Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents
Abstract
1. Introduction
2. Results
2.1. Detection of Heavy Metals in Collected Wastewater Samples
2.2. Phytochemical Screening of Nigella sativa Seed Extract
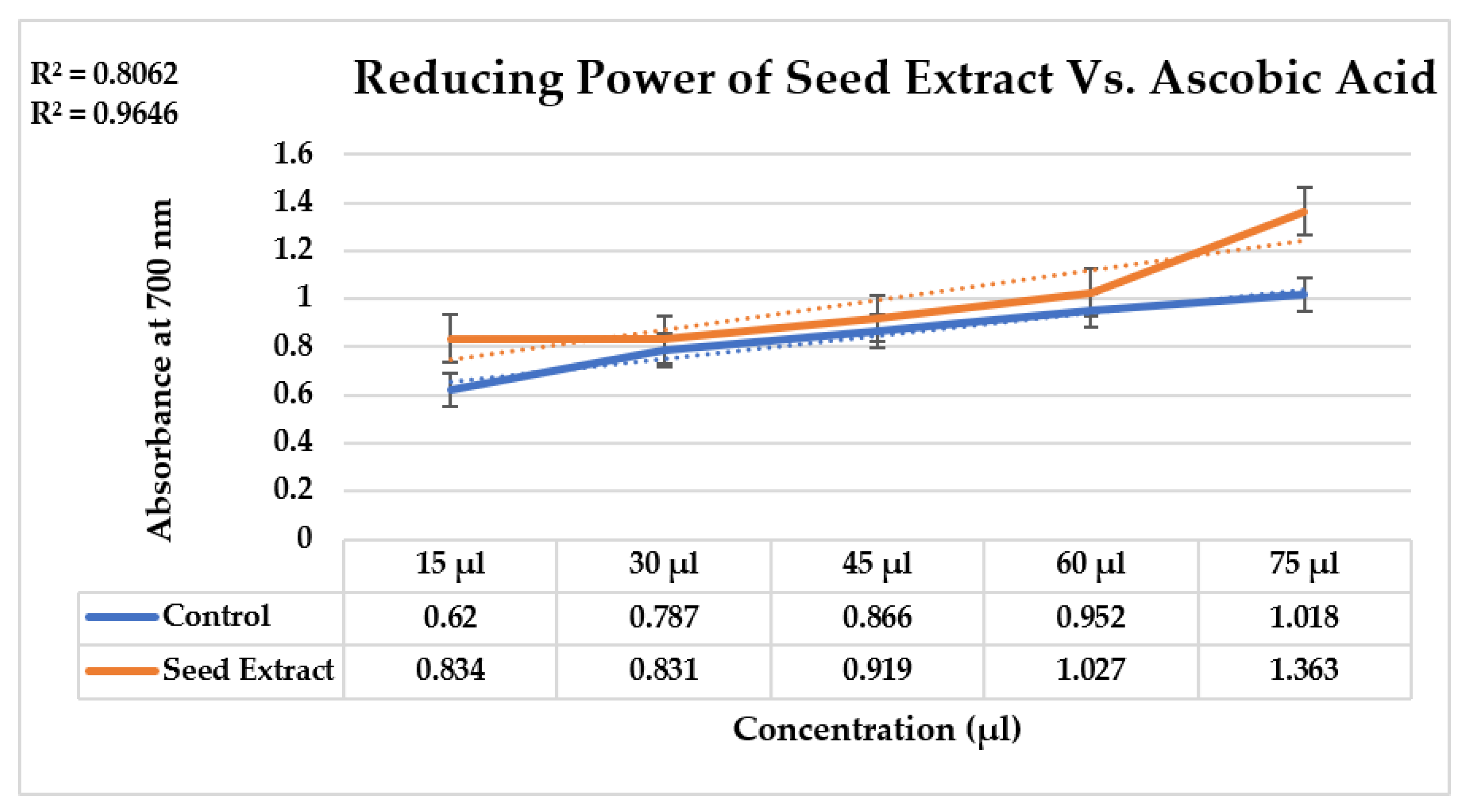
2.3. Determination of Reducing Power of Nigella sativa Extract
2.4. Biosynthesis of ZVI-NPs
2.5. Characterization of ZVI-NPs
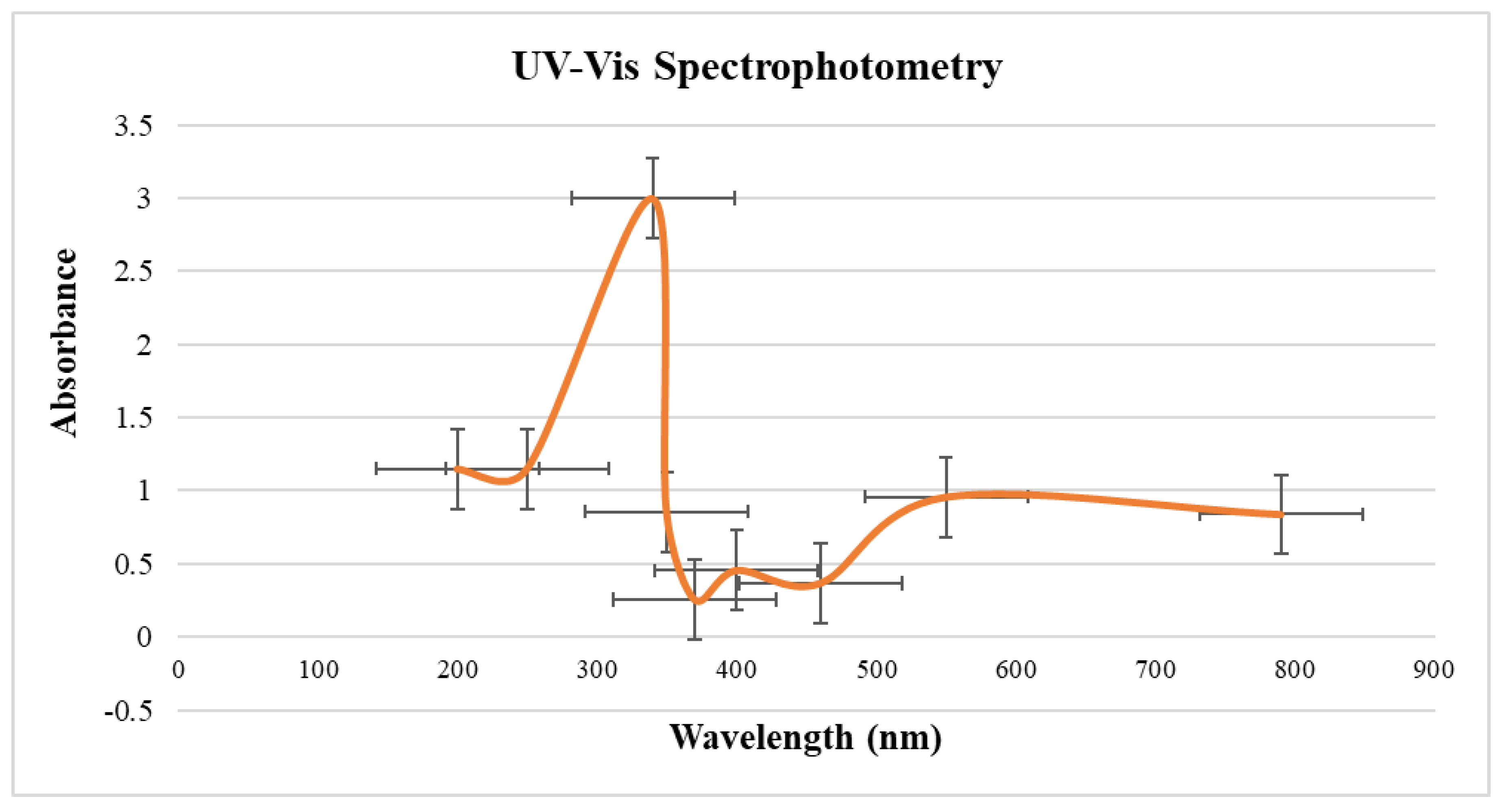
2.5.1. UV-Visible Spectrophotometry
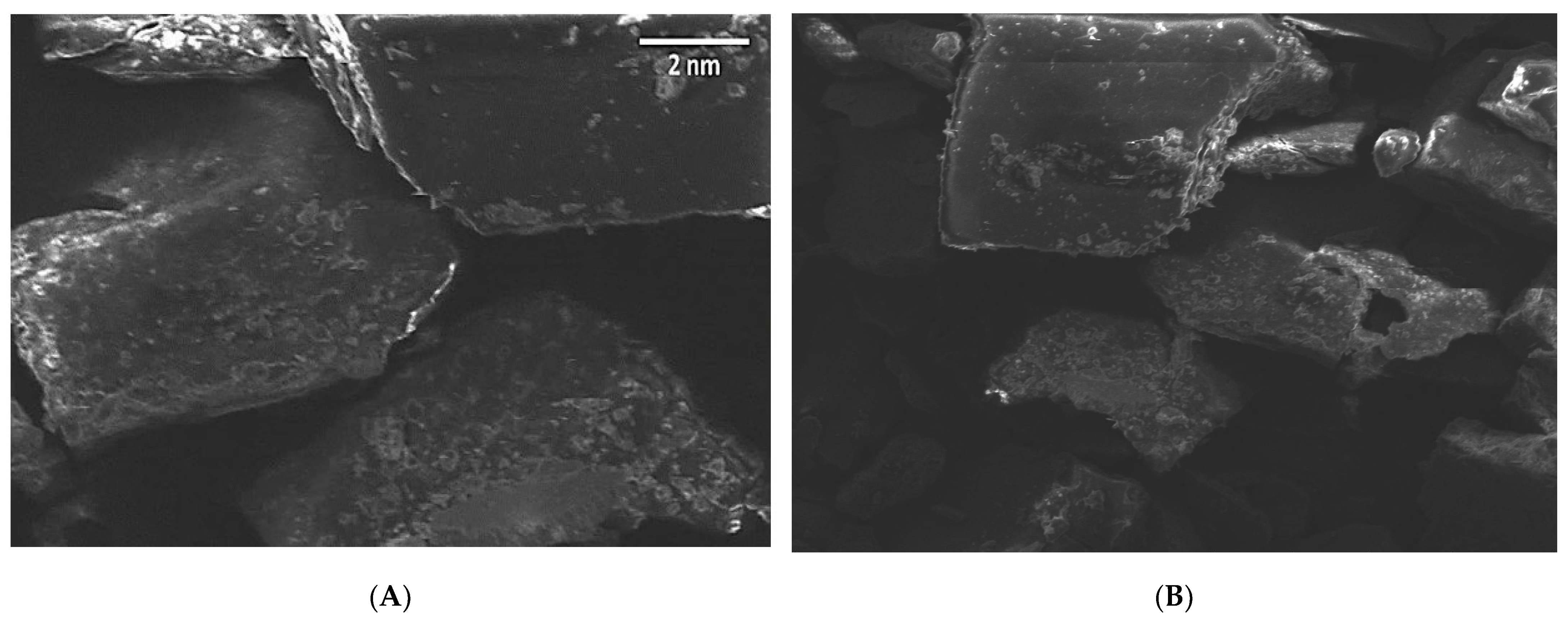
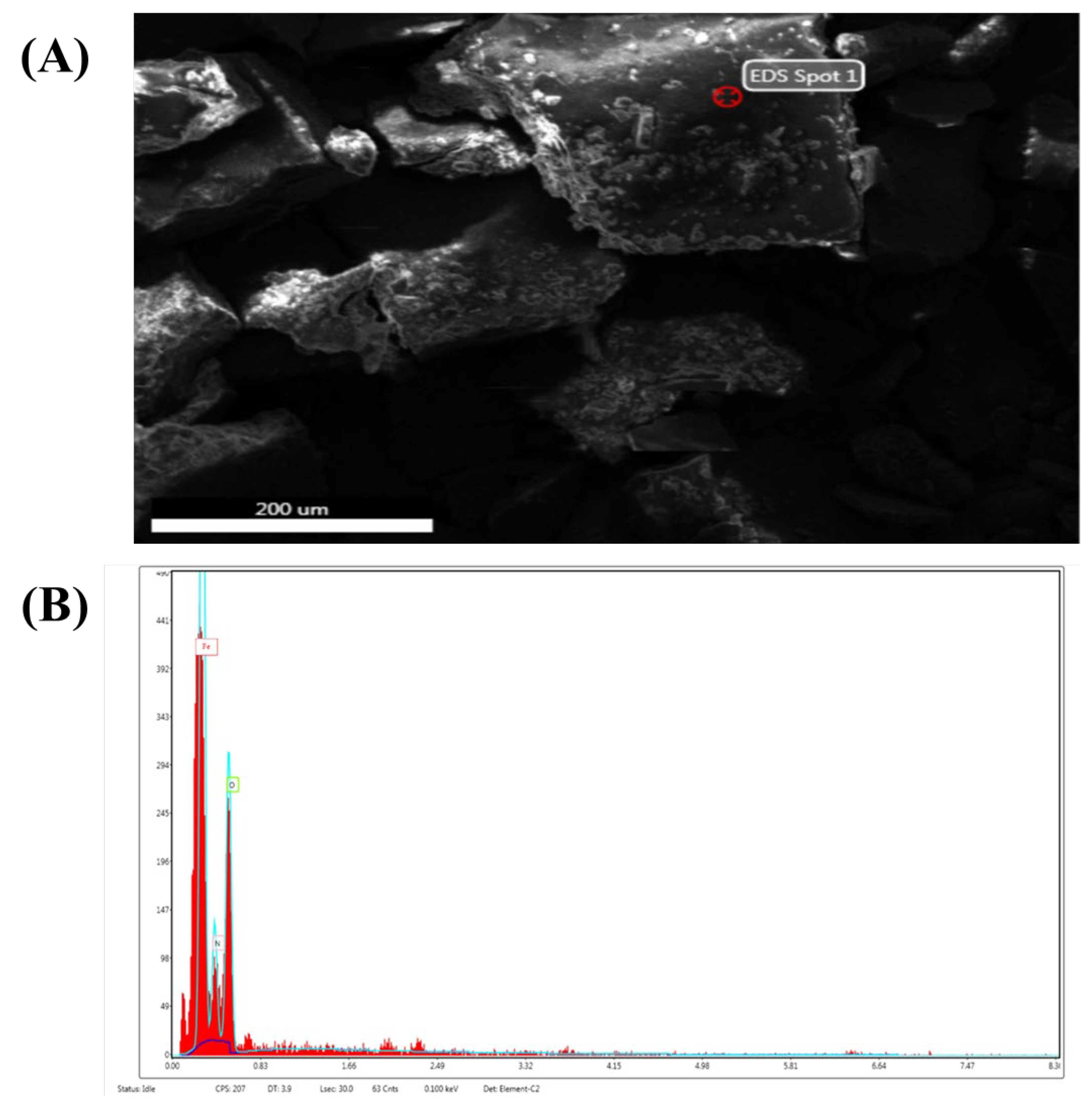
2.5.2. Scanning Electron Microscopy (SEM)
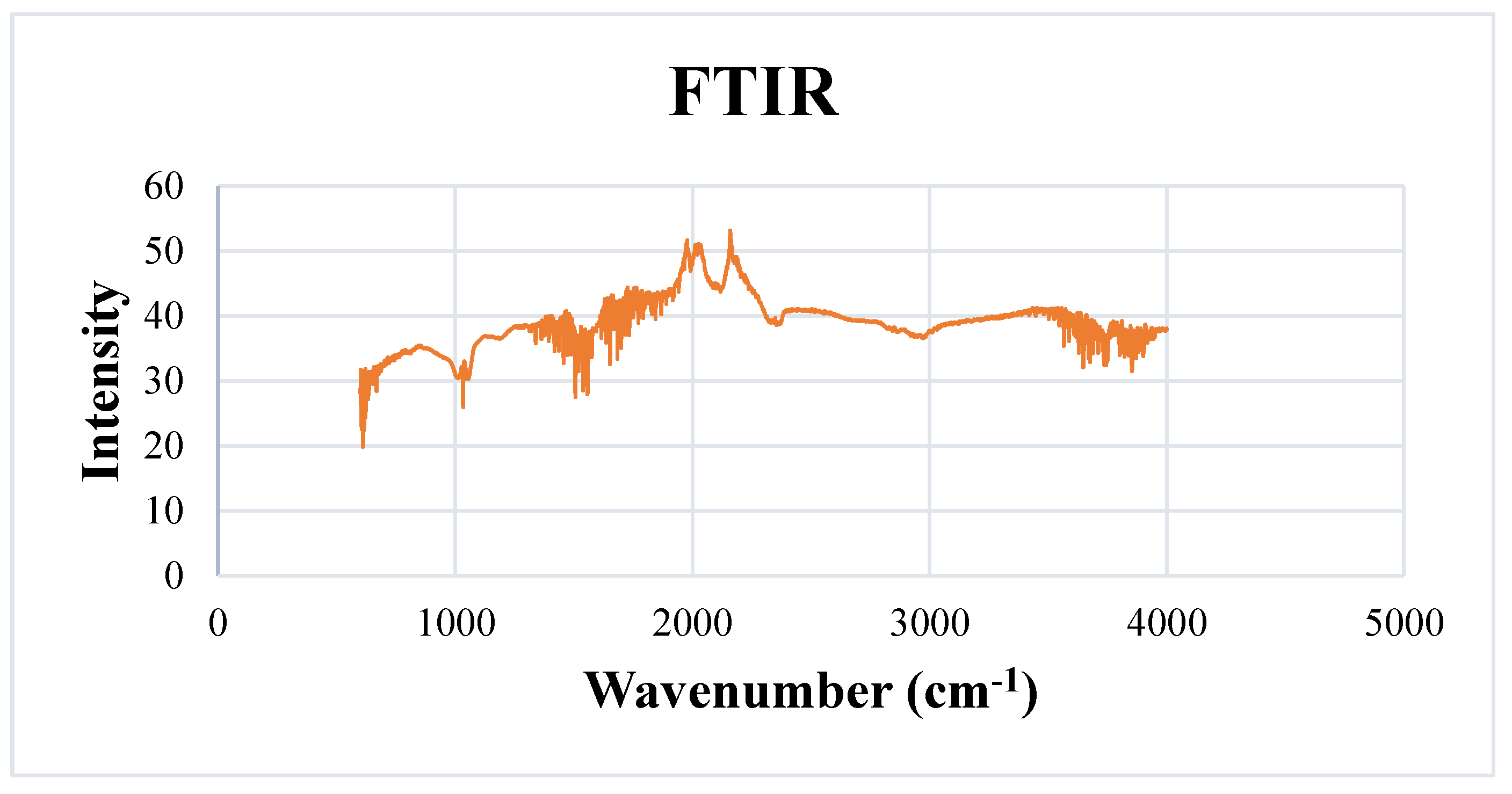
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.4. Energy-Dispersive X-ray Spectroscopy (EDX)
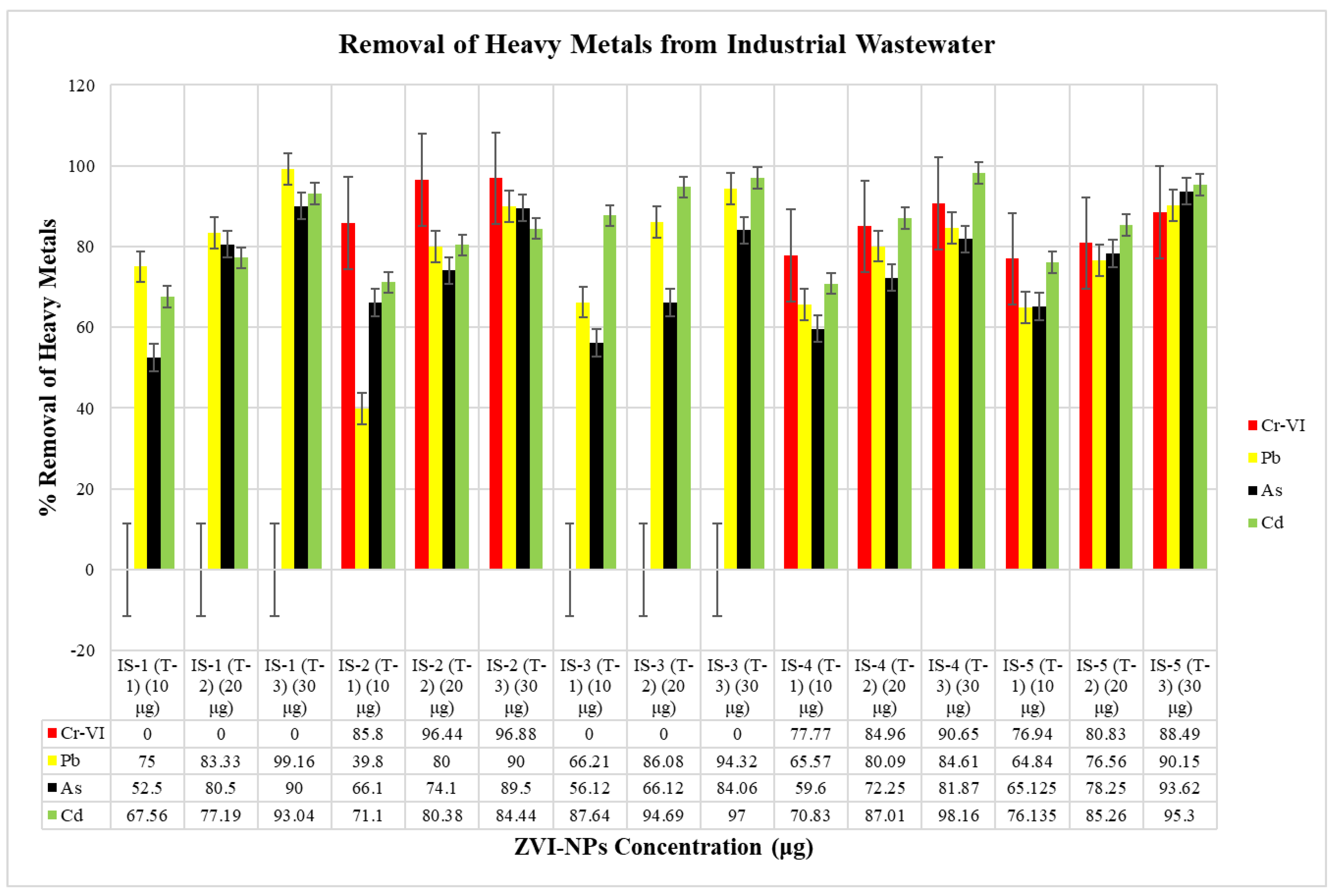
2.6. Remediation of Heavy Metals in Wastewater by ZVI-NPs
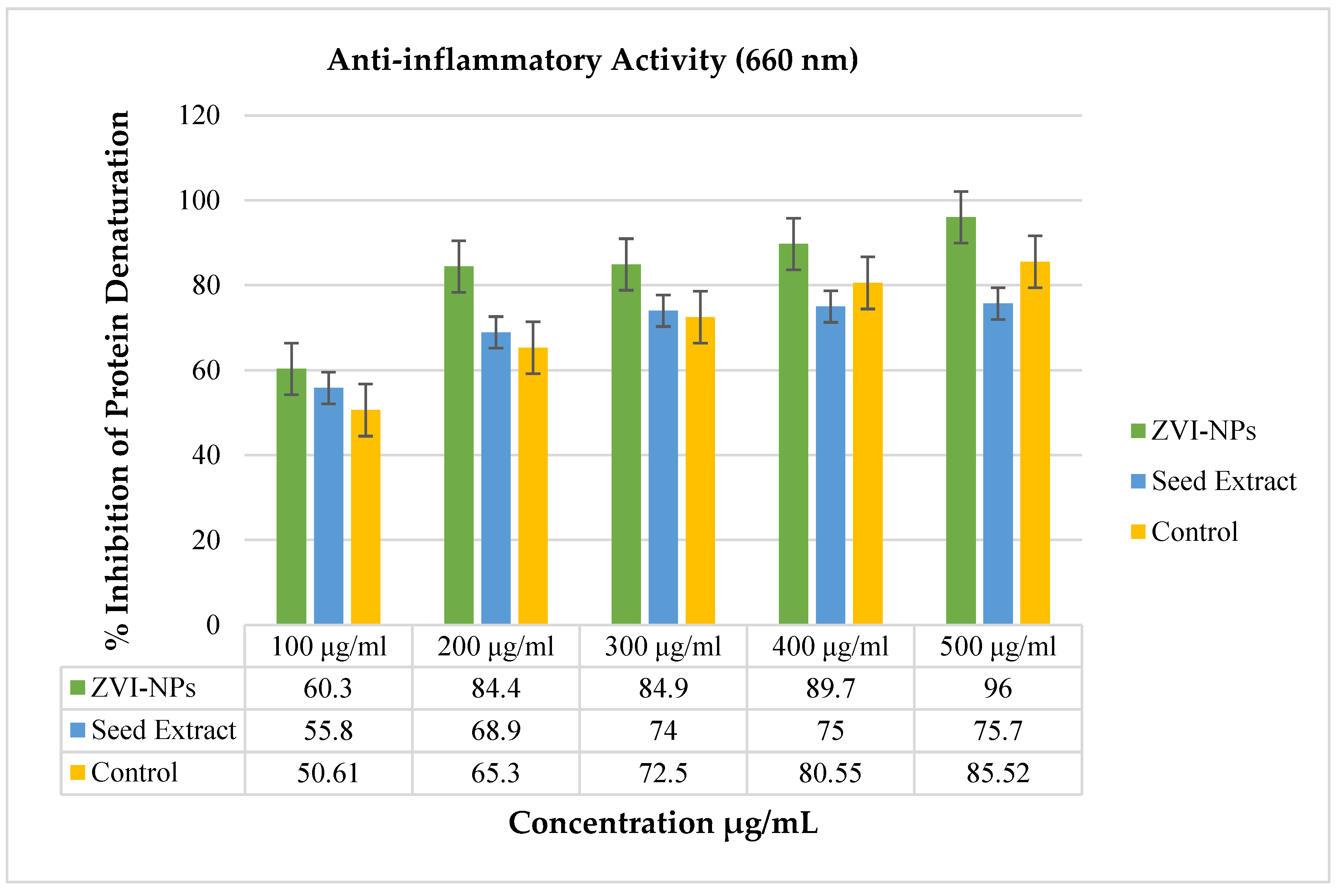
2.7. Anti-Inflammatory Analysis of ZVI-NPs
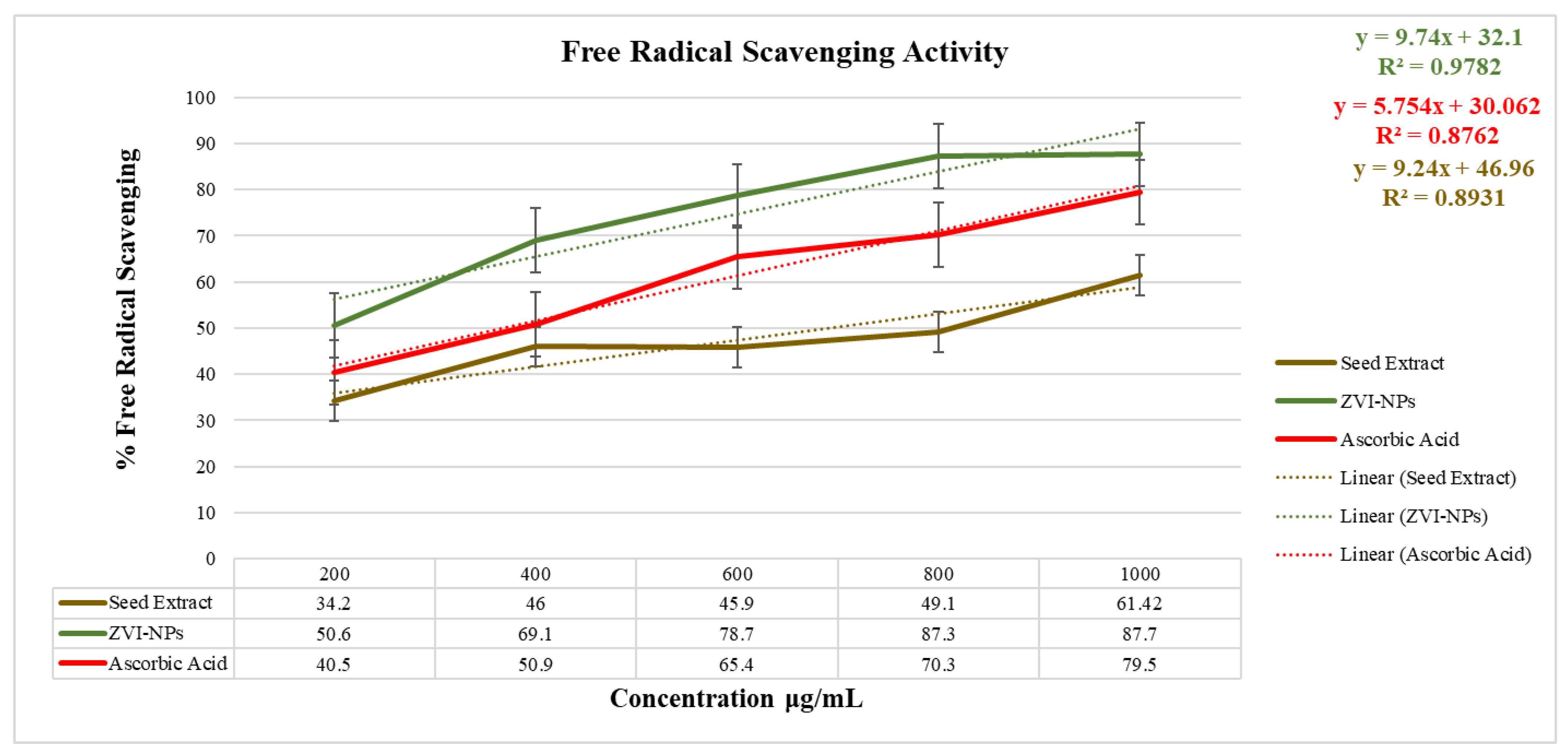
2.8. Antioxidant Analysis
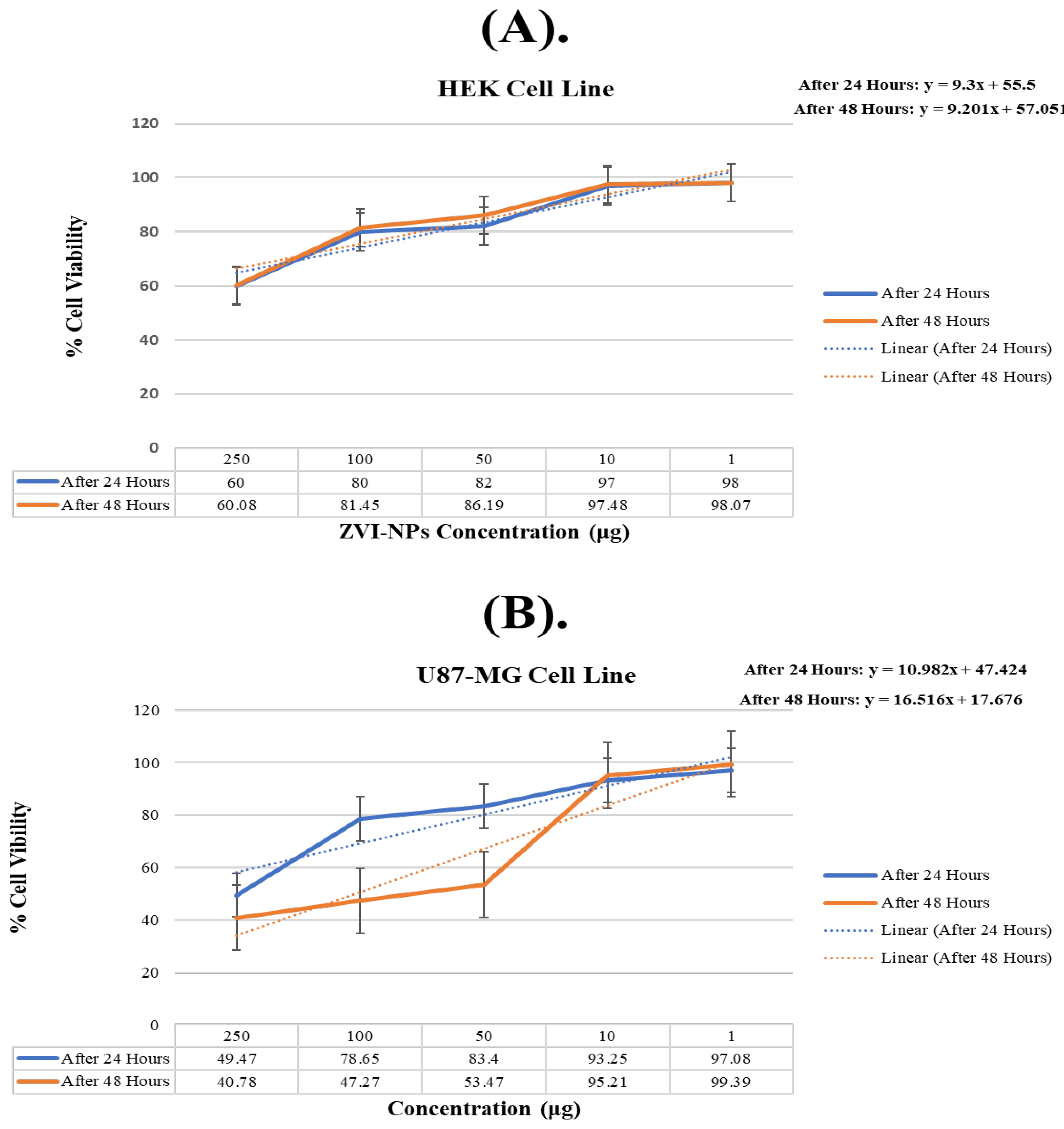
2.9. Cytotoxicity Testing of ZVI-NPs
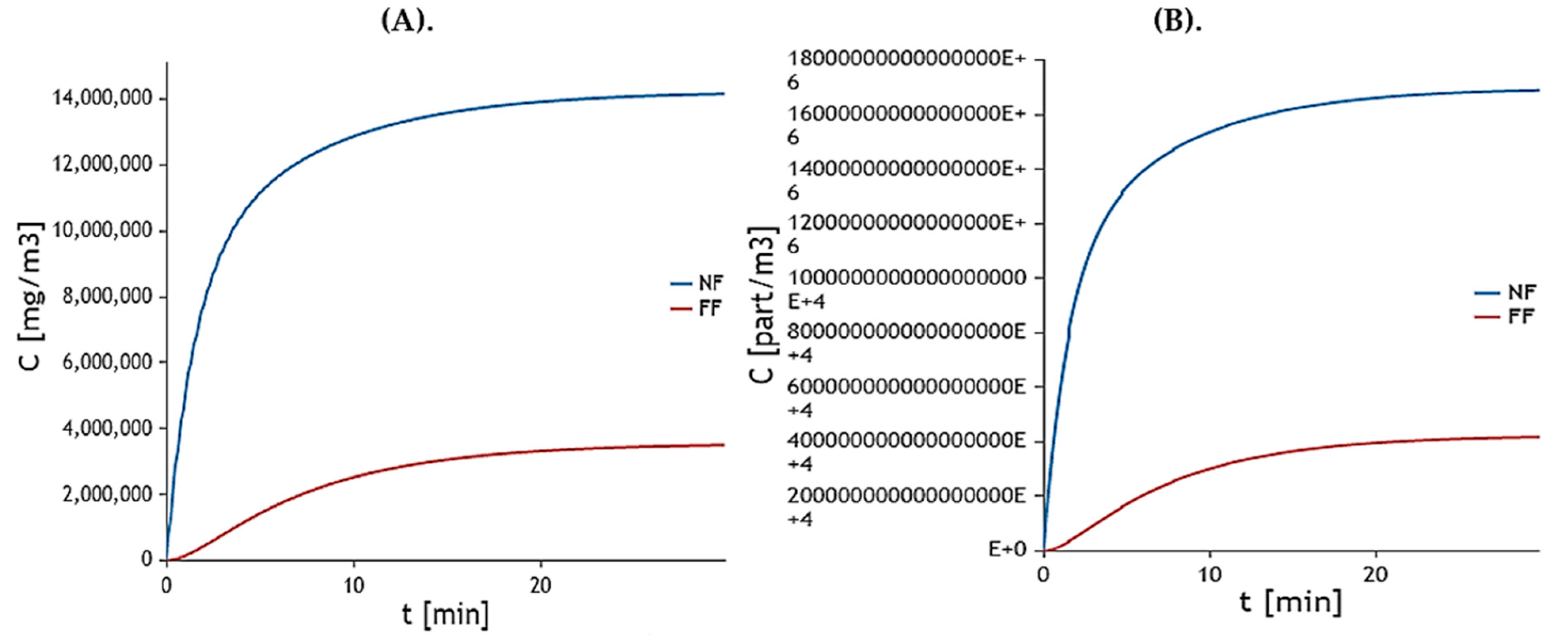
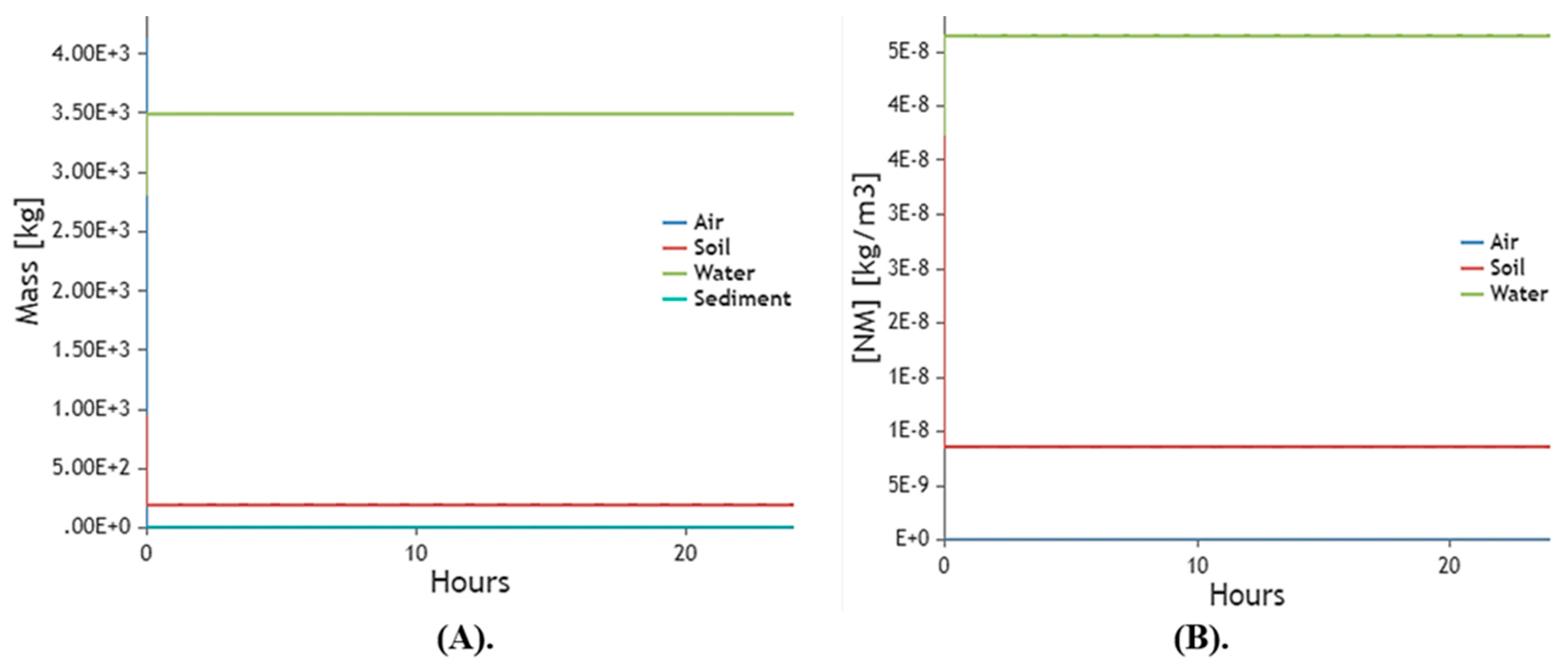
2.10. Mathematical Models
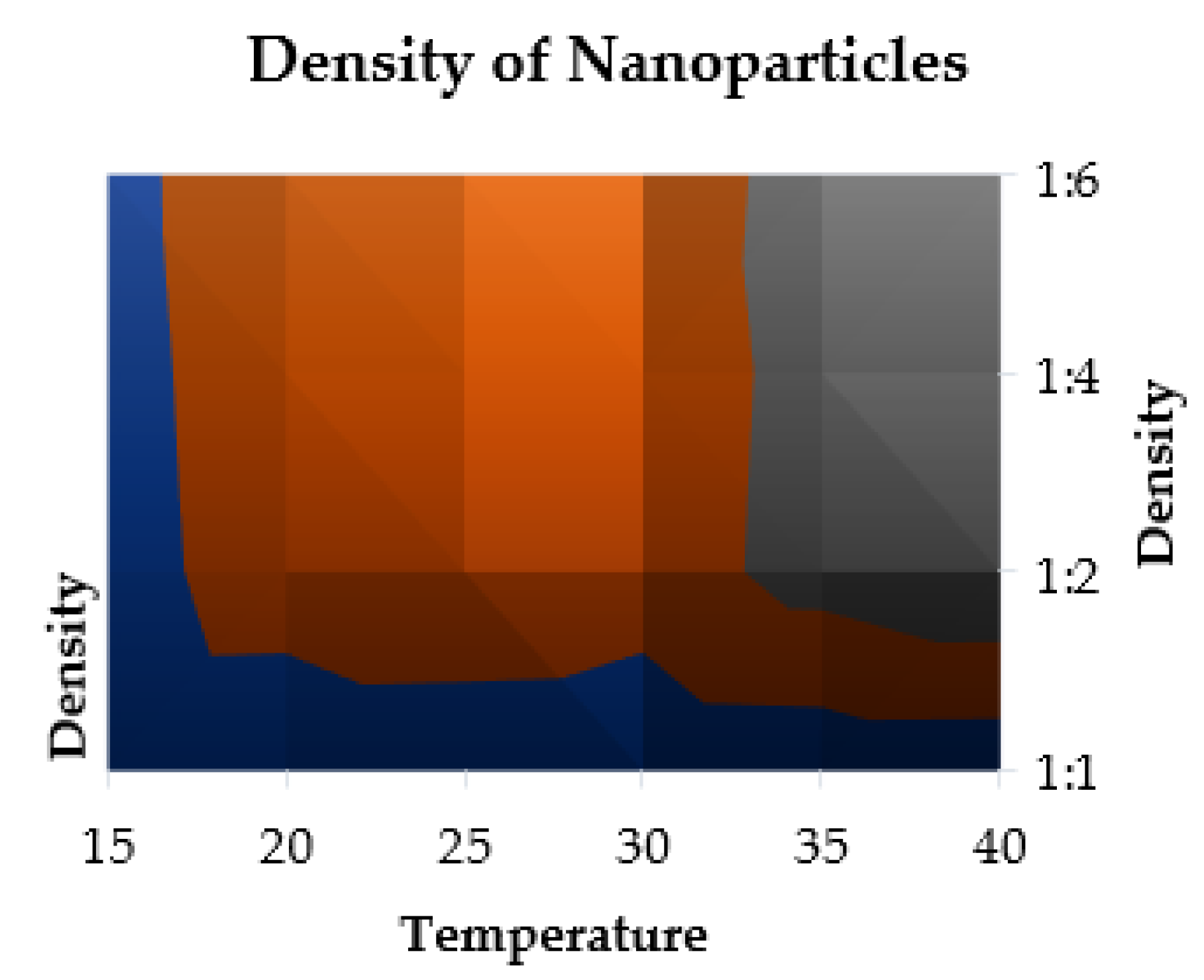
2.10.1. Density Parameter
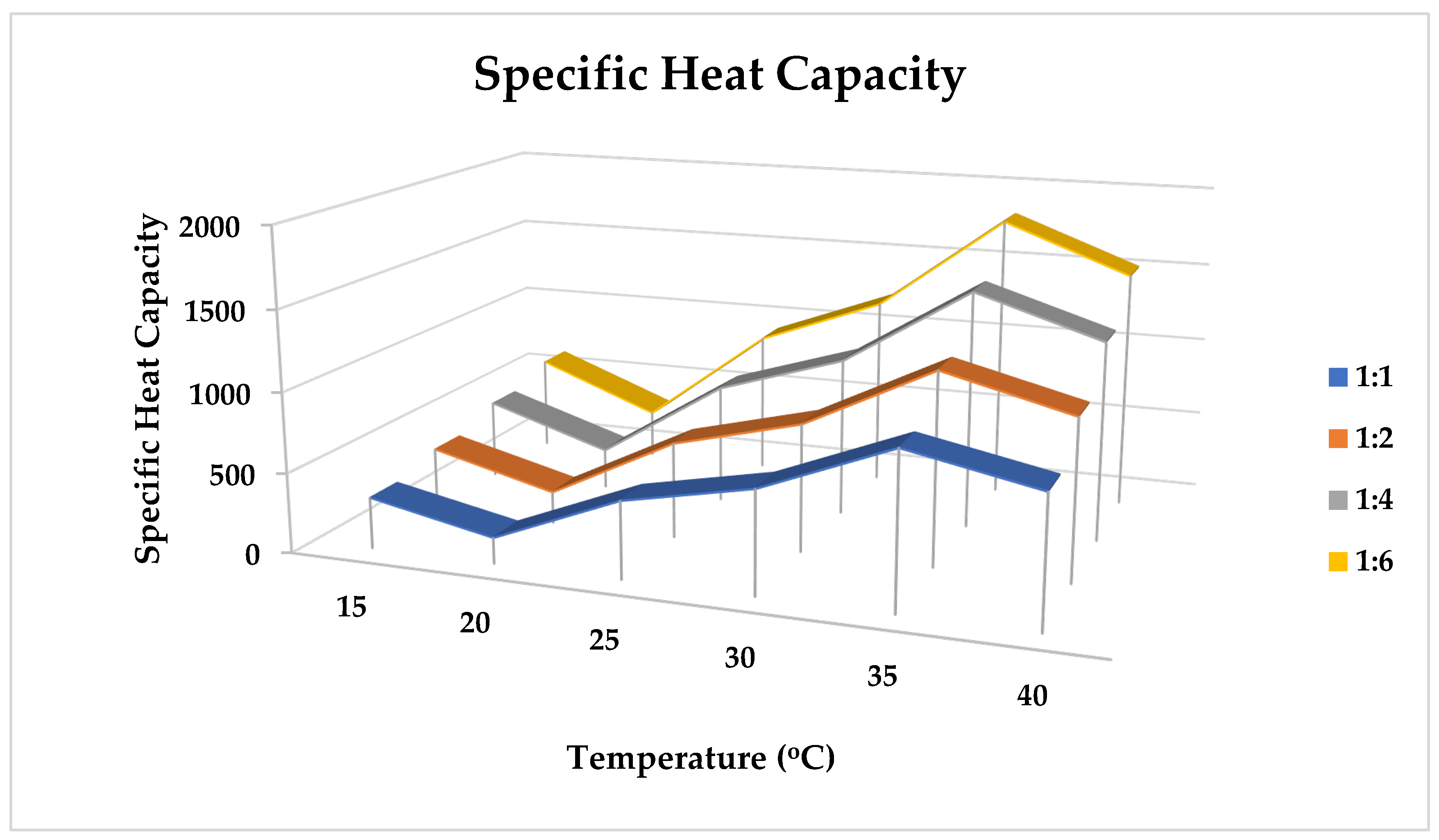
2.10.2. Specific Heat Capacity
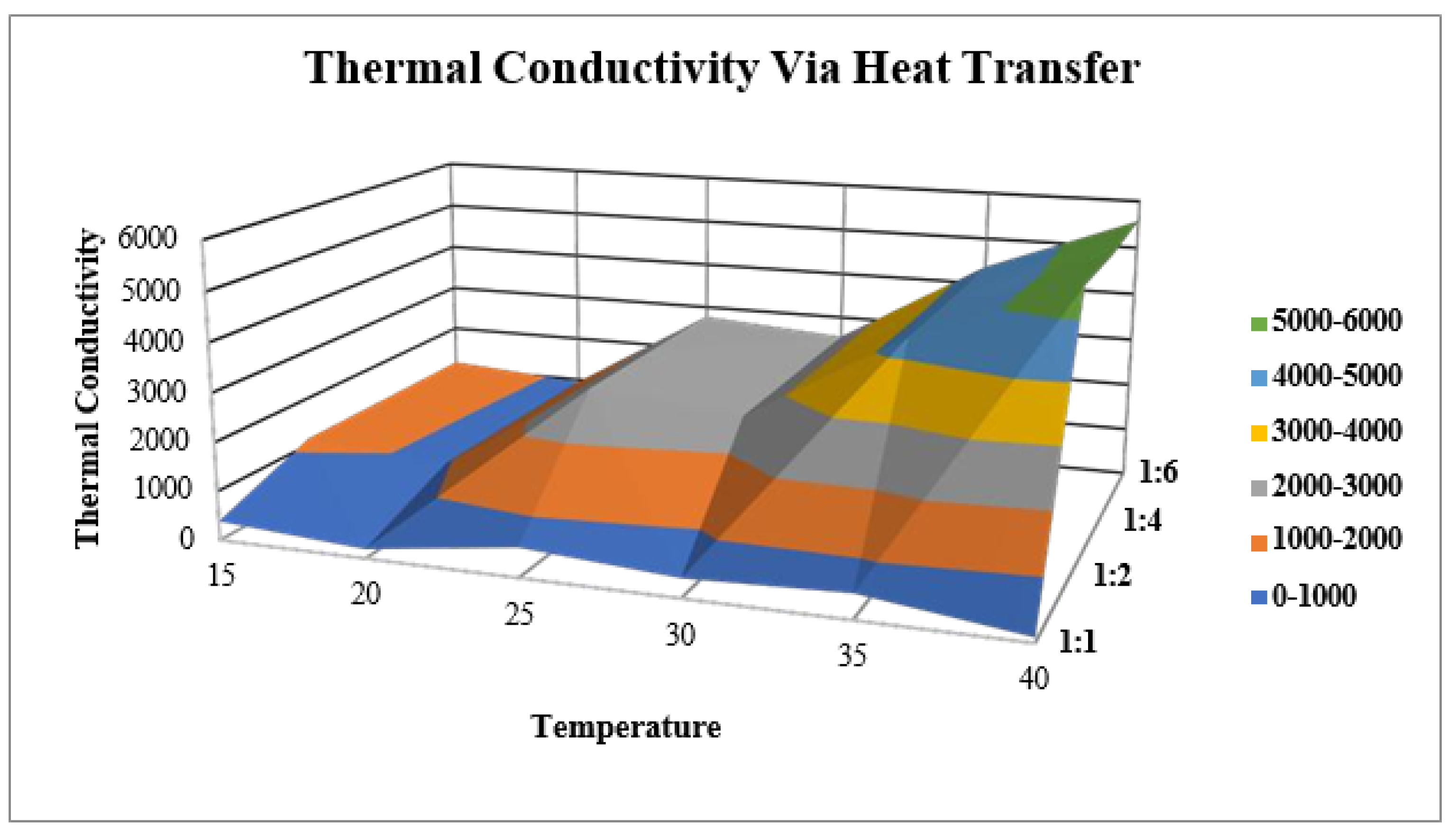
2.10.3. Thermal Conductivity via Heat Transfer
2.10.4. Worker’s Model
2.10.5. Environmental Model
3. Materials and Methods
3.1. Collection of Industrial Wastewater Samples
3.2. Detection of Heavy Metals in Collected Wastewater Samples
