Characterization of the Nonpolar and Polar Extractable Components of Glanded Cottonseed for Its Valorization
Abstract
1. Introduction
2. Results and Discussion
2.1. 1H NMR Spectral Features of Nonpolar Oil Fraction
2.2. 13C NMR Spectral Features of Oil Extracts
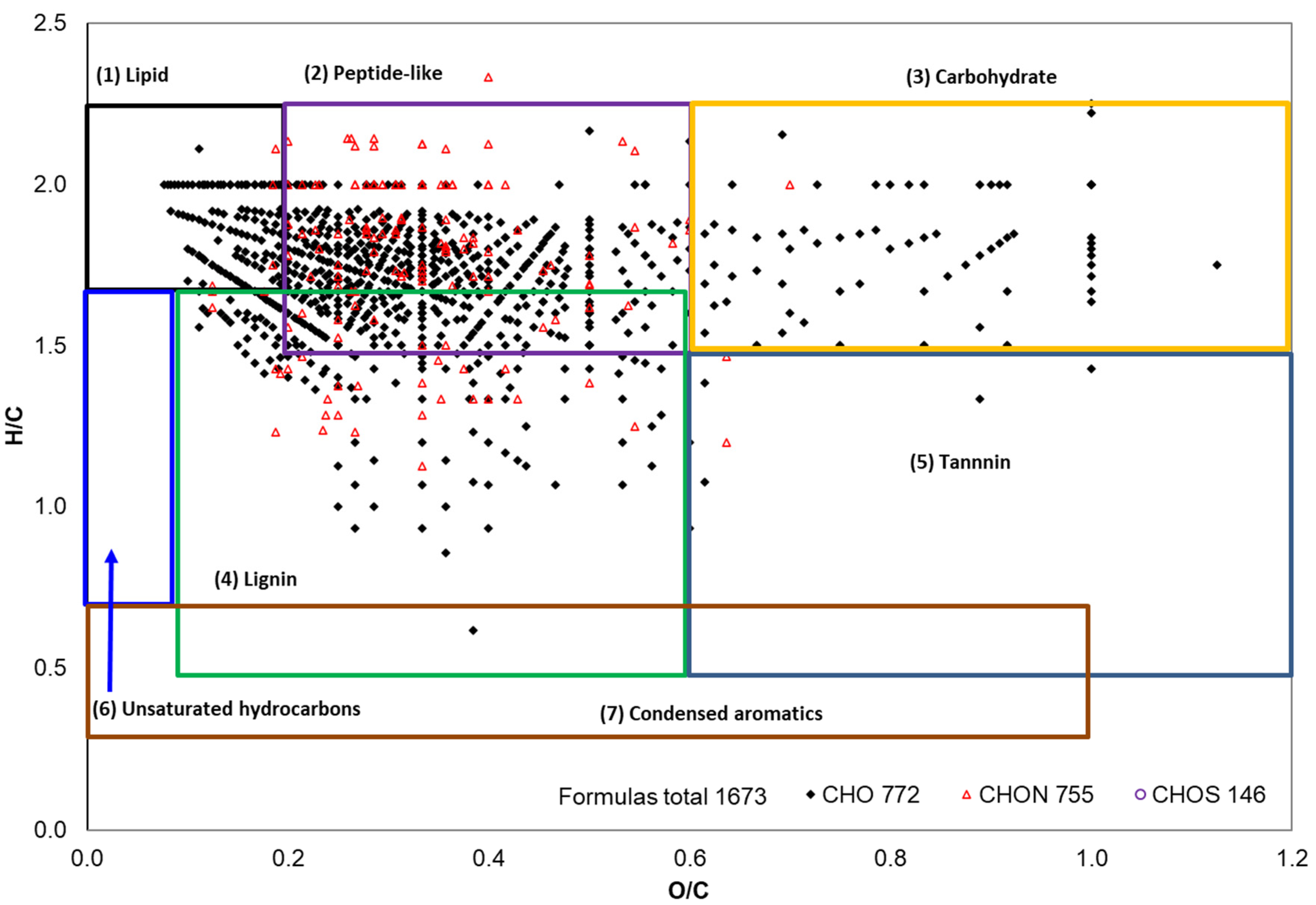
2.3. ESI FT-ICR Mass Spectral Analysis of Gd Cottonseed Extracts
2.4. Selected Potential Bioactive Compounds in the Polar Extracts of Gd Cottonseed
| MS Peak (m/z) | Theoretic Mass | [M − H]− Formula | DBE | Abundance (%) | Compound Name and Potential Function | Reference | |
|---|---|---|---|---|---|---|---|
| Gd | Gl [55] | ||||||
| 293.2123 | 293.2122 | C18H29O3 | 4 | 0.609 | 0.787 | Hydroxy-octadecatrienoic acid; anti-inflammatory | [74,75,76] |
| 392.3173 | 392.3170 | C24H42O3N | 4 | 0.478 | 0.162 | 3-Methoxy-1-methoxymethyl-3-phenylpropyl)dodecanamide; ceramide trafficking inhibitor analogue | [77] |
| 305.0779 | 305.0779 | C14H13O6N2 | 9 | 0.382 | 0.625 | 2,5-Dihydroxy-N′-(2,3,4-trihydroxybenzylidene)benzohydrazide; hexokinase 2 inhibitor | [70] |
| 426.3017 | 426.3014 | C27H40O3N | 8 | 0.291 | ND a | N-Docosahexaenoyl valine or N-linoleoyl phenylalanine; N-acylamides | [78] |
| 378.3015 | 378.3014 | C23H40O3N | 4 | 0.233 | 0.070 | N-linoleoyl valine; N-acylamides | [78] |
| 290.0882 | 290.0881 | C11H16O8N | 4 | 0.204 | 0.138 | Pyroglutamic acid hexose; bioactive metabolite | [79] |
| 277.2173 | 277.2173 | C18H29O2 | 4 | 0.155 | 0.182 | Linolenic acid isomer; nutrient | [74,75] |
| 309.2073 | 309.2071 | C18H29O4 | 4 | 0.120 | 0.121 | Hydroperoxy-octadecatrienoic acid, anti-inflammatory | [69,74] |
| 278.0670 | 278.0670 | C13H12O6N | 8 | 0.117 | 0.208 | N-coumaroyl aspartic acid; bioactive amino derivatives | [80] |
| 499.3279 | 499.3276 | C27H47O8 | 4 | 0.104 | 0.076 | Cholestane octaol; Polar Steroid | [81] |
| 387.1663 | 387.1661 | C18H27O9 | 5 | 0.100 | 0.234 | Tuberonic acid hexoside; tuber-forming substance | [82] |
| 431.2288 | 431.2287 | C21H35O9 | 4 | 0.091 | 0.048 | Neorehmannioside; carotenoid glycoside | [83] |
| 402.3015 | 402.3014 | C25H40O3N | 6 | 0.084 | ND a | N-palmitoyl phenylalanine; N-acylamides | [78] |
| 319.0937 | 319.0936 | C15H15O6N2 | 9 | 0.084 | 0.128 | 5-Phenyluridine; fluorescent nucleotide | [84] |
| 307.1915 | 307.1915 | C18H27O4 | 5 | 0.084 | 0.054 | Dihydrocapsiate; thermogenic | [85] |
3. Materials and Methods
3.1. Materials
3.2. Sequential Extraction of Polar and Nonpolar Fractions of Gd Cottonseed
3.3. NMR Spectral Analysis
3.4. ESI FT-ICR MS Spectrometry
3.5. Van Krevelen (V-K) Diagrams of ESI FT-ICR MS Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- He, Z.; Uchimiya, S.M.; Guo, M. (Eds.) Production and characterization of biochar from agricultural by-products: Overview and use of cotton biomass residues. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 63–86. [Google Scholar]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A review of cottonseed protein chemistry and non-food applications. Sustain. Chem. 2020, 1, 256–274. [Google Scholar] [CrossRef]
- He, Z.; Olk, D.C.; Tewolde, H.; Zhang, H.; Shankle, M. Carbohydrate and amino acid profiles of cotton plant biomass products. Agriculture 2020, 10, 2. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Tewolde, H.; Shankle, M. Chemical characterization of cotton plant parts for multiple uses. Agric. Environ. Lett. 2017, 2, 110044. [Google Scholar] [CrossRef]
