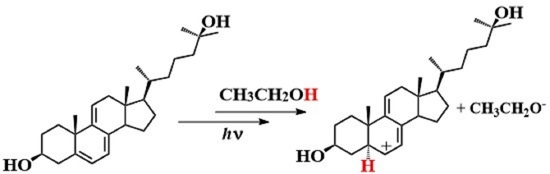
Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol in Ethanol
Abstract
1. Introduction

2. Results
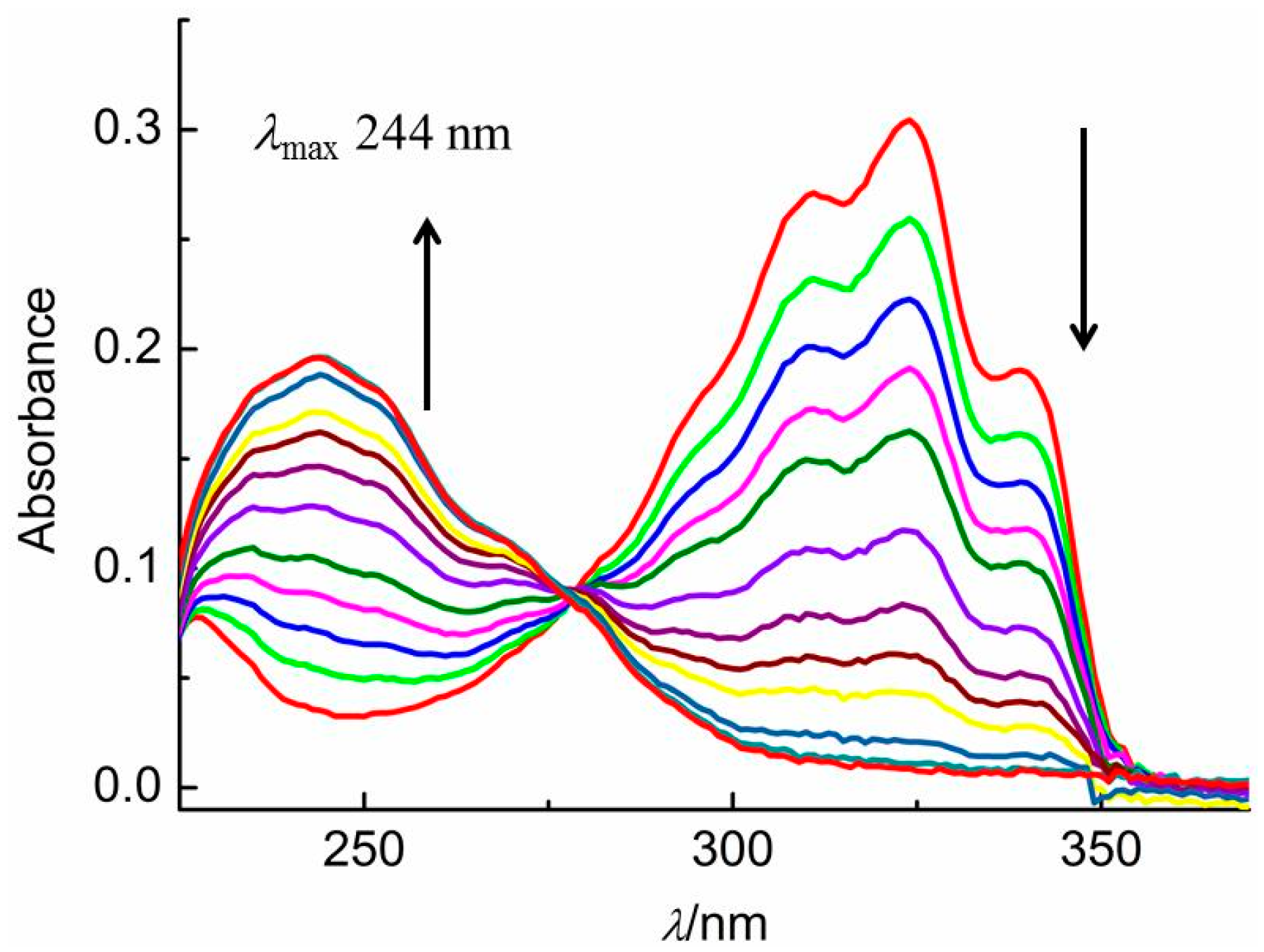
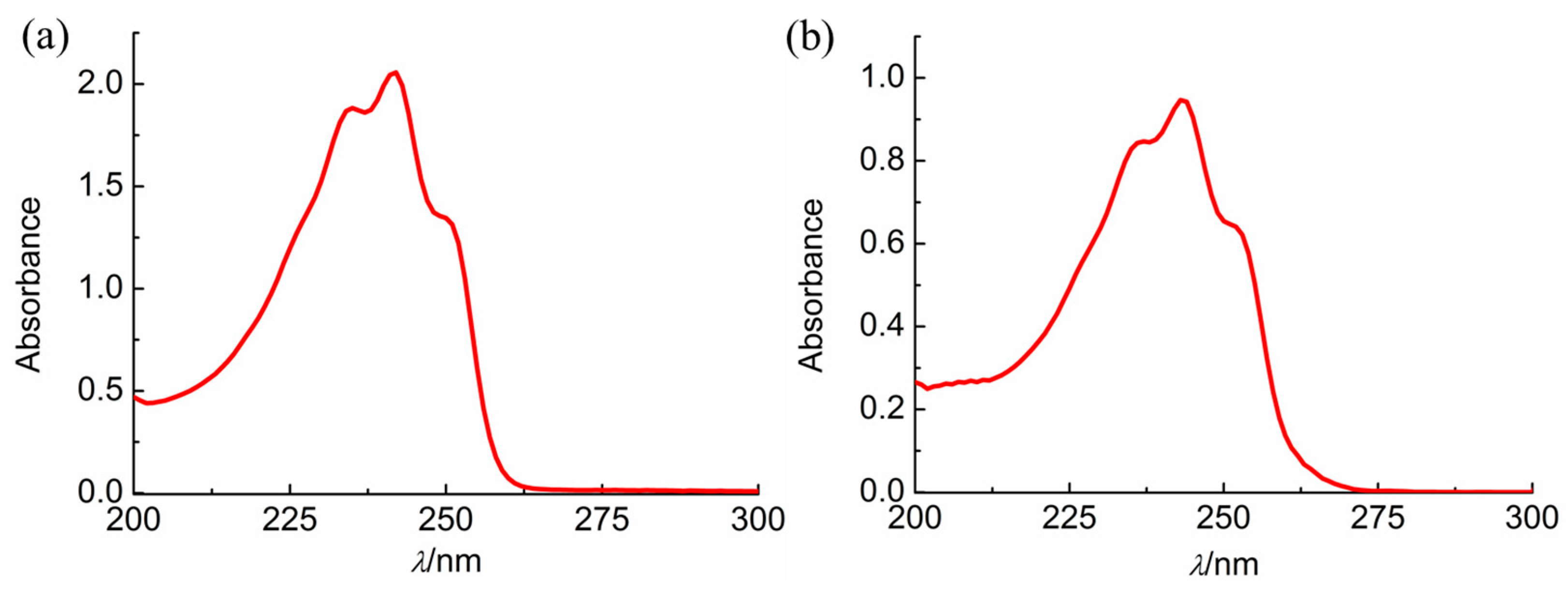
2.1. Photochemical Observations
2.2. Photoproducts

2.3. X-ray Structures
2.4. Photoproduct Quantum Yields
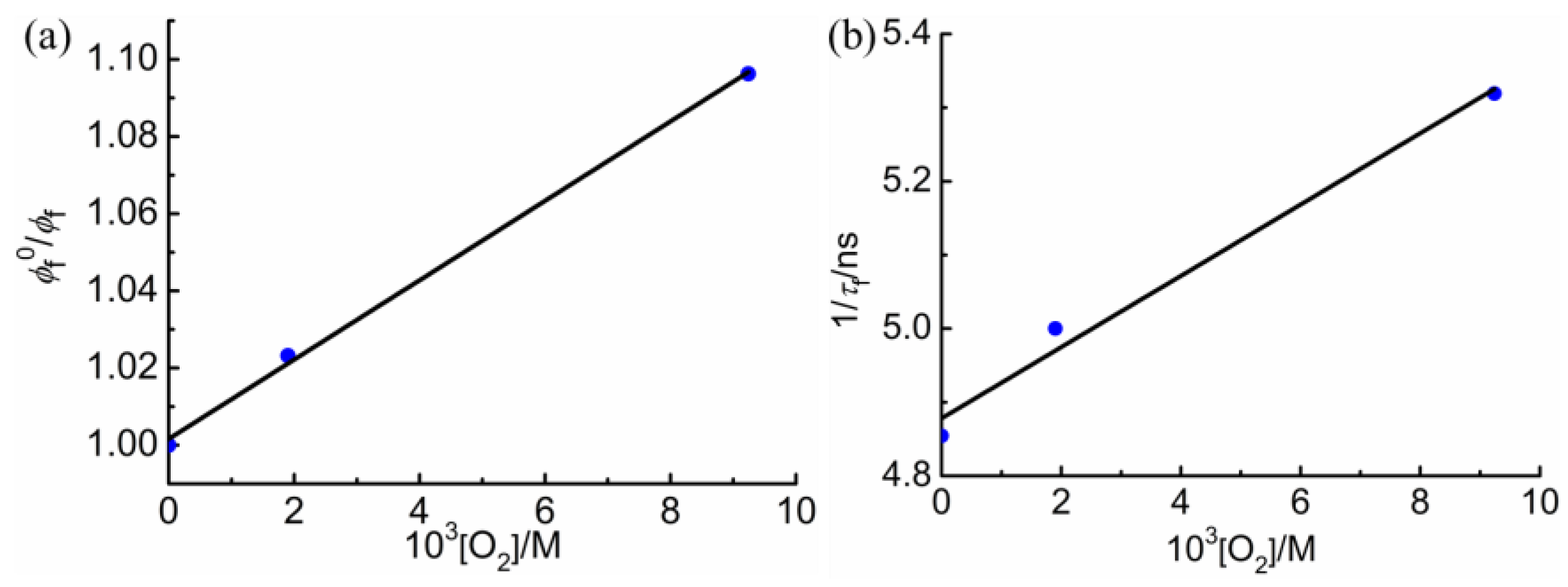
2.5. Fluorescence Measurements
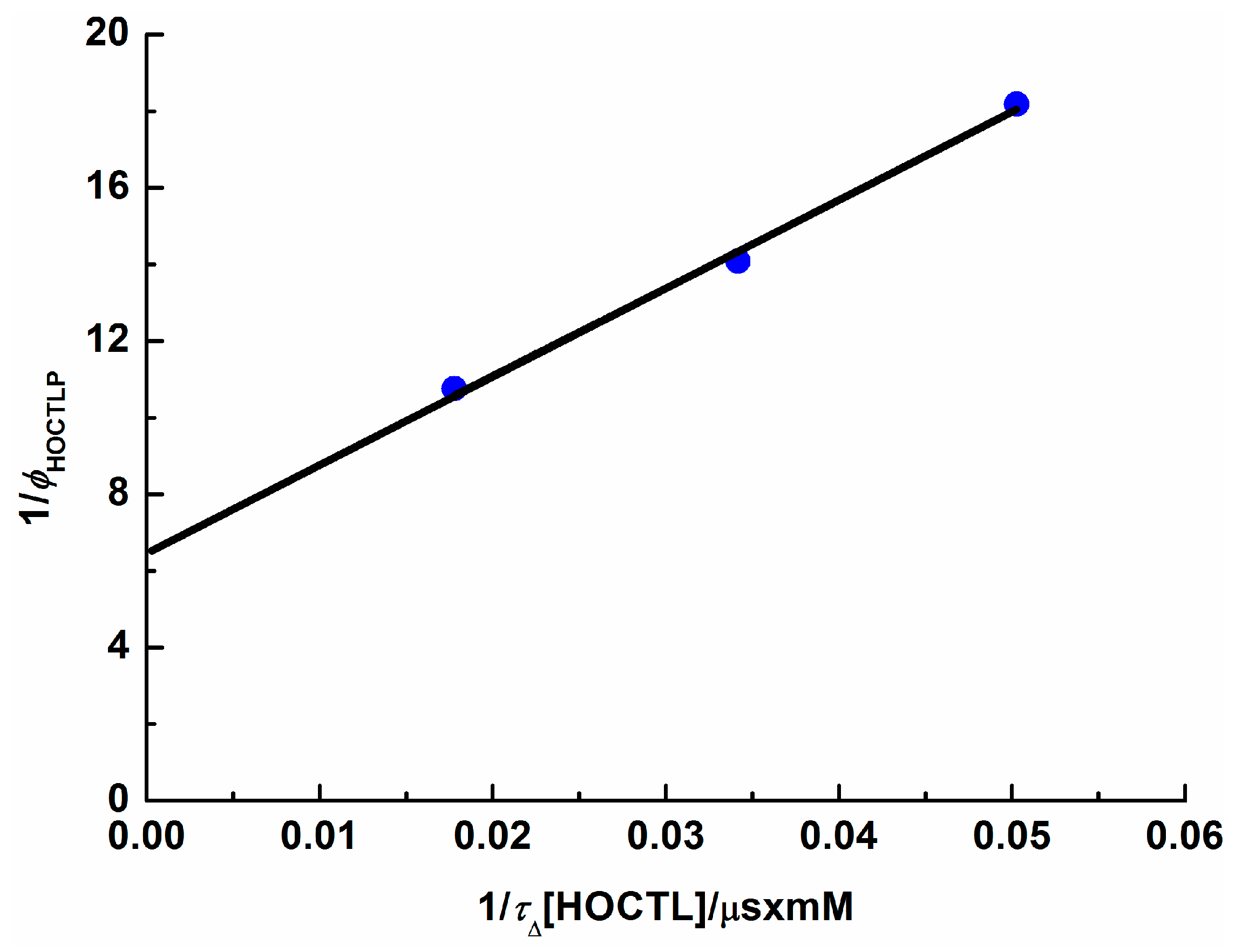
2.6. HOCTL Triplet
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Crystallography
3.4. Irradiation Procedures
4. Discussion
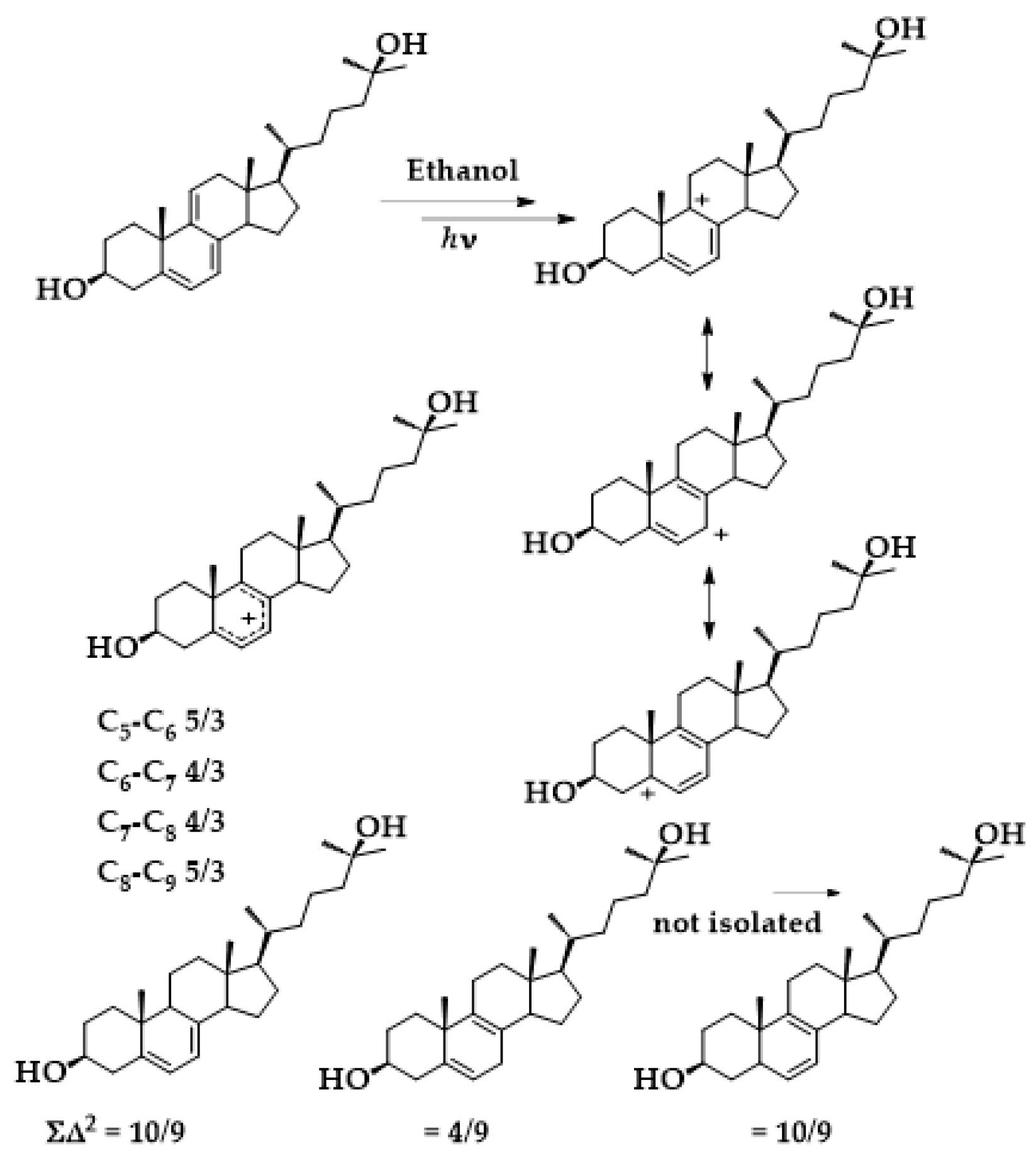
4.1. Mechanism
4.2. Photochemical Kinetics
4.3. Photophysics
4.4. Photochemistry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ruan, B.; Wilson, W.K.; Pang, J.; Gerst, N.; Pinkerton, F.D.; Tsai, J.; Kelley, R.I.; Whitby, F.G.; Milewicz, D.M.; Garbern, J.; et al. Sterols in Blood of Normal and Smith-Lemli-Opitz Subjects. J. Lipid Res. 2001, 42, 799–812. [Google Scholar] [CrossRef]
- Ruan, B.; Wilson, W.K.; Tsai, J.; Schroepfer, G.J., Jr. Aberrant Pathways in the Late Stages of Cholesterol Biosynthesis. Origin and Metabolic Fate of Unsaturated Sterols Relevant to the Smith-Lemli-Opitz Syndrome. J. Lipid Res. 2000, 41, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Delseth, C.; Kashman, Y.; Djerassi, C. Ergosta-5,7,9(11),22-tetraen-3β-ol and its 24-Ethyl Homolog. Two New Marine Sterols from the Red Sea Sponge Biemna fortis. Helv. Chim. Acta 1979, 62, 2037–2045. [Google Scholar] [CrossRef]