3.3. Preparation of Seed Extract of Nigella sativa
3.4. Phytochemical Screening of Nigella Sativa Seed Extract
3.4.1. Wagner’s Test
3.4.2. Foam Test
3.4.3. Ferric Chloride Test
3.4.4. Braymer’s Test
3.4.5. Salkowski’s Test
3.4.6. Bontrager’s Test
3.4.7. Keller–Killian’s Test
3.4.8. Glycosides Test
3.4.9. Alkaline Reagent Test
3.4.10. Precipitate Test
3.5. Determination of Reducing Power of Nigella sativa Extract
3.5.1. Preparation of Standard Solution
3.5.2. Preparation of Test Sample
3.5.3. Protocol for Determination of Reducing Power
3.6. Biosynthesis of ZVI-NPs
3.7. Characterization of ZVI-NPs
3.7.1. UV-Visible Spectrophotometry
3.7.2. Scanning Electron Microscopy (SEM)
3.7.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.7.4. Energy-Dispersive X-ray Spectroscopy (EDX)
3.8. Remediation of Heavy Metals in Wastewater by ZVI-NPs
3.9. Anti-Inflammatory Analysis
3.10. Anti-Oxidant Analysis of ZVI-NPs
3.11. Cytotoxicity Testing of ZVI-NPs
3.12. Physiochemical Properties
Mathematical Formulation
- (a).
- Density Formulation
- (b).
- Specific Heat Equation
- (c).
- Thermal Conductivity Via Heat Transfer
- (d).
- Exposure Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Weiss, C.; Valsami-Jones, E. A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design NMs. Nano Today 2014, 9, 266–270. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Yoon, T.H. Development of Theoretical Descriptors for Cytotoxicity Evaluation of Metallic NPs. Chem. Res. Toxicol. 2017, 30, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Li, B.; Celi, N.; Cai, J.; Zhang, D. Efficient Removal of Pb(II) from Aqueous Systems Using Spirulina-Based Biohybrid Magnetic Helical Microrobots. ACS Appl. Mater. Interfaces 2021, 13, 53131–53142. [Google Scholar] [CrossRef]
