Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara
Abstract
1. Introduction
2. Results
2.1. Physiological Characteristics
2.2. Physicochemical Parameters
2.3. Phytochemical Analysis
2.4. Biological Activity
2.4.1. Assessment of Antioxidant Efficiency
2.4.2. Assessment of Antibacterial Activity
2.4.3. Evaluation of Anti-Inflammatory Efficacy
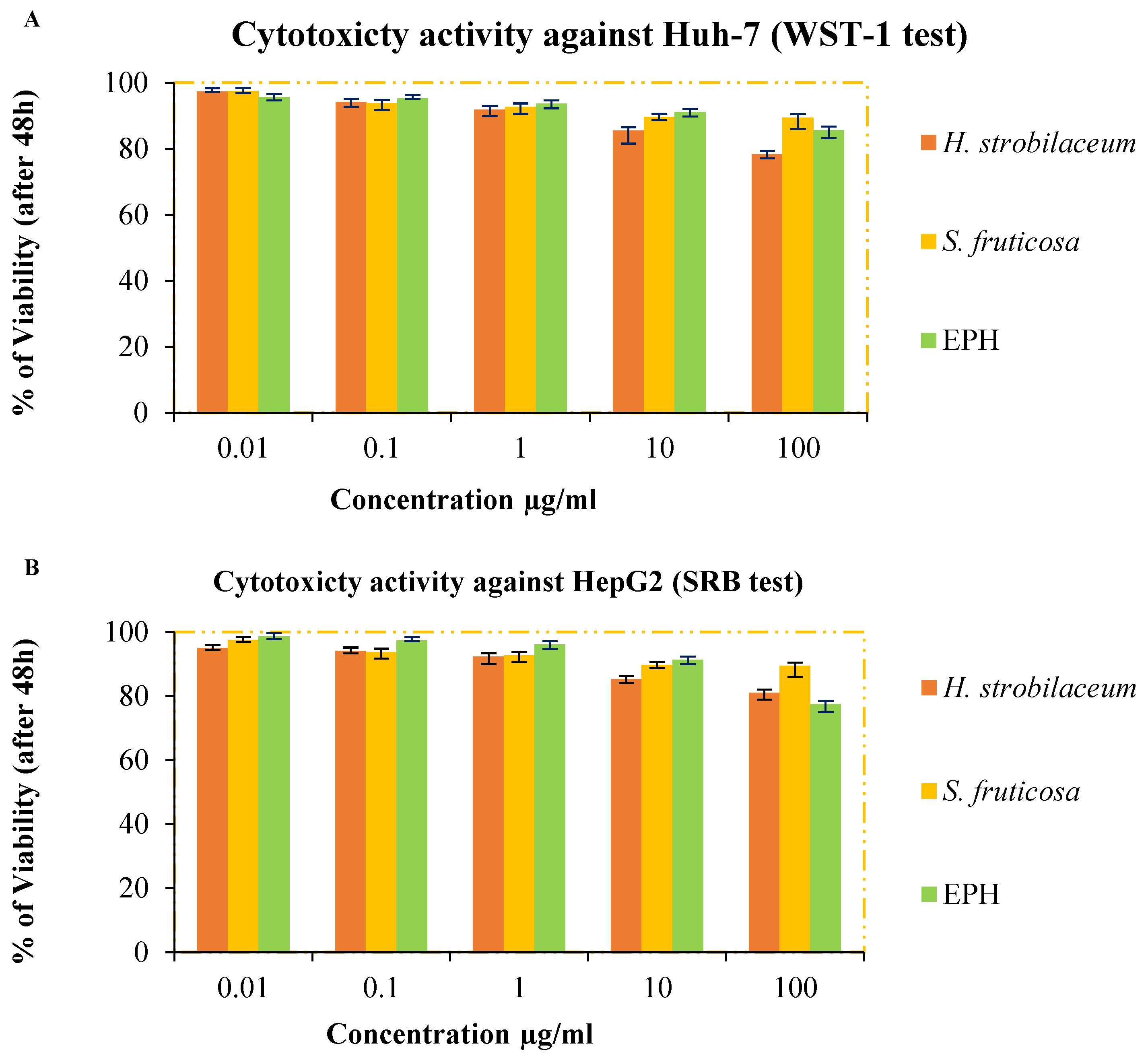
2.4.4. Assessment of Anticancer Potential
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Determination of Physiological Indicators
4.3.1. Determination of Malondialdehyde (MDA) Content
4.3.2. Determination of Proline Content
4.3.3. Determination of Water Content
4.3.4. Determination of the Content of Photosynthetic Pigments
4.4. Determination of Physicochemical Characterization
4.4.1. Ash Material
4.4.2. Determination of Mineral Elements in Plants
4.4.3. pH and Conductivity
4.4.4. Macronutrients Content (Carbohydrates, Lipids, and Proteins)
4.5. Phytochemical Study
4.5.1. Quantification of Phenolic Compounds
4.5.2. RP-HPLC Analysis
4.6. Biological Activity
4.6.1. Antioxidant Activity
4.6.2. Evaluation of Antibacterial Activity
4.6.3. In Vitro Anti-Inflammatory Activity
4.6.4. Cytotoxicity Assay
Huh-7 Cell Viability Was Assessed by WST-1 Assay
HepG2 Cell Viability Was Assessed by SRB Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
References
- Demnati, F.; Samraoui, B.; Farid, A.; Sandoz, A.; Ernoul, L. A literature review of Algerian salt lakes: Values, threats and implications. Environ. Earth Sci. 2017, 76, 127. [Google Scholar] [CrossRef]
- Hacini, M.; Kherici, N.; Oelkers, E.H. Mineral precipitation rates during the complete evaporation of the Merouane Chott ephemeral lake. Geochim. Cosmochim. Acta 2008, 72, 1583–1597. [Google Scholar] [CrossRef]
- Kumar, A. Palynology of the recent intertidal sediments of the Southern Red Sea Coast of Saudi Arabia. Palynology 2021, 45, 143–163. [Google Scholar] [CrossRef]
- Al-Amro, A.M.; Al-Qahtani, S.M. Plant diversity in Sabkha ecosystems of arid region: Spatial and environmental drivers. Braz. J. Biol. 2022, 82, e262331. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R890–R896. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Ghanem, A.-M.F.M.; Mohamed, E.; Kasem, A.M.M.A.; El-Ghamery, A.A. Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Maatallah Zaier, M.; Ciudad-Mulero, M.; Cámara, M.; Pereira, C.; Ferreira, I.C.F.R.; Achour, L.; Kacem, A.; Morales, P. Revalorization of Tunisian wild Amaranthaceae halophytes: Nutritional composition variation at two different phenotypes stages. J. Food Compos. Anal. 2020, 89, 103463. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Feng, W.; Lindner, H.; Robbins, N.E., 2nd; Dinneny, J.R. Growing Out of Stress: The Role of Cell- and Organ-Scale Growth Control in Plant Water-Stress Responses. Plant Cell 2016, 28, 1769–1782. [Google Scholar] [CrossRef]
- Glenn, E.; O’Leary, J. Relationship between salt accumulation and water content of dicotyledonous halophytes. Plant Cell Environ. 2006, 7, 253–261. [Google Scholar]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Bhattacharya, A. Physiological Processes in Plants Under Low Temperature Stress, 1st ed.; Springer: Singapore, 2022; p. 734. [Google Scholar]
- Wang, N.; Zhang, W.; Qin, M.; Li, S.; Qiao, M.; Liu, Z.; Xiang, F. Drought tolerance conferred in soybean (Glycine max L.) by GmMYB84, a novel R2R3-MYB transcription factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef] [PubMed]
- Yasseen, B.; Al-Thani, R. Halophytes and associated properties of natural soils in the Doha area, Qatar. Aquat. Ecosyst. Health Manag. 2007, 10, 320–326. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A. Interspecific root interactions and rhizosphere effects on salt ions and nutrient uptake between mixed grown peanut/maize and peanut/barley in original saline–sodic–boron toxic soil. J. Plant Physiol. 2008, 165, 490–503. [Google Scholar] [CrossRef]
- Mitra, G.N. Regulation of Nutrient Uptake by Plants: A Biochemical and Molecular Approach, 1st ed.; Springer: New Delhi, India, 2015; p. 195. [Google Scholar]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of Antioxidant Activity of Garden Blackberries (Rubus fruticosus L.) Extracts Obtained with Different Extraction Solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Rocha, M.I.; Rodrigues, M.J.; Pereira, C.; Pereira, H.; da Silva, M.M.; da Rosa Neng, N.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Biochemical profile and in vitro neuroprotective properties of Carpobrotus edulis L., a medicinal and edible halophyte native to the coast of South Africa. S. Afr. J. Bot. 2017, 111, 222–231. [Google Scholar] [CrossRef]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Effect of Salinity on Growth, Ion Accumulation and Mineral Nutrition of Different Accessions of a Crop Wild Relative Legume Species, Trifolium fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K. Plant salt tolerance. In Plant Responses to Abiotic Stress; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 241–270. [Google Scholar]
