Synthesis of Transition-Metal-Doped Nanocatalysts with Antibacterial Capabilities Using a Complementary Green Method
Abstract
1. Introduction
2. Results and Discussion
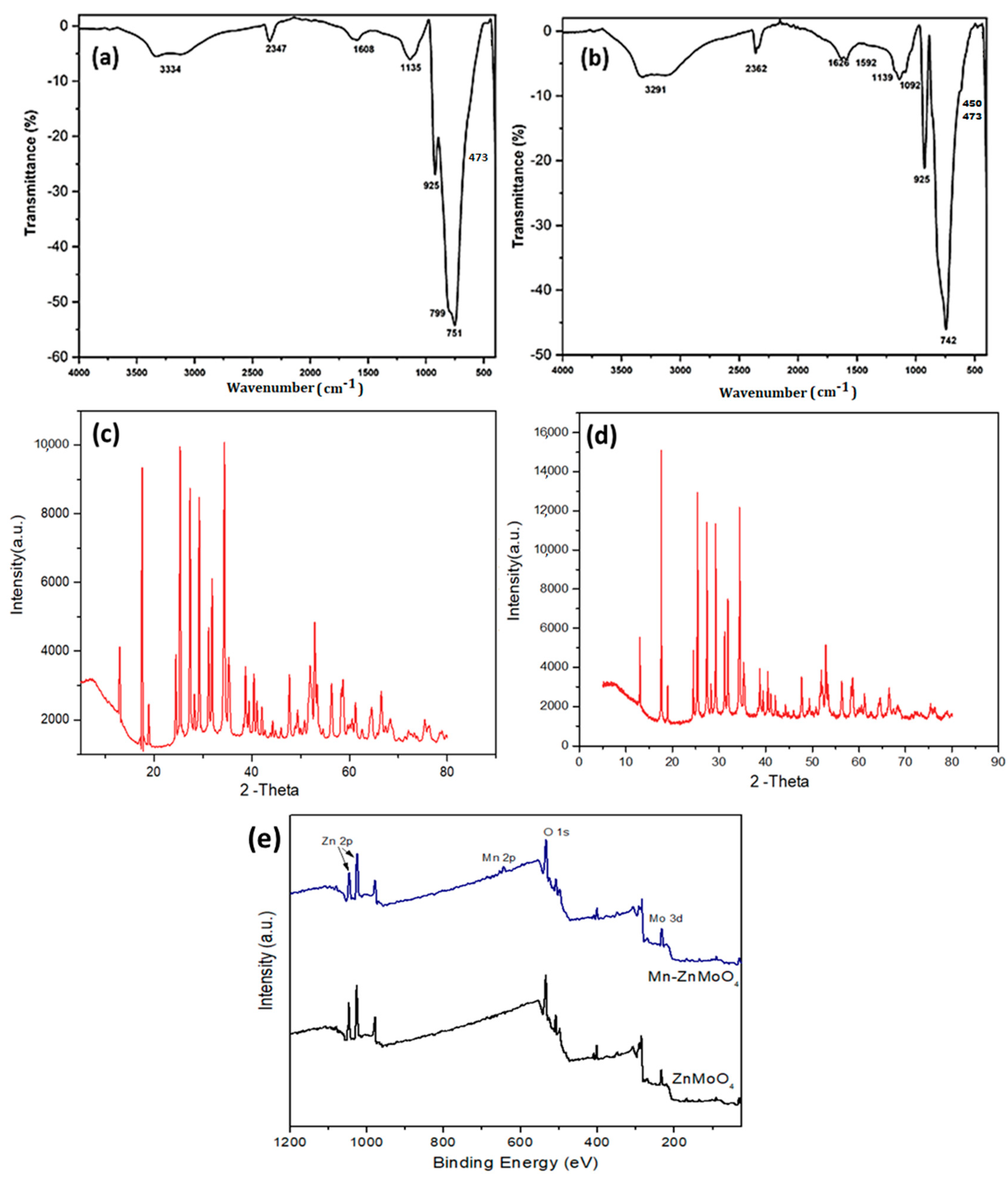
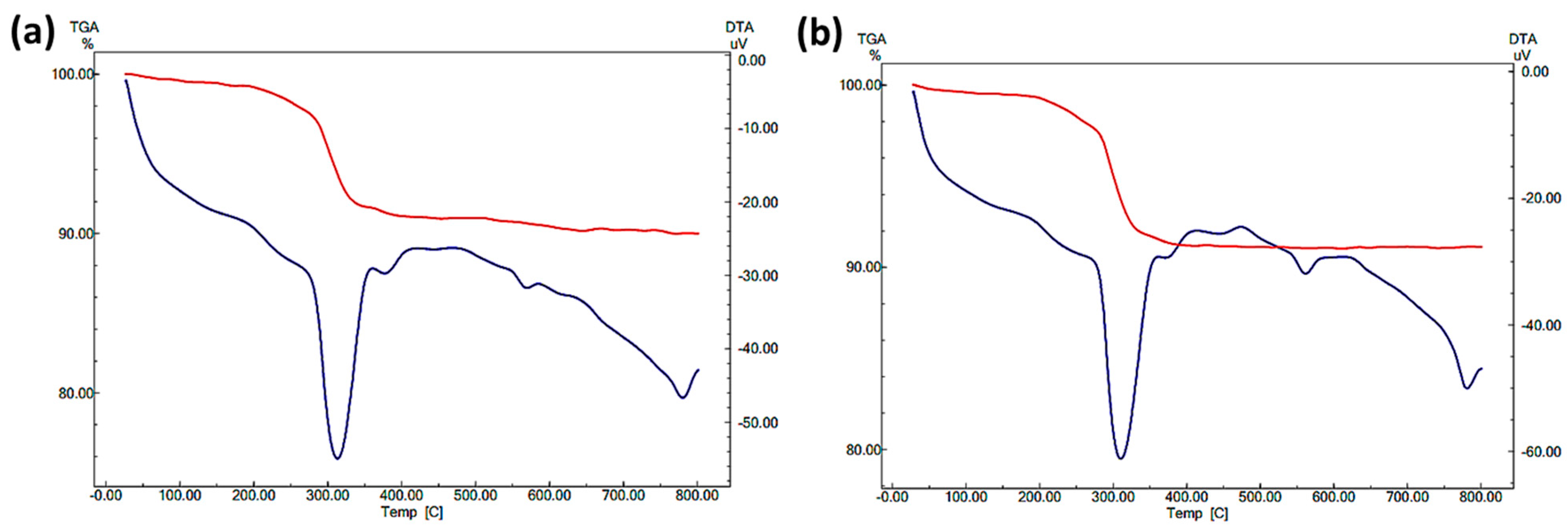
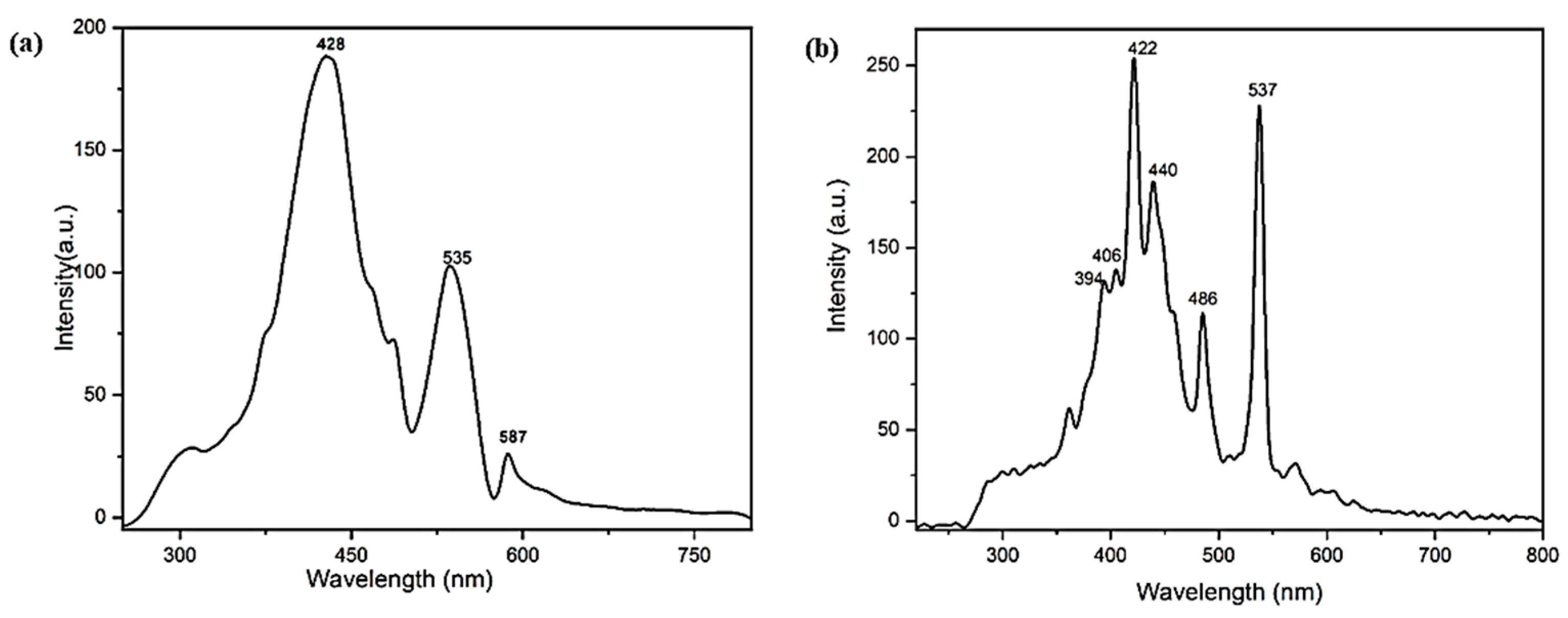
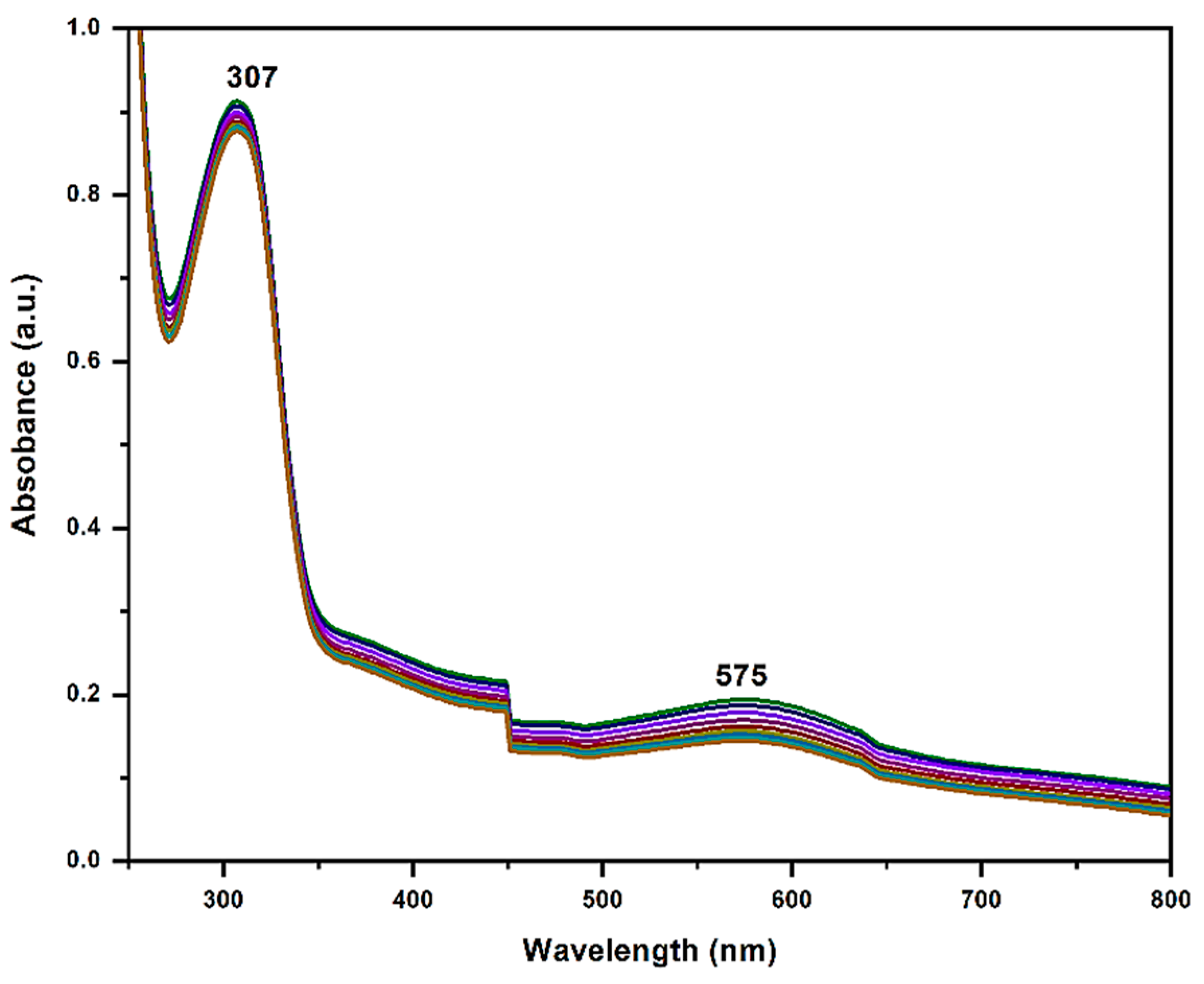
2.1. Characterization of Nanocomposites
2.2. Antibacterial Activities of ZnMoO4 and Mn-Doped ZnMoO4
2.2.1. Bacterial Species Collection
2.2.2. Antibacterial Effect
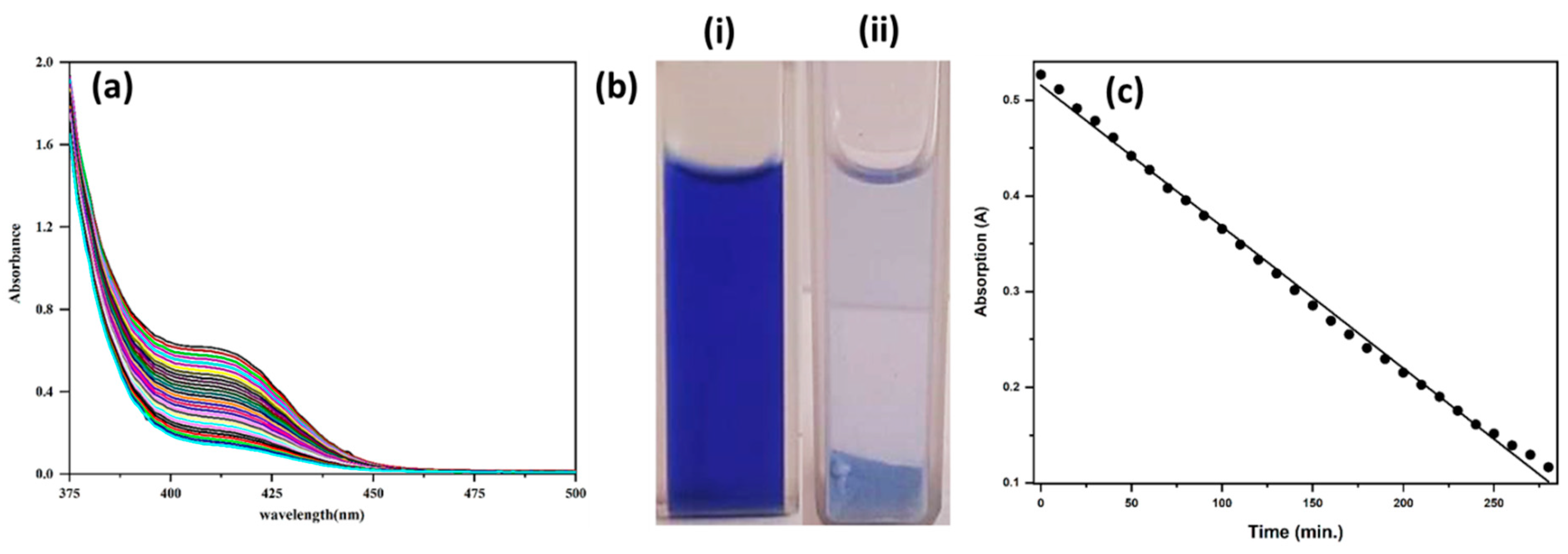
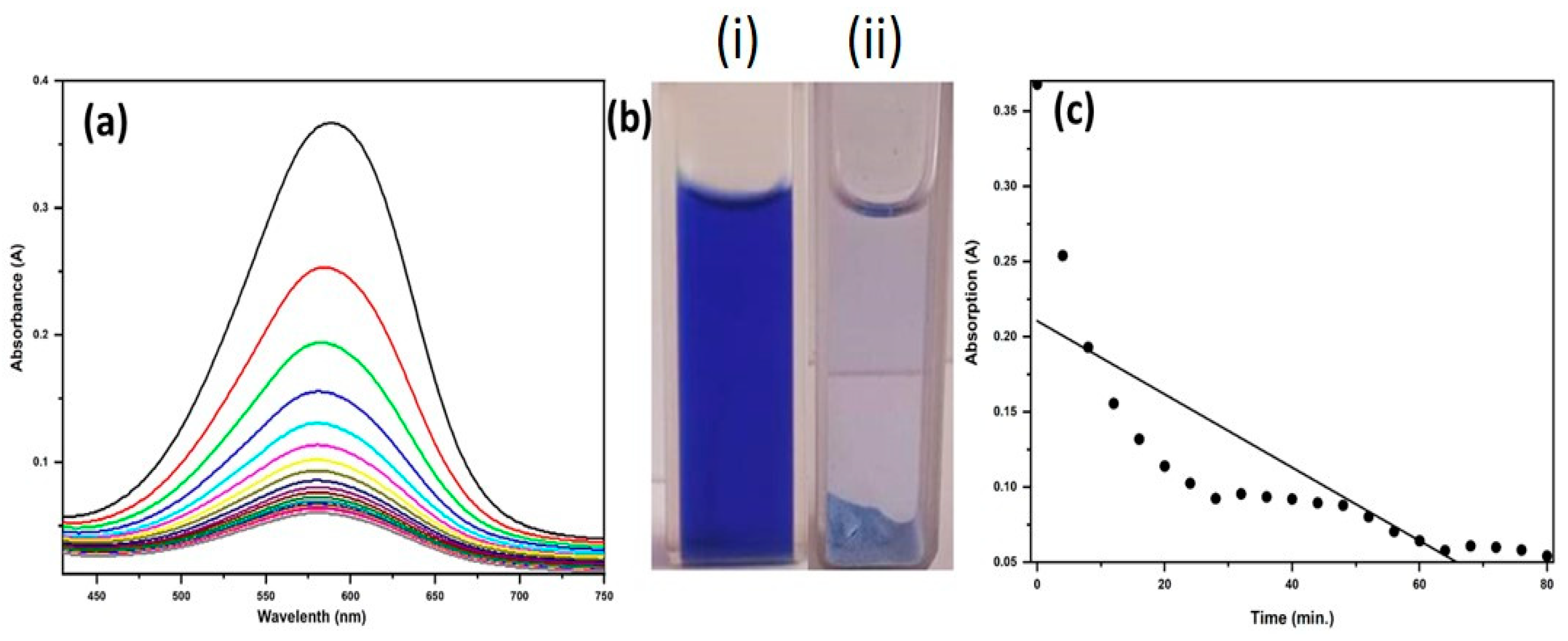
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
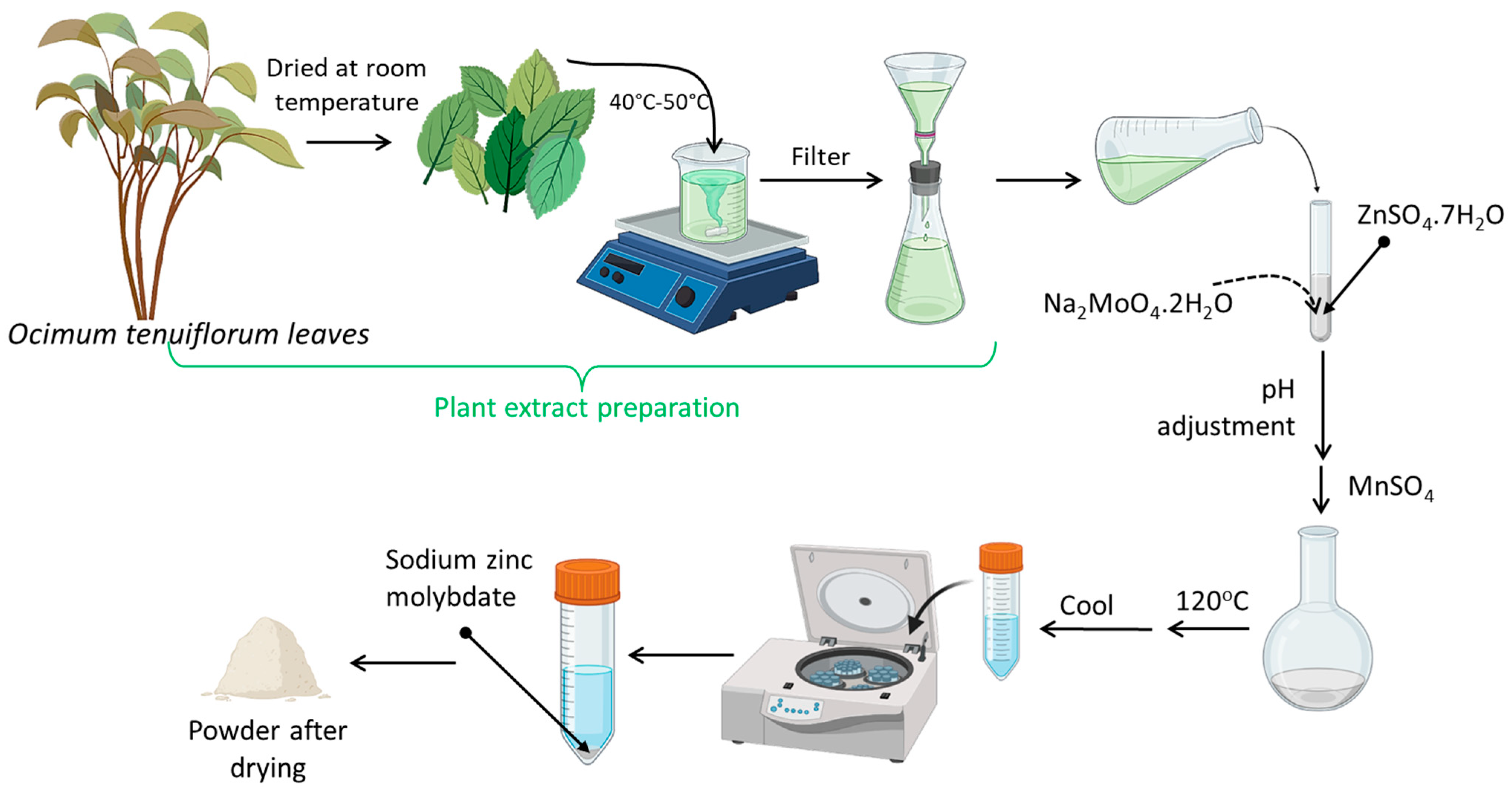
3.3. Extraction of Ocimum Tenuiflorum Leaves
3.4. Synthesis of Sodium Zinc Molybdate
3.5. Synthesis of Mn-Doped Sodium Zinc Molybdate
3.6. Antibacterial Activity
3.7. Dye Remediation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cornu, L.; Jubera, V.; Demourgues, A.; Salek, G.; Gaudon, M. Luminescence properties and pigment properties of A-doped (Zn, Mg) MoO4 triclinic oxides (with A = Co, Ni, Cu or Mn). Ceram. Int. 2017, 43, 13377–13387. [Google Scholar] [CrossRef]
- Wang, D.; Huang, M.; Zhuang, Y.; Jia, H.-L.; Sun, J.; Guan, M. Phase-and Morphology-Controlled Synthesis of Zinc Molybdate for Excellent Photocatalytic Properties. Eur. J. Inorg. Chem. 2017, 2017, 4939–4946. [Google Scholar] [CrossRef]
- Ramezani, M.; Hosseinpour-Mashkani, S.M.; Sobhani-Nasab, A.; Ghasemi Estarki, H. Synthesis, characterization, and morphological control of ZnMoO4 nanostructures through precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2015, 26, 7588–7594. [Google Scholar] [CrossRef]