- Hadi, F.; Arifeen, M.Z.U.; Aziz, T.; Nawab, S.; Nabi, G. Phytoremediation of Cadmium by Ricinus communis L. in Hydrophonic Condition. Am. -Eurasian J. Agric. Environ. Sci. 2015, 15, 1155–1162. [Google Scholar]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic Effects of Zinc Oxide NPs and Bacteria Reduce Heavy Metals Toxicity in Rice (Oryza sativa L.) Plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. Chem. Biol. Eng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.-P. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable catalysis. Chemicals 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. New trends in sustainable nanocatalysis: Emerging use of earth abundant metals. Curr. Opin. Green Sustain. 2017, 7, 39–45. [Google Scholar] [CrossRef]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 2200356. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Pumera, M. Smart micro- and nanorobots for water purification. Nat. Rev. Bioeng. 2023, 4, 1–16. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Efficient removal of heavy metals from artificial wastewater using biochar. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100602. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. 4-Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Gopalakannan, V.; Viswanathan, N. Synthesis of magnetic alginate hybrid beads for efficient chromium (VI) removal. Int. J. Biol. Macromol. 2015, 72, 862–867. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liang, F.; Zhang, W.-X. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. -Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. AYU 2013, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver NPs by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Bukhari, B.; Aziz, T.; Zaib, S.; Mansoor, M.A.; Khan, A.A.; Shahzad, M.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Green Synthesis of Silver NPs Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Rehman, S.U.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver NPs Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Essien, A.; Kavaz, D.; Solomon, M.M. Olive leaves extract mediated zero-valent iron NPs: Synthesis, characterization, and assessment as adsorbent for nickel (II) ions in aqueous medium. Chem. Eng. Commun. 2018, 205, 1568–1582. [Google Scholar] [CrossRef]
- Dermirezen, D.A.; Yilmaz, S.; Yilmz, D.D. Green systhesis and characterization of iron NPs using Ficus Carica dried fruit extract. In Proceedings of the International Eurasian Conference on Biological and Chemical Sciences, Ankara, Turkey, 26–27 April 2018. [Google Scholar]
- Bukhari, B.; Naveed, M.; Makhdoom, S.I.; Jabeen, K.; Asif, M.F.; Batool, H.; Ahmed, N.; Chan, Y.Y. A Comparison Between Organic and Inorganic NPs: Prime NPs for Tumor Curation. Nano 2021, 16, 2130011. [Google Scholar] [CrossRef]