- Heald, S.I.; Jeffs, P.W.; Wheat, R.W. The identification of ergosterol and Δ9(11)-dehydroergosterol from mycelia of Coccidioides immitis by reverse-phase high-performance liquid and gas chromatography and ultraviolet and mass spectrometry. Exper. Mycol. 1981, 5, 162–166. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and Trace Sterols in Marine Invertebrates. Isolation and Structure Elucidation of Nine New 5.alpha.,8.alpha.-Epidioxy Sterols from Four Marine Organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Sica, D.; Boniforti, L.; DiGiacomo, G. Sterols of Candida Tropicalis Grown on n-Alkanes. Phytochemistry 1982, 21, 234–236. [Google Scholar] [CrossRef]
- Fischer, R.T.; Stephenson, F.A.; Shafiee, A.; Schroeder, F. Δ5,7,9(11)-Cholestatrien-3β-ol: A Fluorescent Cholesterol Analogue. Chem. Phys. Lipids 1984, 36, 1–14. [Google Scholar] [CrossRef]
- Fischer, R.T.; Cowlen, M.S.; Dempsey, M.E.; Schroeder, F. Fluorescence of Δ5,7,9(11),22-Ergostatetraen-3β-ol in Micelles, Complexes, and Plasma Membranes. Biochemistry 1985, 24, 3322–3331. [Google Scholar] [CrossRef]
- Schroeder, F. Fluorescent Sterols-Probe Molecules of Membrane Structure and Function. Prog. Lipid Res. 1984, 23, 97–113. [Google Scholar] [CrossRef]