- Lv, L.; Tong, W.; Zhang, Y.; Su, Y.; Wang, X. Metastable monoclinic ZnMoO4: Hydrothermal synthesis, optical properties and photocatalytic performance. J. Nanosci. Nanotechnol. 2011, 11, 9506–9512. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, L.X.; Oliveira, M.C.; Andrés, J.; Gracia, L.; Li, M.S.; Longo, E.; Tranquilina, R.L.; Paskocimasa, C.A.; Bomioa, M.R.D.; Motta, F.V. Structure, morphology and photoluminescence emissions of ZnMoO4: RE3+ = Tb3+ − Tm3+ − X Eu3+ (x = 1, 1.5, 2, 2.5 and 3 mol%) particles obtained by the sonochemical method. J. Alloys Compd. 2018, 750, 55–70. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, S.-M.; Yoon, J.-W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M = Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705. [Google Scholar] [CrossRef]
- Zazhigalov, V.; Sachuk, O.; Kopachevska, N.; Starchevskyy, V.; Sawlowicz, Z. Effect of ultrasonic treatment on formation of nanodimensional structures in ZnO–MoO3 system. Theor. Exp. Chem. 2017, 53, 53–59. [Google Scholar] [CrossRef]
- Chernyak, D.; Danevich, F.; Degoda, V.Y.; Dmitruk, I.; Ferri, F.; Galashov, E.; Giuliani, A.; Ivanov, I.; Kobychev, V.; Mancuso, M.; et al. Optical, luminescence and thermal properties of radiopure ZnMoO4 crystals used in scintillating bolometers for double beta decay search. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 729, 856–863. [Google Scholar] [CrossRef]