- Rojo-Gutiérrez, E.; Buenrostro-Figueroa, J.; López-Martínez, L.; Sepúlveda, D.; Baeza-Jiménez, R. Biotechnological potential of cottonseed, a by-product of cotton production. In Valorisation of Agro-Industrial residues–Volume II: Non-Biological Approaches; Zainul, A.Z., Aguilar, C.N., Kusumaningtyas, R.D., Binod, P., Eds.; Springer: Amsterdam, The Netherlands, 2020; pp. 63–82. [Google Scholar]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Guo, M.; Xiao, P.; Li, H. Valorization of agricultural byproducts through conversion to biochar and bio-oil. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Benjamin, K.S., Alberta, N.A.A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 501–522. [Google Scholar]
- Kantarelis, E.; Zabaniotou, A. Valorization of cotton stalks by fast pyrolysis and fixed bed air gasification for syngas production as precursor of second generation biofuels and sustainable agriculture. Bioresour. Technol. 2009, 100, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Primaz, C.T.; Ribes-Greus, A.; Jacques, R.A. Valorization of cotton residues for production of bio-oil and engineered biochar. Energy 2021, 235, 121363. [Google Scholar] [CrossRef]
- Grewal, J.; Tiwari, R.; Khare, S. Secretome analysis and bioprospecting of lignocellulolytic fungal consortium for valorization of waste cottonseed cake by hydrolase production and simultaneous gossypol degradation. Waste Biomass Valoriz. 2020, 11, 2533–2548. [Google Scholar] [CrossRef]
- Sihag, M.K.; Patel, A.; Kumar, V. Cottonseed (Gossypium hirsutum). In Oilseeds: Health Attributes and Food Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 73–92. [Google Scholar]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1219–1237. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y. Fourier transform infrared spectroscopic analysis in applied cotton fiber and cottonseed research: A review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Zhang, H.; Olanya, O.M. Chemical composition and thermogravimetric behaviors of glanded and glandless cottonseed kernels. Molecules 2022, 27, 316. [Google Scholar] [CrossRef]
- Zhang, J.; Wedegaertner, T. Genetics and breeding for glandless upland cotton with improved yield potential and disease resistance: A review. Front. Plant Sci. 2021, 12, 753426. [Google Scholar] [CrossRef] [PubMed]
- Delgado, E.; Valverde-Quiroz, L.; Lopez, D.; Cooke, P.; Valles-Rosales, D.; Flores, N. Characterization of soluble glandless cottonseed meal proteins based on electrophoresis, functional properties, and microscopic structure. J. Food Sci. 2019, 84, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cheng, H.N.; He, J. Initial formulation of novel peanut butter-like products from glandless cottonseed. Foods 2023, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; He, Z.; Zhang, D.; Dupre, R.; Lloyd, S.W. Cross-serological reaction of glandless cottonseed proteins to peanut and tree nut allergic ige. Molecules 2023, 28, 1587. [Google Scholar] [CrossRef]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Cooke, P.; Licon, E.D.; Soto, A.S.; González, I.A.; Carreón, F.O.C.; Roldán, H.M. Effect of glandless cottonseed meal content on the microstructure of extruded corn-based snacks. Adv. Food Sci. 2014, 36, 125–130. [Google Scholar]