- Hess, J.; Ray, A.; Rials, T. Editorial: Advancements in Biomass Feedstock Preprocessing: Conversion Ready Feedstocks. Front. Energy Res. 2019, 7, 140. [Google Scholar] [CrossRef]
- Edwards, G.E.; Franceschi, V.R.; Voznesenskaya, E.V. Single-cell C4 photosynthesis versus the dual-cell (kranz) paradigm. Annu. Rev. Plant Biol. 2004, 55, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Biel, K.; Fomina, I. Benson-Bassham-Calvin cycle contribution to the organic life on our planet. Photosynthetica 2015, 53, 161–167. [Google Scholar] [CrossRef]
- Kumar, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Choudhary, P.; Parameswari, E.; Alhariri, A.; Samota, M.K.; Damale, R.D.; et al. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar]
- Akhtar, M.S. Salt Stress, Microbes, and Plant Interactions: Causes and Solution, 1st ed.; Springer: Singapore, 2019; p. 297. [Google Scholar]
- Handoussa, H.; AbdAllah, W.; AbdelMohsen, M. UPLC–ESI-PDA–MSn profiling of phenolics involved in biological activities of the medicinal plant Halocnemum strobilaceum (Pall.). Iran. J. Pharm. Res. 2019, 18, 422–429. [Google Scholar]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; Canini, A.; El Haci, I.A.; Atik Bekkara, F. Phytochemical analysis and antioxidant activity of Tamarix africana, Arthrocnemum macrostachyum and Suaeda fruticosa, three halophyte species from Algeria. Plant Biosyst. 2019, 153, 843–852. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Singh, A.; Singh, I. Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology, 1st ed.; Springer: Singapore, 2020; p. 464. [Google Scholar]
- AbdelRazek, M.M.; Moussa, A.Y.; Elshanawany, M.M.; Singab, A.-N. Effect of Changing Culture Media on Metabolites of Endophytic Fungi from Halocnemum strobilaceum. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 135–144. [Google Scholar]
- Gheraissa, N.; Chemsa, A.E.; Elsharkawy, E.R.; Cherrada, N. Phenolic compound profile, and evaluation of biological properties of Bassia muricata (L.) Asch. aerial part. Int. J. Second. Metab. 2022, 9, 335–347. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Megahed, B.M.; Gamal, M.; Safwat, G. Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of Beta vulgaris L. root. Fresenius Environ. Bull. 2018, 27, 6369–6378. [Google Scholar]