- Wüstner, D. Fluorescent Sterols as Tools in Membrane Biophysics and Cell Biology. Chem. Phys. Lipids 2007, 146, 1–25. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Wüstner, D. Analysis of Cholesterol Trafficking with Fluorescent Probes. Methods Cell Biol. 2012, 108, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Lin, S.X.; Karylowski, O.J.; Wüstner, D.; McGraw, T.E.; Maxfield, F.R. Vesicular and Non-vesicular Sterol Transport in Living Cells. The Endocytic Recycling Compartment is a Major Sterol Organelle. J. Biol. Chem. 2002, 277, 609–617. [Google Scholar] [CrossRef]
- Saltiel, J.; Krishnan, S.B.; Gupta, S.; Hernberg, E.A.; Clark, R.J. Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol—A Fluorescent Analogue of Cholesterol. Photochem. Photobiol. Sci. 2022, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Windaus, A.; Linsert, O. Ultraviolet Irradiation of Dehydroergosterol. Justus Liebigs Ann. Chem. 1928, 465, 148–166. [Google Scholar] [CrossRef]
- Windaus, A.; Gaede, J.; Köser, J.; Stein, G. Crystallized Irradiation Products from Ergosterol and Dehydroergosterol. Justus Liebigs Ann. Chem. 1930, 483, 17–30. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Kende, A.S. The Constitution of a Steroidal Irradiation Product. J. Chem. Soc. 1958, 688–692. [Google Scholar] [CrossRef]