| Detected Heavy Metals | Cadmium (Cd) (ppm) | Mercury(Hg) (ppm) | Arsenic (As) (ppm) | Chromium-IV (Cr-IV) (ppm) | Lead (Pb) (ppm) |
|---|---|---|---|---|---|
| Conc. of heavy metals by WHO | 0.003 | 0.001 | 0.01 | 0.05 | 0.01 |
| S-1 | 29.6 | N.D | 0.05 | N.D | 2.4 |
| S-2 | 52 | N.D | 0.08 | 1316.5 | 5 |
| S-3 | 76.4 | N.D | 0.07 | N.D | 7.4 |
| S-4 | 100.2 | N.D | 0.06 | 0.45 | 10.4 |
| S-5 | 124.2 | N.D | 0.08 | 442.5 | 12.8 |
| Phytochemical Test | Screened Phytochemical | Interference | Result | |
|---|---|---|---|---|
| 1 | Wagner’s Test | Alkaloids | Appearance of reddish brown color with Wagner’s reagent | Present |
| 2 | Foam Test | Saponins | Formation of stable foam | Present |
| 3 | Ferric Chloride Test | Phenols | Indication of blue green color with ferric chloride | Present |
| 4 | Braymer’s Test | Tannins | Formation of green precipitates | Present |
| 5 | Salkowski’s Test | Terpenoids | Appearance of yellow color | Present |
| 6 | Bontrager’s Test | Quinones | Occurrence of red color in alkaline phase | Absent |
| 7 | Keller–Killani’s Test | Cardiac Glycosides | Formation of pink to blood red coloration | Present |
| 8 | Glycosides Test | Glycosides | Indication of pink color | Absent |
| 9 | Alkaline Reagent Test | Flavonoids | Formation of yellow color which becomes colorless on addition of acid | Present |
| 10 | Precipitate Test | Phlobatannins | Appearance of red precipitates | Absent |
| SR#. | Cr-VI (ppm) | Pb (ppm) | As (ppm) | Cd (ppm) |
|---|---|---|---|---|
| IS-1 (T-1) (10 μg) | Nil | 0.6 | 0.74 | 9.6 |
| IS-1 (T-2) (20 μg) | Nil | 0.4 | 0.39 | 6.74 |