- Shalaby, H.S.; Hassenin, A.S.H. Effects of Fortification Stirred Yoghurt with Red Beet Powder (RBP) on Hypercholesterolemia Rats. Eur. J. Agric. Food Sci. 2020, 2, 5. [Google Scholar] [CrossRef]
- Labdelli, A.; Rebiai, A.; Tahirine, M.; Adda, A.; Merah, O. Nutritional Content and Antioxidant Capacity of the Seed and the Epicarp in Different Ecotypes of Pistacia atlantica Desf. Subsp. atlantica. Plants 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Saada, M.; Kasmi, M.; Ben Jemaa, M.; Ksouri, R. Antioxidant and antimicrobial activities of Halocnemum strobilaceum fractions and their related bioactive molecules identification by GC/MS and HPLC. Res. J. Recent Sci. 2018, 7, 1–9. [Google Scholar]
- Naija, D.S.; Bouzidi, A.; Boussaada, O.; Helal, A.N.; Mahjoub, M.A.; Echafai, N.; Mighri, Z. The antioxidant and free-radical scavenging activities of Tamarix boveana and Suaeda fruticosa fractions and related active compound. Eur. Sci. J. 2014, 10, 18. [Google Scholar]
- Oueslati, S.; Trabelsi, N.; Boulaaba, M.; Legault, J.; Abdelly, C.; Ksouri, R. Evaluation of antioxidant activities of the edible and medicinal Suaeda species and related phenolic compounds. Ind. Crops Prod. 2012, 36, 513–518. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef]
- Ashokkumar, D.; Thamilselvan, V.; Gp, S.; Mazumder, U.K.; Gupta, M. Antioxidant and Free Radical Scavenging Effects of Lippia nodiflora. Pharm. Biol. 2008, 46, 762–771. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Jacobo-Velázquez, D.A.; Benavides, J. Aqueous Two-Phase System Strategies for the Recovery and Partial Purification of Bioactive Low Molecular Weight Compounds. In Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products; Rito-Palomares, M., Benavides, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 79–96. [Google Scholar]
- Faraone, I.; Rai, D.K.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant Activity and Phytochemical Characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. [Google Scholar] [CrossRef]
- Baik, K.Y.; Huh, Y.H.; Kim, Y.H.; Kim, J.; Kim, M.S.; Park, H.-K.; Choi, E.H.; Park, B. The Role of Free Radicals in Hemolytic Toxicity Induced by Atmospheric-Pressure Plasma Jet. Oxidative Med. Cell. Longev. 2017, 2017, 1289041. [Google Scholar] [CrossRef]