- Fieser, L.F. Steric Course of Reactions of Steroids. Experientia 1950, 6, 312–315. [Google Scholar] [CrossRef]
- Chignell, C.F.; Kukielczak, B.M.; Sik, R.H.; Bilski, P.J.; He, Y.-Y. Ultraviolet A Sensitivity in Smith–Lemli–Opitz Syndrome: Possible Involvement of Cholesta-5,7,9(11)-trien-3β-ol. Free Rad. Biol. Med. 2006, 41, 339–346. [Google Scholar] [CrossRef]
- Morton, R.A.; Heilbron, I.M.; Spring, F.S. Absorption Spectra in Relation to Vitamin, A. Biochem. J. 1930, 24, 136–140. [Google Scholar] [CrossRef]
- Windaus, A.; Linsert, O.; Eckhardt, H.J. Über Iso-dehydro-cholesterin (Δ6,Δ8-cholestadien-3-ol). Justus Liebigs Ann. Chem. 1938, 534, 22–41. [Google Scholar] [CrossRef]
- Nussim, M.; Mazur, Y.; Sondheimer, F. The Hydration of Unsaturated Steroids by the Brown Hydroboration Reaction. II. Steroidal Conjugated Dienes. J. Org. Chem. 1964, 29, 1131–1136. [Google Scholar] [CrossRef]
- Schroepfer, G.J., Jr.; Parish, E.J.; Kandutsch, A.A. Inhibitors of sterol biosynthesis. Synthesis and activities of ring C oxygenated sterols. Chem. Phys. Lipids 1988, 46, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.K.; Sumpter, R.M.; Warren, J.J.; Rogers, P.S.; Ruan, B.; Schroepfer, G.J., Jr. Analysis of unsaturated C27 sterols by nuclear magnetic resonance spectroscopy. J. Lipid Res. 1996, 37, 1529–1555. [Google Scholar] [CrossRef] [PubMed]