- Beeman, J.; Danevich, F.; Degoda, V.; Galashov, E.; Giuliani, A.; Ivanov, I.; Mancuso, M.; Marnieros, S.; Nones, C.; Pessina, G.E.; et al. An improved ZnMoO4 scintillating bolometer for the search for neutrinoless double beta decay of 100 Mo. J. Low Temp. Phys. 2012, 167, 1021–1028. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Dayalan, A.; Nagaraja, K. Co-precipitation synthesis, humidity sensing and photoluminescence properties of nanocrystalline Co2+ substituted zinc (II) molybdate (Zn1–xCoxMoO4; x = 0, 0.3, 0.5, 0.7, 1). Solid State Sci. 2017, 67, 46–58. [Google Scholar] [CrossRef]
- Mardare, C.C.; Tanasic, D.; Rathner, A.; Müller, N.; Hassel, A.W. Growth inhibition of Escherichia coli by zinc molybdate with different crystalline structures. Phys. Status Solidi 2016, 213, 1471–1478. [Google Scholar] [CrossRef]
- Luitel, H.N.; Chand, R.; Hamajima, H.; Gaihre, Y.R.; Shingae, T.; Yanagita, T.; Watari, T. Highly efficient NIR to NIR upconversion of ZnMoO4: Tm3+, Yb3+ phosphors and their application in biological imaging of deep tumors. J. Mater. Chem. B 2016, 4, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-P.; Huang, K.-J.; Zhang, C.-X.; Song, S.-S.; Wu, X. High-performance symmetric supercapacitor based on flower-like zinc molybdate. J. Alloys Compd. 2018, 731, 1151–1158. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Fujikawa, N.; Ueda, W.; Moro-Oka, Y.; Lee, K.-W. Propane oxidation over various metal molybdate catalysts. Catal. Today 1995, 24, 327–333. [Google Scholar] [CrossRef]