- De Graft-Johnson, J.; Nowak, D. Effect of Selected Plant Phenolics on Fe2+-EDTA-H₂O₂ System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Elmokasabi, F.; Al-Sanousi, M.; El-Mabrouk, R. Taxonomy and Ethnobotany of Medicinal Plants in Eastern Region of Libya. IOSR J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 14–23. [Google Scholar]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M.; et al. A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk: An edible halophyte medicinal plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef] [PubMed]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Mansour, S.; Djebli, N.; Bouakline, H.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; et al. Antimicrobial and anti-inflammatory activities of three halophyte plants from Algeria and detection of some biomolecules by HPLC-DAD. Nat. Prod. Res. 2021, 35, 2107–2111. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Tatlow, D.; Poothencheri, S.; Bhangal, R.; Tatlow, C. Novel method for rapid reversal of drug toxicity: A case report. Clin. Exp. Pharmacol. Physiol. 2015, 42, 389–393. [Google Scholar] [CrossRef]
- Ksouri, R.; Smaoui, A.; Isoda, H.; Abdelly, C. Utilization of halophyte species as new sources of bioactive substances. J. Arid Land Stud. 2012, 22, 41–44. [Google Scholar]
- Akhtar, M.S.; Swamy, M. Anticancer Plants: Natural Products and Biotechnological Implements, 1st ed.; Springer: Singapore, 2018; pp. 133, 564. [Google Scholar]
- Rauf, A.; Imran, M.; Khan, B.; Rehman, M.; Gilani, S.A.; Mehmood, Z. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Casella, M.L.; Parody, J.P.; Ceballos, M.P.; Quiroga, A.D.; Ronco, M.T.; Francés, D.E.; Monti, J.A.; Pisani, G.B.; Carnovale, C.E.; Carrillo, M.C.; et al. Quercetin prevents liver carcinogenesis by inducing cell cycle arrest, decreasing cell proliferation and enhancing apoptosis. Mol. Nutr. Food Res. 2014, 58, 289–300. [Google Scholar] [CrossRef]
- Chouikh, A.; Chemsa, A.E.; Aounallah, C.; Aounallah, I.; Alia, F. Phytochemical study, nutritive value, antioxidant and anti-inflammatory activities of phenolic extracts from desert plant Calligonum comosum L’Hér. Alger. J. Biosci. 2020, 1, 68–75. [Google Scholar] [CrossRef]
- Mbaebie, B.O.; Edeoga, H.O.; Afolayan, A.J. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac. J. Trop. Biomed. 2012, 2, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Kumar, T.S.; Gangaprasad, A.; Maggi, F.; Rao, M.V. Phytochemical analysis, antioxidant and antimicrobial activity of wild and in vitro derived plants of Ceropegia thwaitesii Hook—An endemic species from Western Ghats, India. J. Genet. Eng. Biotechnol. 2018, 16, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Nawaz, H.; Shad, M.A.; Shahwar, D.