- De Simone, F.; Dini, A.; Finamore, E.; Minale, L.; Pizza, C.; Riccio, R.; Zollo, F. Starfish Saponins. Part 5. Structure of Steroidal Cyclic Glycoside from Starfish Echinaster sepositus. J. Chem. Soc. Perkin Trans. 1 1981, 1855–1862. [Google Scholar] [CrossRef]
- Moses, F.G.; Liu, R.S.H.; Monroe, B.M. The “Merry-Go-Round” Quantum Yield Apparatus. Mol. Photochem. 1969, 1, 245–249. [Google Scholar]
- Saltiel, J.; Marinari, A.; Chang, D.W.-L.; Mitchener, J.C.; Megarity, E.D. Trans-Cis Photoisomerization of the Stilbenes and a Reexamination of the Positional Dependence of the Heavy-Atom Effect. J. Am. Chem. Soc. 1979, 101, 2982–2996. [Google Scholar] [CrossRef]
- IUPAC. Analytical Chemistry Division, Commission on Solubility Data, Oxygen and Ozone. In Solubility Data Series; Battino, R., Ed.; Pergamon: Oxford, UK, 1981; Volume 7. [Google Scholar]
- CRC. Handbook of Chemistry and Physics, 57th ed.; Weast, R.C., Ed.; CRC Press: Boca Raton, FL, USA, 1976. [Google Scholar]
- Scatchard, G.; Satkiewicz, F.G. Vapor-Liquid Equilibrium.XII. The System Ethanol-Cyclohexane from 5 to 65°. J. Am. Chem. Soc. 1964, 86, 130–133. [Google Scholar] [CrossRef]
- Godfrey, T.S.; Hilpern, J.W.; Porter, G. Triplet-Triplet Absorption Spectra of Benzophenone and its Derivatives. Chem. Phys. Lett. 1967, 1, 490–492. [Google Scholar] [CrossRef]