- Velazquez-Martinez, V.; Quintero-Quiroz, J.; Rodriguez-Uribe, L.; Valles-Rosales, D.V.; Reyes-Jaquez, D.; Klasson, T.; Delgado, E. Effect of glandless cottonseed meal protein and maltodextrin as microencapsulating agents on spray-drying of sugarcane bagasse phenolic compounds. J. Food Sci. 2022, 87, 750–763. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, X.; Ding, L.; Xu, B.; Gao, Y.; Cheng, Y.; Dai, F.; Liu, B.; Si, Z.; Fang, L. Development of the engineered “glanded plant and glandless seed” cotton. Food Chem. Mol. Sci. 2022, 5, 100130. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Klasson, K.T. Oxidative stability of cottonseed butter products under accelerated storage conditions. Molecules 2023, 28, 1599. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Uzun, B.B.; Onal, E.; Putun, A.E. Synthetic fuel production from cottonseed: Fast pyrolysis and a tga/ft-ir/ms study. J. Anal. Appl. Pyrol. 2014, 105, 83–90. [Google Scholar] [CrossRef]
- DePaolis, M.; De Respino, S.; Samineni, L.; Brighton, S.; Kumar, M. Cottonseed extract as a coagulant for water treatment. Environ. Sci. Adv. 2023, 2, 227–234. [Google Scholar] [CrossRef]
- Li, L.; Yue, H.; Wu, Q.; Fernández-Blázquez, J.P.; Shuttleworth, P.S.; Clark, J.H.; Guo, J. Unveiling the reinforcement effects in cottonseed protein/polycaprolactone blend biocomposites. Compos. Sci.Technol. 2022, 225, 109480. [Google Scholar] [CrossRef]
- Li, J.; Pradyawong, S.; Sun, X.S.; Wang, D.; He, Z.; Zhong, J.; Cheng, H.N. Improving adhesion performance of cottonseed protein by the synergy of phosphoric acid and water soluble calcium salts. Int. J. Adhes. Adhes. 2021, 108, 102867. [Google Scholar] [CrossRef]
- Chen, N.; Huang, J.; Li, K. Investigation of a formaldehyde-free cottonseed flour-based adhesive for interior plywood. BioResources 2020, 15, 5546–5557. [Google Scholar] [CrossRef]
- Villalpando, A.; Easson, M.; Cheng, H.; Condon, B. Use of cottonseed protein as a strength additive for nonwoven cotton. Text. Res. J. 2019, 89, 1725–1733. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N. Preparation and utilization of water washed cottonseed meal as wood adhesives. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 156–178. [Google Scholar]
- He, Z.; Cheng, H.N.; Nam, S. Comparison of the wood bonding performance of water-and alkali-soluble cottonseed protein fractions. J. Adhes. Sci. Technol. 2021, 35, 1500–1517. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K. Identification of bcl2 as a stably expressed qpcr reference gene for human colon cancer cells treated with cottonseed-derived gossypol and bioactive extracts and bacteria-derived lipopolysaccharides. Molecules 2022, 27, 7560. [Google Scholar] [CrossRef]
- Zia, M.; Shah, S.; Shoukat, S.; Hussain, Z.; Khan, S.; Shafqat, N. Physicochemical features, functional characteristics, and health benefits of cottonseed oil: A review. Braz. J. Biol. 2021, 82, e243511. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.-k. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Song, W.; Kong, X.; Hua, Y.; Li, X.; Zhang, C.; Chen, Y. Antioxidant and antibacterial activity and in vitro digestion stability of cottonseed protein hydrolysates. LWT 2020, 118, 108724. [Google Scholar] [CrossRef]
