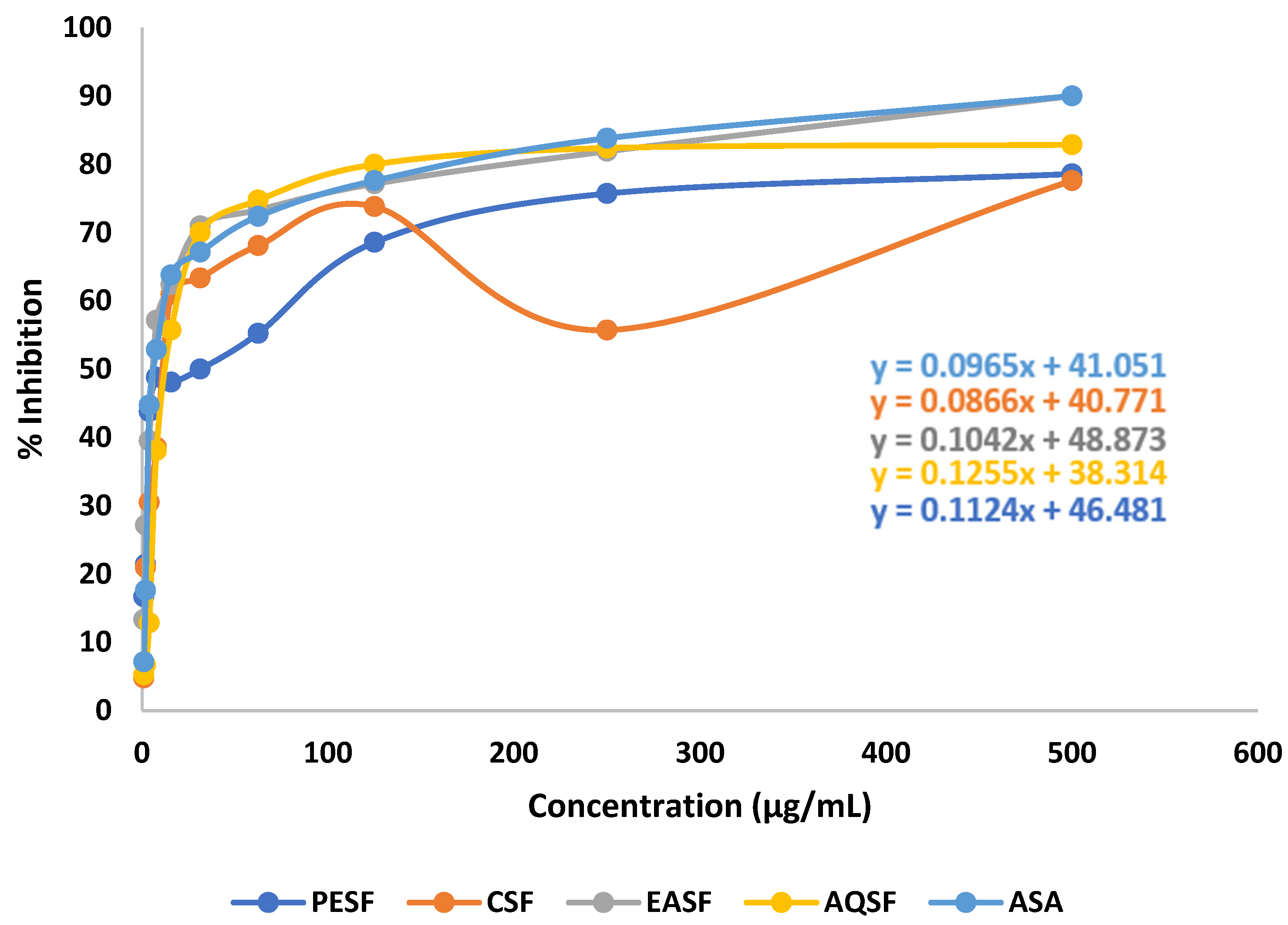
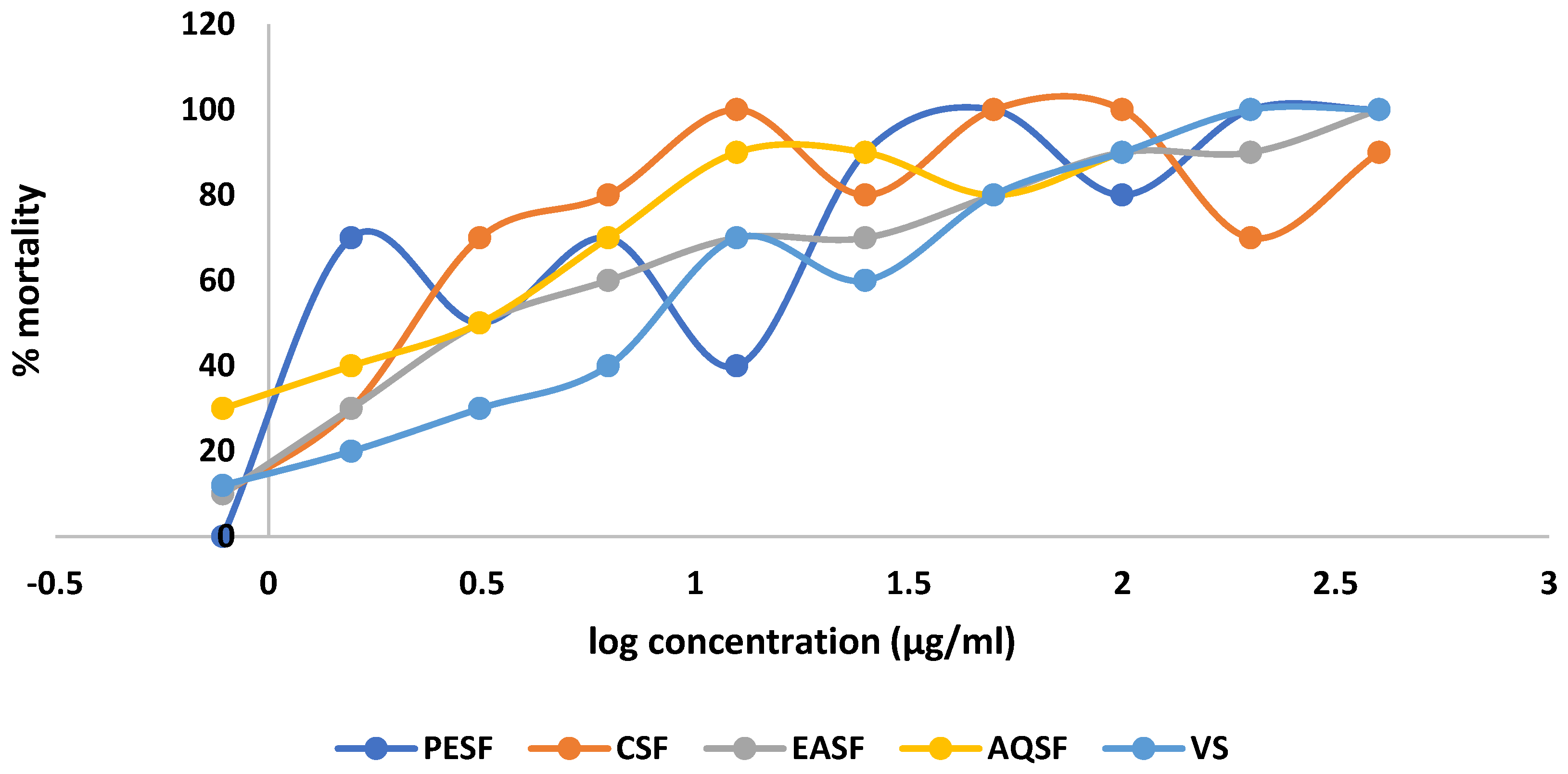
| Sample/ Standard | DPPH Free Radical Scavenging Activity (IC50 µg/mL) | Cytotoxicity (LC50 µg/mL) | % Clot Lysis | |
|---|---|---|---|---|
| Extract | CME | 25.68 ± 0.10 | 3.94 ± 0.16 | 63.77 ± 0.23 |
| PESF | 113.89 ± 3.25 | 3.45 ± 0.40 | 48.62 ± 0.56 | |
| CSF | 85.55 ± 2.74 | 1.94 ± 0.24 | 44.10 ± 0.40 | |
| EASF | 10.78 ± 0.11 | 5.59 ± 0.70 | 48.17 ± 0.58 | |
| AQSF | 93.09 ± 2.19 | 2.07 ± 0.36 | 26.00 ± 0.74 | |
| Standard | Ascorbic acid | 31.25 ± 0.67 | - | - |
| VS | - | 0.464 ±0.02 | - | |
| Blank | - | - | 2.00 ± 0.16 | |
| SK | - | - | 70.78 ±0.46 |
| Test Models | Groups | Fasting Blood Glucose Levels (mmol/L) Mean± SEM | |||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | ||
| Normal | Normal Control | 5.66 ± 0.2379 | 5.74 ± 0.2421 | 5.58 ± 0.1772 | 5.68 ± 0.3308 |
| Diabetic | Diabetic Control | 16.56 ± 2.164 | 20.06 ± 2.355 | 21.54 ± 2.808 | 22.52 ± 2.161 |