- Topp, M. Activation-Controlled Hydrogen Abstraction by Benzophenone Triplet. Chem. Phys. Lett. 1975, 32, 144–149. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Woodward, R.B. Structure and absorption spectra. III. Normal conjugated dienes. J. Am. Chem. Soc. 1942, 64, 72–75. [Google Scholar] [CrossRef]
- Fieser, L.F.; Fieser, M. Advanced Organic Chemistry; Reinhold Publishing Corp: New York, NY, USA, 1961; p. 204. [Google Scholar]
- Kropp, P.J. Photochemistry of Alkenes in Solution. Pure Appl. Chem. 1970, 24, 585–598. [Google Scholar] [CrossRef]
- Marshall, J.A. Photosensitized Ionic Additions to Cyclohexenes. Acc. Chem. Res. 1969, 2, 33–40. [Google Scholar] [CrossRef]
- Kropp, P.J.; Reardon, E.J., Jr.; Gaibel, Z.; Williard, K.F.; Hattaway, J.H., Jr. Photochemistry of Alkenes. Direct Irradiation in Hydroxylic Media. J. Am. Chem. Soc. 1973, 95, 7058–7067. [Google Scholar] [CrossRef]
- Dauben, W.G.; Wipke, W.T. Photochemistry of Dienes. Pure Appl. Chem. 1964, 9, 533–539. [Google Scholar] [CrossRef]
- Dauben, W.G.; Smith, J.H.; Saltiel, J. The Mass Spectra of Cyclobutyl and Cyclopropylcarbinyl Methyl Ethers and the Methanolysis of Bicyclobutane. J. Org. Chem. 1969, 34, 261–266. [Google Scholar] [CrossRef]
- Saltiel, J.; Redwood, C.E. Photochemistry of the 1,4-Diphenyl-1,3-butadienes in Ethanol. Trapping Conical Intersections. J. Phys. Chem. A 2016, 120, 2832–2840. [Google Scholar] [CrossRef]