- Maggiore, R.; Galvagno, S.; Bart, J.C.; Giannetto, A.; Crisafulli, C.; Toscano, G. Catalytic oxidation of propene over zinc, cadmium and nickel molybdates. Z. Phys. Chem. 1983, 137, 111–118. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Moraes, E.; Almeida, M.; Dalmaschio, C.; Batista, N.; Varela, J.A.; Longo, E.; Siu Li, M.; Andrés, J.; Beltrán, A. A combined theoretical and experimental study of electronic structure and optical properties of β-ZnMoO4 microcrystals. Polyhedron 2013, 54, 13–25. [Google Scholar] [CrossRef]
- Pasiński, D.; Sokolnicki, J. Broadband orange phosphor by energy transfer between Ce3+ and Mn2+ in Ca3Al2Ge3O12 garnet host. J. Alloys Compd. 2019, 786, 808–816. [Google Scholar] [CrossRef]
- Mallikarjun, S.; Rao, A.; Rajesh, G.; Shenoy, R.; Pai, M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: An in vitro study. J. Indian Soc. Periodontol. 2016, 20, 145. [Google Scholar] [PubMed]
- Hussain, E.H.; Jamil, K.; Rao, M. Hypoglycaemic, hypolipidemic and antioxidant properties of tulsi (Ocimum sanctum Linn) on streptozotocin induced diabetes in rats. Indian J. Clin. Biochem. 2001, 16, 190–194. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-inspired polynorepinephrine/MXene-based magnetic nanohybrid for electromagnetic interference shielding in X-band and strain-sensing performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized thin coating of polypyrrole/graphene nanofiber/iron oxide onto nonpolar plastic for flexible electromagnetic radiation shielding, strain sensing, and non-contact heating applications. Adv. Mater. Interfaces 2021, 8, 2101255. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Sanderson, K. Quantum dots go large: A small industry could be on the verge of a boom. Nature 2009, 459, 760–762. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Perelshtein, I.; Margel, S.; Gedanken, A. Acoustic green synthesis of graphene-gallium nanoparticles and PEDOT: PSS hybrid coating for textile to mitigate electromagnetic radiation pollution. ACS Appl. Nano Mater. 2022, 5, 1644–1655. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Frost, R.L.; Palmer, S.J. Raman spectrum of decrespignyite [(Y, REE) 4Cu(CO3) 4Cl(OH)5 2H2O] and its relation with those of other halogenated carbonates including bastnasite, hydroxybastnasite, parisite and northupite. J. Raman Spectrosc. 2011, 42, 2042–2048. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Li, H.; Yang, G. ZnMoO4 micro-and nanostructures synthesized by electrochemistry-assisted laser ablation in liquids and their optical properties. Cryst. Growth Des. 2012, 12, 4487–4493. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Determination of vanadium-oxygen bond distances and bond orders by Raman spectroscopy. J. Phys. Chem. 1991, 95, 5031–5041. [Google Scholar] [CrossRef]
- Pholnak, C.; Sirisathitkul, C.; Harding, D.J. Characterizations of octahedral zinc oxide synthesized by sonochemical method. J. Phys. Chem. Solids 2011, 72, 817–823. [Google Scholar] [CrossRef]