- Mohammadrezaei, M.; Navidshad, B.; Gheisari, A.; Toghyani, M. Cottonseed meal bioactive peptides as an alternative to antibiotic growth promoters in broiler chicks. Int. J. Pept. Res. Ther. 2020, 27, 329–340. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Nie, C.; Ge, W.; Wang, Y.; Zhang, W. Oligopeptide derived from solid-state fermented cottonseed meal significantly affect the immunomodulatory in balb/c mice treated with cyclophosphamide. Food Sci. Biotechnol. 2018, 27, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sethumadhavan, K. Cottonseed extracts and gossypol regulate diacylglycerol acyltransferase gene expression in mouse macrophages. J. Agric. Food Chem. 2018, 66, 6022–6030. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sethumadhavan, K.; Bland, J.M. Isolation of cottonseed extracts that affect human cancer cell growth. Sci. Rep. 2018, 8, 10458. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.F.; Kwan, S.H.; Lee, C.S.; Soh, Y.N.A.; Ho, Y.S.; Bi, X. Cottonseed meal protein isolate as a new source of alternative proteins: A proteomics perspective. Int. J. Mol. Sci. 2022, 23, 10105. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Bertolo, M.R.V.; Gautério, G.V.; de Mendonça, G.M.N.; Lemes, A.C.; Egea, M.B. Bioactive phytochemicals from cotton (Gossypium hirsutum) seed oil processing by-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–16. [Google Scholar]
- Cao, H.; Sethumadhavan, K.; Wu, X.; Zeng, X.; Zhang, L. Cottonseed extracts regulate gene expression in human colon cancer cells. Sci. Rep. 2022, 12, 1039. [Google Scholar] [CrossRef]
- Taylor, R.E.; French, A.D.; Gamble, G.R.; Himmelsbach, D.S.; Stipanovic, R.D.; Thibodeaux, D.P.; Wakelyn, P.J.; Dybowski, C. 1h and 13c solid-state nmr of gossypium barbadense (pima) cotton. J. Mol. Struct. 2008, 878, 177–184. [Google Scholar] [CrossRef]
- He, Z.; Zhong, J.; Cheng, H.N. Conformational change of metal phytates: Solid state 1d 13c and 2d 1h-13c nmr spectroscopic investigations. J. Food Agric. Environ. 2013, 11, 965–970. [Google Scholar]
- Liu, S.; Zhu, Y.; Wu, F.; Meng, W.; Wang, H.; He, Z.; Guo, W.; Song, F.; Giesy, J.P. Using solid 13c nmr coupled with solution 31p nmr spectroscopy to investigate molecular species and lability of organic carbon and phosphorus from aquatic plants in tai lake, china. Environ. Sci. Pollut. Res. 2017, 24, 1880–1889. [Google Scholar] [CrossRef]
- Sacchi, R.; Addeo, F.; Paolillo, L. 1h and 13c nmr of virgin olive oil. An overview. Magn. Reson. Chem. 1997, 35, S133–S145. [Google Scholar] [CrossRef]
- He, Z.; Sleighter, R.L.; Hatcher, P.G.; Liu, S.; Wu, F.; Zou, H.; Olanya, O.M. Molecular level comparison of water extractives of maple and oak with negative and positive ion esi ft-icr mass spectrometry. J. Mass Spectrom. 2019, 54, 655–666. [Google Scholar] [CrossRef]
- Liu, S.; He, Z.; Tang, Z.; Liu, L.; Hou, J.; Li, T.; Zhang, Y.; Shi, Q.; Giesy, J.P.; Wu, F. Linking the molecular composition of autochthonous dissolved organic matter to source identification for freshwater lake ecosystems by combination of optical spectroscopy and ft-icr-ms analysis. Sci. Total Environ. 2020, 703, 134764. [Google Scholar] [CrossRef] [PubMed]