- Saltiel, J.; Gupta, S. Photochemistry of the Stilbenes in Methanol. Trapping the Common Phantom Singlet State. J. Phys. Chem. A 2018, 122, 6089–6099. [Google Scholar] [CrossRef]
- Dauben, W.G.; Ritscher, J.S. Photochemistry of Ethylidenecyclooctenes. Mechanism of Bicyclobutane Formation. J. Am. Chem. Soc. 1970, 92, 2925–2926. [Google Scholar] [CrossRef]
- Salem, L. The Sudden Polarization Effect and Its Possible Role in Vision. Acc. Chem. Res. 1979, 12, 87–92. [Google Scholar] [CrossRef]
- Quenneville, J.; Martínez, T.J. Ab Initio Study of Cis-Trans Photoisomerization in Stilbene and Ethylene. J. Phys. Chem. A 2003, 107, 829–837. [Google Scholar] [CrossRef]
- Minezawa, N.; Gordon, M.S. Photoisomerization of Stilbene: A Spin-Flip Density Functional Theory Approach. J. Phys. Chem. A 2011, 115, 7901–7911. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, I.N.; Granovsky, A.A. Photoisomerization of Stilbene: The Detailed XMCQDPT2 Treatment. J. Chem. Theory Comput. 2013, 9, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Garavelli, M.; Orlandi, G. Tracking the Stilbene Photoisomerization in the S1 State Using RASSCF. Phys. Chem. Chem. Phys. 2013, 15, 19763–19773. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.L.; Hilinski, E.F. Dependence of the Lifetime of the Twisted Excited Singlet State of Tetraphenylethylene on Solvent Polarity. J. Am. Chem. Soc. 1988, 110, 2296–2298. [Google Scholar] [CrossRef]