| IS-1 (T-3) (30 μg) | Nil | 0.02 | 0.2 | 2.06 |
| IS-2 (T-1) (10 μg) | 186.8 | 3.01 | 3.39 | 15.01 |
| IS-2 (T-2) (20 μg) | 46.8 | 1 | 2.59 | 10.2 |
| IS-2 (T-3) (30 μg) | 41 | 0.5 | 1.05 | 8.09 |
| IS-3 (T-1) (10 μg) | Nil | 2.5 | 7.02 | 9.44 |
| IS-3 (T-2) (20 μg) | Nil | 1.03 | 5.42 | 4.05 |
| IS-3 (T-3) (30 μg) | Nil | 0.42 | 2.55 | 2.29 |
| IS-4 (T-1) (10 μg) | 20 | 3.58 | 6.45 | 29.22 |
| IS-4 (T-2) (20 μg) | 13.53 | 2.07 | 4.44 | 13.01 |
| IS-4 (T-3) (30 μg) | 8.41 | 1.6 | 2.9 | 1.84 |
| IS-5 (T-1) (10 μg) | 102 | 4.5 | 5.58 | 29.64 |
| IS-5 (T-2) (20 μg) | 84.8 | 3 | 3.48 | 18.3 |
| IS-5 (T-3) (30 μg) | 50.9 | 1.26 | 1.02 | 5.83 |
| % Inhibition of Protein Denaturation Anti-Inflammatory Activity (660 nm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blank: Distilled Water | Control Aspirin: 2.370 Abs | Seed Extract of Nigella sativa | ZVI-NPs | |||||||||
| Conc. | R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean | R1 | R2 | R3 | Mean |
| 100 μg/mL | 50.55 | 50.6 | 50.7 | 50.61 | 55.7 | 55.8 | 55.9 | 55.8 | 60.3 | 60.4 | 60.4 | 60.3 |
| 200 μg/mL | 65.3 | 65.4 | 65.2 | 65.3 | 68.9 | 68.9 | 69 | 68.9 | 84.4 | 84.5 | 84.4 | 84.4 |
| 300 μg/mL | 72.5 | 72.7 | 72.4 | 72.5 | 74.1 | 74 | 74 | 74 | 85.1 | 84.9 | 84.8 | 84.9 |
| 400 μg/mL | 80.56 | 80.4 | 80.7 | 80.55 | 75 | 75.1 | 75 | 75 | 89.7 | 89.7 | 89.8 | 89.7 |
| 500 μg/mL | 85.7 | 85.6 | 85.4 | 85.52 | 75.7 | 75.8 | 75.7 | 75.7 | 96 | 96.1 | 96.16 | 96 |
| Anti-oxidant Activity (517 nm) | ||||||
|---|---|---|---|---|---|---|
| Concentration (μg/mL) | % Free Radical Scavenging | IC50 | ||||