- Terrell, E.; Garcia-Perez, M. Novel strategy to analyze fourier transform ion cyclotron resonance mass spectrometry data of biomass pyrolysis oil for oligomeric structure assignment. Energy Fuels 2020, 34, 8466–8481. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, J.N.; Jiang, B.; Zhan, Z.W. Characterisation of copal resin and amber by negative-ion electrospray ionisation fourier transform ion cyclotron resonance mass spectrometry. J. Mass Spectrom. 2021, 56, e4710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vermillion, K.; Jin, C.; Wang, X.; Zhao, W. Nmr study on the oxidation of vegetable oils for assessing the antioxidant function of trehalose. Biocatal. Agric. Biotechnol. 2021, 36, 102134. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Nanocrystalline lithium ion impregnated calcium oxide as heterogeneous catalyst for transesterification of high moisture containing cotton seed oil. Energy Fuels 2010, 24, 2091–2097. [Google Scholar] [CrossRef]
- He, Z.; Guo, M.; Fortier, C.; Cao, X.; Schmidt-Rohr, K. Fourier transform infrared and solid state 13c nuclear magnetic resonance spectroscopic characterization of defatted cottonseed meal-based biochars. Mod. Appl. Sci. 2021, 15, 108–121. [Google Scholar] [CrossRef]
- He, Z.; Guo, M.; Sleighter, R.L.; Zhang, H.; Fortier, C.A.; Hatcher, P.G. Characterization of defatted cottonseed meal-derived pyrolysis bio-oil by ultrahigh resolution electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. J. Anal. Appl. Pyrol. 2018, 136, 96–106. [Google Scholar] [CrossRef]
- Melo, J.A.; de Sá, M.S.; Moral, A.; Bimbela, F.; Gandía, L.M.; Wisniewski, A. Renewable hydrocarbon production from waste cottonseed oil pyrolysis and catalytic upgrading of vapors with mo-co and mo-ni catalysts supported on γ-al2o3. Nanomaterials 2021, 11, 1659. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Nam, S.; Klasson, K.T.; Cheng, H.N. Molecular level characterization of the effect of roasting on the extractable components of glandless cottonseed by fourier transform ion cyclotron resonance mass spectrometry. Food Chem. 2023, 403, 134404. [Google Scholar] [CrossRef]
- Kurkuri, N.J.; Annarao, S.; Miyapadavu, P.; Kamaiah, J. 1h-nmr based lipid profiling of gossypium hirsutum seed oil at different developmental stages. Cur. Res. Green Sustain. Chem. 2021, 4, 100216. [Google Scholar] [CrossRef]
- Ye, Y.; Khushvakov, J.; Boboev, A.; Akramova, R.; Yunusov, O.; Dalimova, D.; Turdikulova, S.; Mirzaakhmedov, S.; Engelsen, S.B.; Khakimov, B. Effect of refinement and production technology on the molecular composition of edible cottonseed oils from a large industrial scale production. J. Funct. Foods 2022, 99, 105326. [Google Scholar] [CrossRef]
- Hama, J.R.; Fitzsimmons-Thoss, V. Determination of unsaturated fatty acids composition in walnut (Juglans regia L.) oil using nmr spectroscopy. Food Anal. Met. 2022, 15, 1226–1236. [Google Scholar] [CrossRef]
- Thoss, V.; Murphy, P.J.; Marriott, R.; Wilson, T. Triacylglycerol composition of british bluebell (hyacinthoides non-scripta) seed oil. RSC Adv. 2012, 2, 5314–5322. [Google Scholar] [CrossRef]
- López-Camacho, P.Y.; Martínez-Espinosa, J.C.; Basurto-Islas, G.; Torres-Zarraga, A.; Márquez-Villa, J.M.; Macías-Alonso, M.; Marrero, J.G. Spondias mombin seed oil compounds identification by raman spectroscopy and nmr. Appl. Sci. 2021, 11, 2886. [Google Scholar] [CrossRef]
- Goldson-Barnaby, A.; Clarke, J.; Warren, D.; Duffus, K. Free radical scavenging capacity, carotenoid content, and nmr characterization of blighia sapida aril oil. J. Lipids 2018, 2018, 1762342. [Google Scholar]