-E.; Andaleeb, H.; Muzaffar, S.; Jabeen, R.; Rehman, T.; Waheed, A.A. Antioxidant Potential of Cell Wall Polysaccharides Extracted from Various Parts of Aerva javanica. Free. Radic. Antioxid. 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Sharma, A.; Marceau, C.; Hamaguchi, R.; Burridge, P.W.; Rajarajan, K.; Churko, J.M.; Wu, H.; Sallam, K.I.; Matsa, E.; Sturzu, A.C.; et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014, 115, 556–566. [Google Scholar] [CrossRef]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]


| Moisture Content (%) | Proline (mg/g) | MDA (mM) | Ch a (mg/g) | Ch b (mg/g) | Carotene (mg/g) | |
|---|---|---|---|---|---|---|
| H. strobilaceum | 46.94 ± 5.04 | 0.366 ± 0.06 | 0.24 ± 0.06 | 2.79 ± 0.01 | 0.6 ± 0.02 | 0.88 ± 0.02 |
| S. fruticosa | 82.7 ± 3.56 | 0.434 ± 0.09 | 0.12 ± 0.02 | 3.22 ± 0.01 | 0.91 ± 0.03 | 0.99 ± 0.00 |
| Weight (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Oxides | ||||||||||||||
| O | C | Ca | Na | Si | Al | Cl | Mg | K | S | CaO | Na2O | SiO2 | Al2O3 | MgO | |
| H. strobilaceum | 43.05 | 32.22 | 3.23 | 7.51 | 1.11 | 0.41 | 6.15 | 0.17 | 0.42 | 0.86 | 6.61 | 21.3 | 3.67 | 1.31 | 0.48 |
| S. fruticosa | 25.04 | 49.55 | 1.59 | 1.92 | 0.49 | 0.3 | 6.32 | 0.75 | 5.16 | 0.25 | 4.46 | 1.57 | 2.1 | 1.14 | 3.53 |
| Ash (%) | pH | EC (dmS/m) | CCA (mg G/g) | LC (mg SO/g) | PC (mg BSA/g) | |
|---|---|---|---|---|---|---|
| H. strobilaceum | 29.89 ± 1.8 | 7.5 ± 0.005 | 19.4 ± 0.00 | 8.13 ± 0.17 | 2.68 ± 0.02 | 15.2 ± 0.04 |
| S. fruticosa | 19.76 ± 1.21 | 6.15 ± 0.09 | 12.38 ± 0.07 | 9.07 ± 0.22 | 2.25 ± 0.15 | 15.6 ± 0.09 |
| TPC (µg GAE/mg) | TFC (µg QE/mg) | FC (µg QE/mg) | AC (µg C-3-GE/mg) | HTC (µg GAE/mg) | TCT (µg CE/mg) | |
|---|---|---|---|---|---|---|
| H. strobilaceum | 24.97 ± 0.09 | 12.17 ± 0.16 | 5.43 ± 0.06 | 1.87 ± 1.88 | 6.23 ± 0.24 | 3.99 ± 0.09 |
| S. fruticosa | 47.38 ± 0.16 | 14.57 ± 0.12 | 6.70 ± 0.16 | 1.17 ± 0.47 | 8.81 ± 0.32 | 4.68 ± 0.25 |
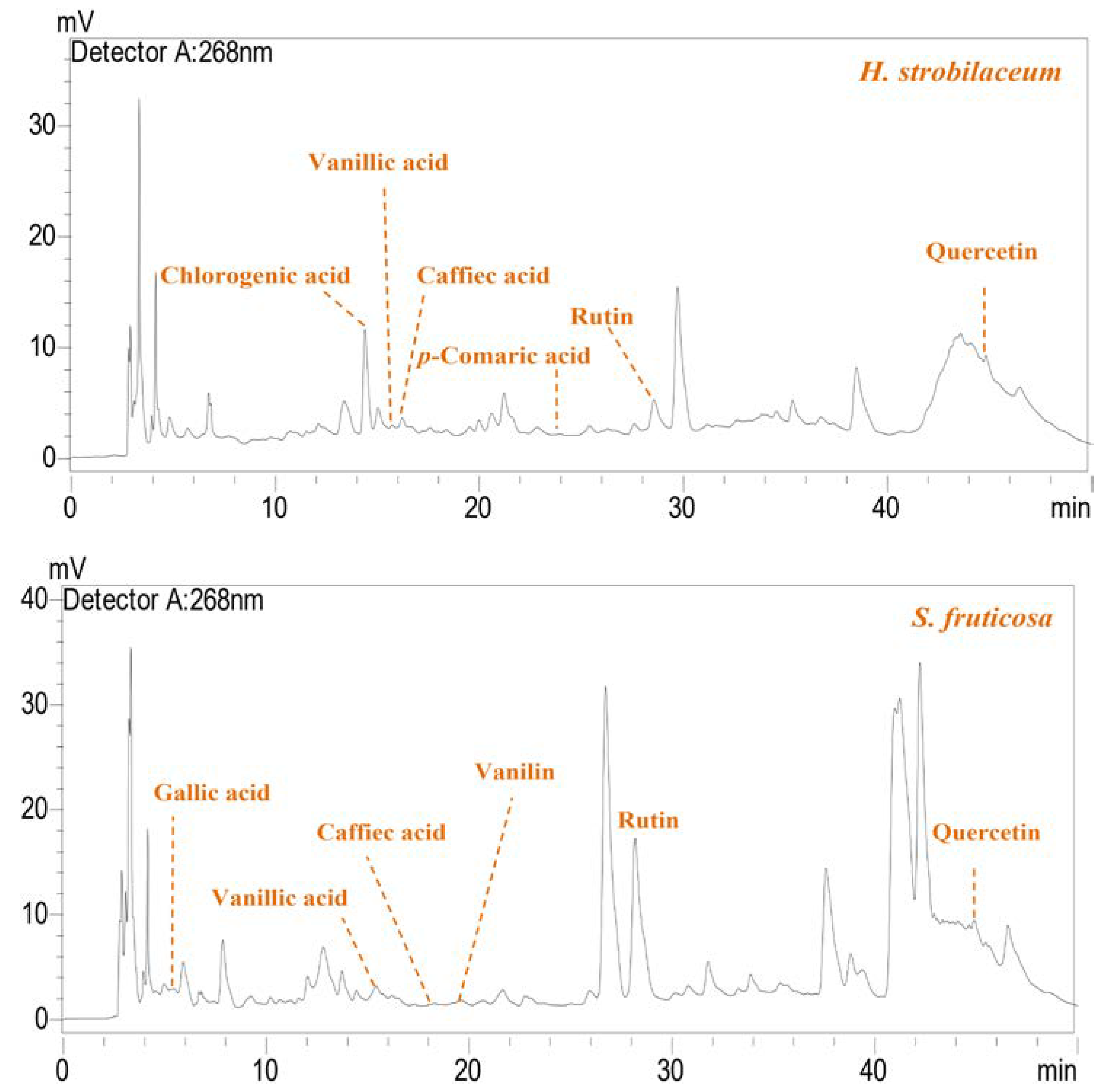
| Compounds | Retention Time (min) | H. strobilaceum | S. fruticosa | |
|---|---|---|---|---|
| Phenolic acid (µg/100 mg ED) | Gallic acid | 5.29 | - | 10.01 |
| Chlorogenic acid | 13.392 | 85.77 | - | |
| Vanillic acid | 15.531 | 1.33 | 20.30 | |
| Caffeic acid | 16.277 | 2.71 | 5.66 | |
| Vanillin | 21.46 | - | 17.36 | |
| p-Coumaric acid | 23.817 | 0.96 | 1.63 | |
| Flavonoide (µg/100 mg ED) | Rutin | 28.37 | 84.42 | 367.56 |
| Naringin | 34.788 | - | - | |
| Quercetin | 45.047 | 207.16 | 93.69 | |