| Ascorbic Acid | Seed Extract | ZVI-NPs | Ascorbic Acid | Seed Extract | ZVI-NPs | |
| 200 | 40.5 | 34.2 | 50.6 | 596.7 | 387.56 | 194.77 |
| 400 | 50.9 | 46 | 69.1 | |||
| 600 | 65.4 | 45.9 | 78.7 | |||
| 800 | 70.3 | 49.1 | 87.3 | |||
| 1000 | 79.5 | 61.42 | 87.7 | |||
| IC50 Values | ||||
|---|---|---|---|---|
| Concentration | U87-MG Cell Line 24 Hours | 48 h | HEK Cell Line 24 Hours | 48 h |
| 250 | 45.15166 | 39.70977 | 54.03226 | 53.87948 |
| 100 | 74.33166 | 46.19977 | 74.03226 | 75.24948 |
| 50 | 79.08166 | 52.39977 | 76.03226 | 79.98948 |
| 10 | 88.93166 | 94.13977 | 91.03226 | 91.27948 |
| 1 | 92.76166 | 98.31977 | 92.03226 | 91.86948 |
| Cell Morphology | ||||||
|---|---|---|---|---|---|---|
| Positive Control |  | Solvent Control |  | MTT Treated Cell | 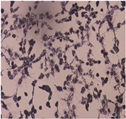 | |
| Concentration | 250 | 100 | 50 | 10 | 1 | |
| U87-MG Cell Line | After 24 Hours | 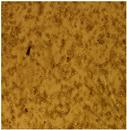 |  |  |  | 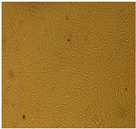 |
| HEK Cell Lines | 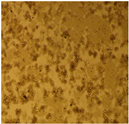 |  | 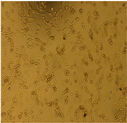 |  |  | |
| Temperature (°C) | ρ1b | ρ2b | ρ3b | ρ4b |
|---|---|---|---|---|
| 15 | 80 | 133.33 | 142.28 | 155.21 |
| 20 | 74.07 | 287.43 | 298.45 | 301.25 |
| 25 | 66.67 | 365.28 | 355.82 | 345.28 |
| 30 | 57.14 | 297.45 | 288.58 | 272.65 |
| 35 | 68.96 | 478.58 | 468.23 | 488.12 |
| 40 | 64.51 | 582.24 | 525.54 | 565.45 |
| Temperature (°C) | ρCp | ρCp2 | ρCp3 | ρCp4 |
|---|---|---|---|---|
| 15 | 320 | 400 | 500 | 600 |