- Wollenberg, K.F. Quantitative high resolution 13c nuclear magnetic resonance of the olefinic and carbonyl carbons of edible vegetable oils. J. Am. Oil Chem. Soc. 1990, 67, 487–494. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. Nmr chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Lou, Y.; Shi, Q.; Zhuang, R.; Zhan, Z.W. Molecular characterization of edible vegetable oils via free fatty acid and triacylglycerol fingerprints by electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Int. J. Food Sci. Technol. 2020, 55, 165–174. [Google Scholar] [CrossRef]
- Wu, Z.; Rodgers, R.P.; Marshall, A.G. Characterization of vegetable oils: Detailed compositional fingerprints derived from electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. J. Agric. Food Chem. 2004, 52, 5322–5328. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Mattison, C.P. Quantitative comparison of the storage protein distribution in glandless and glanded cottonseeds. Agric. Environ. Lett. 2022, 7, e20076. [Google Scholar] [CrossRef]
- Dou, Y.; Mei, M.; Kettunen, T.; Mäkinen, M.; Jänis, J. Chemical fingerprinting of phenolic compounds in finnish berry wines using fourier transform ion cyclotron resonance mass spectrometry. Food Chem. 2022, 383, 132303. [Google Scholar] [CrossRef] [PubMed]
- El-Razek, A.; Mohamed, H.; Mohamed, T.A.; Ali, M.I.; Hamed, A.R. Anti-inflammatory activity of two new acylic c18 hydroxy unsaturated fatty acids from the gum resin of styrax benzoin in raw264. 7 macrophages. Egypt. J. Chem. 2022, 65, 375–384. [Google Scholar] [CrossRef]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Review: Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Therap. Adv. Endocrinol. Metabol. 2010, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Zhang, Y.; Wu, C.; Yang, K.; Gao, S.; Zheng, M.; Li, X.; Li, H.; Chen, L. Structure based discovery of novel hexokinase 2 inhibitors. Bioorg. Chem. 2020, 96, 103609. [Google Scholar] [CrossRef]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef]
- Battista, N.; Bari, M.; Bisogno, T. N-acyl amino acids: Metabolism, molecular targets, and role in biological processes. Biomolecules 2019, 9, 822. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Olanya, O.M. Antioxidant activities of the water-soluble fractions of glandless and glanded cottonseed protein. Food Chem. 2020, 325, 126907. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Comparative assessment of phytochemical profiles of comfrey (Symphytum officinale L.) root extracts obtained by different extraction techniques. Molecules 2020, 25, 837. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New technological approaches for recovering bioactive food constituents from sweet cherry (Prunus avium L.) stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.C.; Kaleva, M.; Georgieva, A. Antimicrobial and antioxidant potential of scenedesmus obliquus microalgae in the context of integral biorefinery concept. Molecules 2022, 27, 519. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsubara, R.; Kitagawa, H.; Kobayashi, S.; Kumagai, K.; Yasuda, S.; Hanada, K. Stereoselective synthesis and structure—Activity relationship of novel ceramide trafficking inhibitors.(1 r, 3 r)-n-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl) dodecanamide and its analogues. J. Med. Chem. 2003, 46, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M. Applications of Chemical Methodology in Environmental Science, Systems Biology, and Interdisciplinary Chemical Education; University of Tennessee: Knoxville, TN, USA, 2019; Available online: https://trace.tennessee.edu/utk_graddiss/5400 (accessed on 15 May 2023).