| Glibenclamide | 14.24 ± 2.106 | 5.66 ± 0.3906 | 4.88 ± 0.3734 | 5.16 ± 0.3669 | |
| CME | 13.70 ± 1.204 | 5.86 ± 0.2159 | 7.38 ± 0.3007 | 6.12 ± 0.2131 | |
| PESF | 23.72 ± 3.031 | 7.18 ± 0.4259 | 5.98 ± 0.2818 | 5.78 ± 0.2956 | |
| CSF | 13.72 ± 2.213 | 6.02 ± 0.3137 | 4.24 ± 0.2768 | 5.08 ± 0.2059 | |
| EASF | 17.6 ± 2.385 | 11.72 ± 2.835 | 6.44 ± 0.5819 | 6.2 ± 0.6050 | |
| AQSF | 19.74 ± 1.076 | 9.8 ± 2.852 | 7.66 ± 2.040 | 5.6 ± 0.2665 | |
| % Reduction in Blood Glucose Level (BGL) | ||||
|---|---|---|---|---|
| Sample/Standard | After 7 Day | After 14 Day | After 21 Day | % Reduction in BGL (Average) |
| Glibenclamide (STD) | 60.25 | 65.73 | 63.76 | 63.24 |
| CME | 57.22 | 45.93 | 55.32 | 52.82 |
| PESF | 69.73 | 67.20 | 74.20 | 70.37 |
| CSF | 56.12 | 69.09 | 62.97 | 62.72 |
| EASF | 33.40 | 63.40 | 64.77 | 53.85 |
| AQSF | 50.35 | 60.99 | 71.63 | 60.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobaer, M.A.; Ashrafi, S.; Ahsan, M.; Hasan, C.M.; Rashid, M.A.; Islam, S.N.; Masud, M.M. Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature. Molecules 2023, 28, 4186. https://doi.org/10.3390/molecules28104186
Jobaer MA, Ashrafi S, Ahsan M, Hasan CM, Rashid MA, Islam SN, Masud MM. Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature. Molecules. 2023; 28(10):4186. https://doi.org/10.3390/molecules28104186
Chicago/Turabian StyleJobaer, Md. Abu, Sania Ashrafi, Monira Ahsan, Choudhury Mahmood Hasan, Mohammad Abdur Rashid, Sheikh Nazrul Islam, and Mohammad Mehedi Masud. 2023. "Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature" Molecules 28, no. 10: 4186. https://doi.org/10.3390/molecules28104186
APA StyleJobaer, M. A., Ashrafi, S., Ahsan, M., Hasan, C. M., Rashid, M. A., Islam, S. N., & Masud, M. M. (2023). Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature. Molecules, 28(10), 4186. https://doi.org/10.3390/molecules28104186