| 20 | 160 | 200 | 250 | 300 |
| 25 | 480 | 600 | 750 | 900 |
| 30 | 640 | 800 | 1000 | 1200 |
| 35 | 960 | 1200 | 1500 | 1800 |
| 40 | 800 | 1000 | 1250 | 1500 |
| Temperature (°C) | Knf1 | Knf2 | Knf3 | Knf4 |
|---|---|---|---|---|
| 15 | 390.65 | 1132.31 | 1145.18 | 1158.25 |
| 20 | 188.63 | 915.38 | 928.67 | 945.14 |
| 25 | 598.16 | 2791.94 | 2787.96 | 2777.51 |
| 30 | 388.21 | 2526.11 | 2537.43 | 2558.28 |
| 35 | 501.98 | 4354.68 | 4342.24 | 4377.45 |
| 40 | 102.4 | 5562.8 | 5505.21 | 5578.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, M.; Makhdoom, S.I.; Rehman, S.u.; Aziz, T.; Bashir, F.; Ali, U.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents. Molecules 2023, 28, 3299. https://doi.org/10.3390/molecules28083299
Naveed M, Makhdoom SI, Rehman Su, Aziz T, Bashir F, Ali U, Alharbi M, Alshammari A, Alasmari AF. Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents. Molecules. 2023; 28(8):3299. https://doi.org/10.3390/molecules28083299
Chicago/Turabian StyleNaveed, Muhammad, Syeda Izma Makhdoom, Shafiq ur Rehman, Tariq Aziz, Farzana Bashir, Urooj Ali, Metab Alharbi, Abdulrahman Alshammari, and Abdullah F. Alasmari. 2023. "Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents" Molecules 28, no. 8: 3299. https://doi.org/10.3390/molecules28083299
APA StyleNaveed, M., Makhdoom, S. I., Rehman, S. u., Aziz, T., Bashir, F., Ali, U., Alharbi, M., Alshammari, A., & Alasmari, A. F. (2023). Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents. Molecules, 28(8), 3299. https://doi.org/10.3390/molecules28083299