- Bondia-Pons, I.; Savolainen, O.; Törrönen, R.; Martinez, J.A.; Poutanen, K.; Hanhineva, K. Metabolic profiling of goji berry extracts for discrimination of geographical origin by non-targeted liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Inter. 2014, 63, 132–138. [Google Scholar] [CrossRef]
- Cosson, A.; Meudec, E.; Ginies, C.; Danel, A.; Lieben, P.; Descamps, N.; Cheynier, V.; Saint-Eve, A.; Souchon, I. Identification and quantification of key phytochemicals in peas–linking compounds with sensory attributes. Food Chem. 2022, 385, 132615. [Google Scholar] [CrossRef] [PubMed]
- Popov, R.S.; Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Dmitrenok, P.S. Structural characterization of polar steroid compounds of the far eastern starfish lethasterias fusca by nanoflow liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 743–764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chandra, P.; Bajpai, V.; Singh, A.; Srivastava, M.; Mishra, D.; Kumar, B. Rapid qualitative and quantitative analysis of bioactive compounds from phyllanthus amarus using lc/ms/ms techniques. Ind. Crop. Prod. 2015, 69, 143–152. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernández-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from pistacia lentiscus leaves. J. Pharmac. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-D.; Wang, Z.-L.; Liu, L.; Cheng, L. Aqueous and visible-light-promoted c–h (hetero) arylation of uracil derivatives with diazoniums. J. Org. Chem. 2021, 86, 16434–16447. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Optimization of the extraction of phytochemicals from black mulberry (Morus nigra L.) leaves. J. Ind. Engineer. Chem. 2018, 68, 282–292. [Google Scholar] [CrossRef]
- Ohno, T.; He, Z.; Sleighter, R.L.; Honeycutt, C.W.; Hatcher, P.G. Ultrahigh resolution mass spectrometry and indicator species analysis to identify marker components of soil- and plant biomass-derived organic matter fractions. Environ. Sci. Technol. 2010, 44, 8594–8600. [Google Scholar] [CrossRef]


| Chemical Shift Peak and Position in ppm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 5.35 | 5.25 | 4.3 | 4.1 | 2.75 | 2.30 | 2.00 | 1.6 | 1.3 | 0.95 | 0.87 | Reference | |
| Gd-n | 5.81 | 0.83 | 1.58 | 1.78 | 2.58 | 5.30 | 6.95 | 6.56 | 55.93 | ND a | 12.68 | This work |
| Cottonseed | 7.41 | 1.03 | 1.99 | 2.09 | 3.21 | 6.09 | 8.71 | 6.46 | 53.99 | ND | 9.02 | [50] |
| Corn | 8.27 | 1.02 | 2.01 | 0.25 | 3.34 | 6.06 | 10.27 | 6.63 | 52.99 | ND | 9.15 | [50] |
| Canola | 2.36 | 0.34 | 0.64 | 0.65 | 67.71 | 1.93 | 3.57 | 2.03 | 17.79 | 0.28 | 2.70 | [50] |
| Peanut | 6.25 | 0.97 | 1.92 | 1.99 | 1.72 | 5.82 | 9.24 | 6.13 | 57.40 | ND | 8.56 | [50] |
| Soybean | 8.76 | 1.03 | 2.01 | 2.06 | 3.92 | 6.06 | 9.67 | 6.16 | 51.20 | 0.14 b | [50] | |
| Walnut | 9.61 | 0.97 | 3.79 c | 4.76 | 5.73 | 10.39 | 5.83 | 48.06 | 1.17 | 9.71 | [58] | |
| PUFAs | MUFAs | SFAs | Reference | |
|---|---|---|---|---|
| Gd-n (Cottonseed Oil) | 48.7 | 16.9 | 34.4 | This work |
| Spondias mombin Seed | 43.5 | 29.4 | 27.1 | [61] |
| Walnut Oil | 84.0 | 13.0 | 2.0 | [58] |
| Bluebell Oil a | 11.0 | 79.6 | 9.2 | [59] |
| Chemical Shift (ppm) | Carbon | Assignment |
|---|---|---|
| 14.25, 14.34 | α-CH3 | All acyl chains |