- de Azevedo Marques, A.P.; de Melo, D.M.; Paskocimas, C.A.; Pizani, P.S.; Joya, M.R.; Leite, E.R.; Longo, E. Photoluminescent BaMoO4 nanopowders prepared by complex polymerization method (CPM). J. Solid State Chem. 2006, 179, 671–678. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.-W.; Lim, C.S.; Oh, W.-C.; Shim, K.B. Microwave-assisted synthesis of CaMoO4 nano-powders by a citrate complex method and its photoluminescence property. J. Alloys Compd. 2005, 390, 245–249. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Li, L.; Song, S.; Yuan, X.; Jiao, W.; Hao, Z.; Li, X. Design of a ZnMoO4 porous nanosheet with oxygen vacancies as a better performance electrode material for supercapacitors. New J. Chem. 2021, 45, 9026–9039. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Yu, S.; Alcocer, E.J.; Shahid, M.; Wang, Z.; Pan, W. Synergistic effect of N-decorated and Mn2+ doped ZnO nanofibers with enhanced photocatalytic activity. Sci. Rep. 2016, 6, 32711. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Ezahri, M.; Benlhachemi, A.; Arab, M.; Bakiz, B.; Guinneton, F.; Gavarri, J.-R. Rietveld refinements, impedance spectroscopy and phase transition of the polycrystalline ZnMoO4 ceramics. Ceram. Int. 2015, 41, 15193–15201. [Google Scholar]
- Xianju, Z.; Xiaodong, Y.; Tengjiao, X.; Kaining, Z.; Tianyu, C.; Hao, Y.; Wang, Z. Luminescence properties and energy transfer of host sensitized CaMoO4: Tb3+ green phosphors. J. Rare Earths 2013, 31, 655–659. [Google Scholar]