| H. strobilaceum | S. fruticosa | Ascorbic Acid | α-Tocopherol | |||
|---|---|---|---|---|---|---|
| Radical scavenging activity | DPPH• | IC50 | 81.70 ± 0.64 | 118.8 ± 1.46 | 1.44 ± 0.02 | / |
| HO• | IC50 | >1000 | >1000 | 86.0 ± 0.70 | / | |
| β-carotene bleaching method | EC50 | 58.8 ± 0.94 | 82.8 ± 2.23 | 532.4 ± 2.50 | 2.10 ± 0.08 | |
| Anti-hemolysis activity | Hly50 | 193.3 ± 1.70 | 225.7 ± 27.80 | 154.4 ± 1.70 | / | |
| Reducing power | EC50 | >2000 | 1024 ± 35 | 67.28 ± 2.00 | / | |
| Total antioxidant capacity (mg GAE/g) | 93.94 ± 1.92 | 151.83 ± 2.03 | / | / | ||
| Treatments (mg) | Inhibition Zone Diameter (mm) | |||||
|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | |||||
| B. subtilis ATCC-6633 | L. innocua CLIP-74915 | S. aureus ATCC-6538 | E. coli ATCC-25922 | P. aeruginosa ATCC-9027 | S. typhimurium ATCC-14028 | |
| H. strobilaceum (4 mg) | 10.7 ± 1.2 * | 11.3 ± 4.7 * | 11.3 ± 4.7 * | 15.3 ± 3.2 ** | 8 ± 1.7 | 11.7 ± 1.2 * |
| S. fruticosa (4 mg) | 9.7 ± 0.6 | 14.3 ± 1.5 * | 14.3 ± 1.5 * | 9.7 ± 0.6 | 12.3 ± 4 * | 11.7 ± 0.6 * |
| Gentamicin (1 mg) | 10.67 ± 2.1 * | 10.67 ± 2 * | 11.33 ± 1.1 * | / | 30.33 ± 0.6 *** | 14.33 ± 0.58 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheraissa, N.; Chemsa, A.E.; Cherrada, N.; Erol, E.; Elsharkawy, E.R.; Ghemam-Amara, D.; Zeghoud, S.; Rebiai, A.; Messaoudi, M.; Sawicka, B.; et al. Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules 2023, 28, 3580. https://doi.org/10.3390/molecules28083580
Gheraissa N, Chemsa AE, Cherrada N, Erol E, Elsharkawy ER, Ghemam-Amara D, Zeghoud S, Rebiai A, Messaoudi M, Sawicka B, et al. Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules. 2023; 28(8):3580. https://doi.org/10.3390/molecules28083580
Chicago/Turabian StyleGheraissa, Noura, Ahmed Elkhalifa Chemsa, Nezar Cherrada, Ebru Erol, Eman Ramadan Elsharkawy, Djilani Ghemam-Amara, Soumeia Zeghoud, Abdelkrim Rebiai, Mohammed Messaoudi, Barbara Sawicka, and et al. 2023. "Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara" Molecules 28, no. 8: 3580. https://doi.org/10.3390/molecules28083580
APA StyleGheraissa, N., Chemsa, A. E., Cherrada, N., Erol, E., Elsharkawy, E. R., Ghemam-Amara, D., Zeghoud, S., Rebiai, A., Messaoudi, M., Sawicka, B., Atanassova, M., & Abdel-Kader, M. S. (2023). Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum strobilaceum Pall. and Suaeda fruticosa (L.) Forske., Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules, 28(8), 3580. https://doi.org/10.3390/molecules28083580