| 22.75, 22.90 | β-CH3 | All acyl chains |
| 25.00, 25.07 | C-3 | All acyl chains |
| 25.81 | C-11 | Diallylic |
| 27.39 | C8-11 (oleyl), C8-14 (linoleyl) | Allylic |
| multiple 29.24–29.96 peaks | (CH2)n | All acyl chains |
| 31,72, 32.11, 32.13 | C-16 | R-CH2-CH2-CH3 (stearyl, oleyl, linoleyl) |
| 34.21, 34.24, 34,38 | C-2 | All acyl chains |
| 62.29 | α-CH2O | Glycerol (triacylglycerol) |
| 69.06 | β-CH2O | Glycerol (triacylglycerol) |
| 128.07, 128.08 | C-12 | Linolenyl |
| 128.25, 128.26 | C-13 | Linolenyl |
| 128.89, 129.87 | C-9 | Linolenyl, oleyl |
| 130.16, 130.18, 130.40 | C-10, C-11, C-12 | Linolenyl, gondoyl |
| 173.05 | α-C-1 Glycerol | Carbonyl (triacylglycerols) |
| 173.46, 173.51 | β-C-1 Glycerol | Carbonyl (triacylglycerols) |
| Class | Gd | Gl [55] | |||
|---|---|---|---|---|---|
| Total | Phenolic | Total | Phenolic | ||
| Lipid | Number | 165 | 42 | 111 | 30 |
| % Formulas | 9.9% | 4.0% | 15.2% | 9.0% | |
| % Magnitude | 65.2% | 14.5% | 61.0% | 10.7% | |
| Peptide | Number | 232 | 149 | 74 | 40 |
| % Formulas | 13.9% | 14.0% | 10.1% | 12.1% | |
| % Magnitude | 1.7% | 8.2% | 1.7% | 6.3% | |
| Carbohydrate | Number | 171 | 38 | 92 | 14 |
| % Formulas | 10.2% | 3.6% | 12.6% | 4.2% | |
| % Magnitude | 6.7% | 24.1% | 7.6% | 6.5% | |
| Lignin | Number | 784 | 765 | 195 | 186 |
| % Formulas | 46.9% | 72.0% | 26.7% | 56.0% | |
| % Magnitude | 6.6% | 61.9% | 5.3% | 57.7% | |
| Tannin | Number | 73 | 72 | 18 | 18 |
| % Formulas | 4.4% | 6.8% | 2.5% | 5.4% | |
| % Magnitude | 1.1% | 10.5% | 0.7% | 7.8% | |
| Unsaturated Hydrocarbon | Number | 1 | 1 | 0 | 0 |
| % Formulas | 0.06% | 0.09% | - | - | |
| % Magnitude | <0.01% | <0.01% | - | - | |
| Condensed Aromatic | Number | 0 | 0 | 0 | 0 |
| % Formulas | - | - | - | - | |
| % Magnitude | - | - | - | - | |
| Other a | Number | 247 | 0 | 240 | 44 |
| % Formulas | 14.8% | - | 32.9% | 13.3% | |
| % Magnitude | 18.6% | - | 23.8% | 11.0% | |
| Summary | Number | 1673 | 1067 | 730 | 332 |
| Major Component (g kg−1) | ||||||
| Moisture | Gossypol | Oil | Protein | ADF | ADL | Starch |
| 67.9 ± 0.5 | 3.75 ± 0.02 | 387 ± 18 | 397 ± 8 | 100 ± 18 | 52.3 ± 10.1 | 12.2 ± 1.0 |
| Macro Element and Ash (g kg−1) | ||||||
| P | Ca | K | Mg | Na | S | Ash |
| 9.8 ± 0.8 | 2.0 ± 0.3 | 12.0 ± 0.7 | 5.4 ± 0.4 | 0.6 ± 0.0 | 4.5 ± 0.3 | 46.7 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Nam, S.; Liu, S.; Zhao, Q. Characterization of the Nonpolar and Polar Extractable Components of Glanded Cottonseed for Its Valorization. Molecules 2023, 28, 4181. https://doi.org/10.3390/molecules28104181
He Z, Nam S, Liu S, Zhao Q. Characterization of the Nonpolar and Polar Extractable Components of Glanded Cottonseed for Its Valorization. Molecules. 2023; 28(10):4181. https://doi.org/10.3390/molecules28104181
Chicago/Turabian StyleHe, Zhongqi, Sunghyun Nam, Shasha Liu, and Qi Zhao. 2023. "Characterization of the Nonpolar and Polar Extractable Components of Glanded Cottonseed for Its Valorization" Molecules 28, no. 10: 4181. https://doi.org/10.3390/molecules28104181
APA StyleHe, Z., Nam, S., Liu, S., & Zhao, Q. (2023). Characterization of the Nonpolar and Polar Extractable Components of Glanded Cottonseed for Its Valorization. Molecules, 28(10), 4181. https://doi.org/10.3390/molecules28104181