- Sczancoski, J.; Cavalcante, L.; Marana, N.; da Silva, R.O.; Tranquilin, R.; Joya, M.; Pizani, P.; Varela, J.; Sambrano, J.; Li, M.S.; et al. Electronic structure and optical properties of BaMoO4 powders. Curr. Appl. Phys. 2010, 10, 614–624. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Zhang, L.; Pan, N.; Cao, X.; Li, J. Photocatalytic degradation of methyl orange using a TiO2/Ti mesh electrode with 3D nanotube arrays. ACS Appl. Mater. Interfaces 2012, 4, 171–177. [Google Scholar] [CrossRef]
- Liang, J.-H.; Han, X. Structure-activity relationships and mechanism of action of macrolides derived from erythromycin as antibacterial agents. Curr. Top. Med. Chem. 2013, 13, 3131–3164. [Google Scholar] [CrossRef]
- Jiang, Y.-R.; Lee, W.W.; Chen, K.-T.; Wang, M.-C.; Chang, K.-H.; Chen, C.-C. Hydrothermal synthesis of β-ZnMoO4 crystals and their photocatalytic degradation of Victoria Blue R and phenol. J. Taiwan Inst. Chem. Eng. 2014, 45, 207–218. [Google Scholar] [CrossRef]
- Sarwar, N.; Humayoun, U.B.; Kumar, M.; Zaidi, S.F.A.; Yoo, J.H.; Ali, N.; Jeong, D.I.; Lee, J.H.; Yoon, D.H. Citric acid mediated green synthesis of copper nanoparticles using cinnamon bark extract and its multifaceted applications. J. Clean. Prod. 2021, 292, 125974. [Google Scholar] [CrossRef]
- Sarwar, N.; Bin Humayoun, U.; Kumar, M.; Nawaz, A.; Zafar, M.S.; Rasool, U.; Kim, Y.H.; Yoon, D.H. A bio based immobilizing matrix for transition metal oxides (TMO) crosslinked cotton: A facile and green processing for photocatalytic self-cleaning and multifunctional textile. Mater. Lett. 2022, 309, 131338. [Google Scholar] [CrossRef]

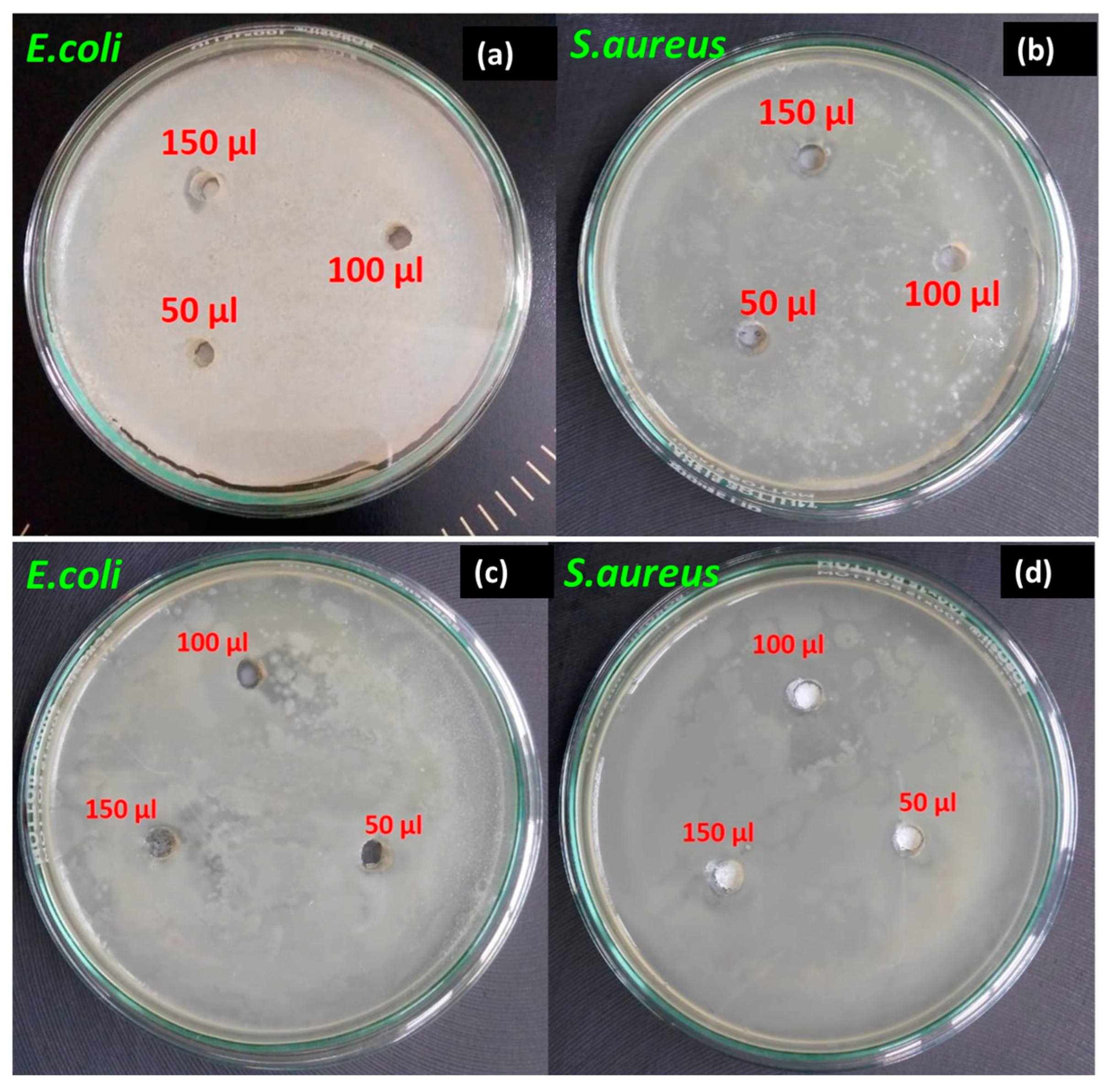

| Bacterial Species | Concentration of Mn-Doped ZnMoO4 | ||
|---|---|---|---|
| 50 µL | 100 µL | 150 µL | |
| E. coli | 3.0 mm | 1.0 mm | 1.5 mm |
| S. aureus | 1.4 mm | 6.0 mm | 8.0 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Ahirwar, R.C.; Borgaonkar, K.; Gupta, N.; Ahsan, M.; Rathore, J.; Das, P.; Ganguly, S.; Rawat, R. Synthesis of Transition-Metal-Doped Nanocatalysts with Antibacterial Capabilities Using a Complementary Green Method. Molecules 2023, 28, 4182. https://doi.org/10.3390/molecules28104182
Singh A, Ahirwar RC, Borgaonkar K, Gupta N, Ahsan M, Rathore J, Das P, Ganguly S, Rawat R. Synthesis of Transition-Metal-Doped Nanocatalysts with Antibacterial Capabilities Using a Complementary Green Method. Molecules. 2023; 28(10):4182. https://doi.org/10.3390/molecules28104182
Chicago/Turabian StyleSingh, Anshul, Ranjana Choudhary Ahirwar, Kavindra Borgaonkar, Neeta Gupta, Muhammad Ahsan, Jyoti Rathore, P. Das, S. Ganguly, and Reena Rawat. 2023. "Synthesis of Transition-Metal-Doped Nanocatalysts with Antibacterial Capabilities Using a Complementary Green Method" Molecules 28, no. 10: 4182. https://doi.org/10.3390/molecules28104182
APA StyleSingh, A., Ahirwar, R. C., Borgaonkar, K., Gupta, N., Ahsan, M., Rathore, J., Das, P., Ganguly, S., & Rawat, R. (2023). Synthesis of Transition-Metal-Doped Nanocatalysts with Antibacterial Capabilities Using a Complementary Green Method. Molecules, 28(10), 4182. https://doi.org/10.3390/molecules28104182