- Sundström, V.; Gillbro, T. Dynamics of the isomerization of trans-stilbene in n-alcohols by ultraviolet picosecond absorption recovery. Chem. Phys. Lett. 1984, 109, 538–543. [Google Scholar] [CrossRef]
- Kim, S.K.; Courtney, S.H.; Fleming, G.R. Isomerization of trans-Stilbene in Alcohols. Chem. Phys. Lett. 1989, 159, 543–548. [Google Scholar] [CrossRef]
- Saltiel, J.; Ko, D.-H.; Fleming, S.A. Differential Medium Effects on the Trans to Cis Photoisomerization of all-trans-1,6-Diphenyl-1,3,5-hexatriene. Competing Diradicaloid vs Zwitterionic Pathways. J. Am. Chem. Soc. 1994, 116, 4099–4100. [Google Scholar] [CrossRef]
- Saltiel, J.; Wang, S.; Watkins, L.P.; Ko, D.-H. Direct Photoisomerization of the 1,6-Diphenyl-1,3,5-hexatrienes. Medium Effect on Triplet and Singlet Contributions. J. Phys. Chem. A 2000, 104, 11443–11450. [Google Scholar] [CrossRef]
- Redwood, C.; Kumar, R.V.K.; Hutchinson, S.; Mallory, F.B.; Mallory, C.W.; Clark, R.J.; Dmitrenko, O.; Saltiel, J. Photoisomerization of cis-1,2-di(1-methyl-2-naphthyl)ethene at 77 K in glassy media. Photochem. Photobiol. 2015, 91, 607–615. [Google Scholar] [CrossRef]
- Squillacote, M.; Wang, J.; Chen, J. Electrostatic Control of the Regioselectivity in the Photoisomerization of trans,trans-1-Fluoro-2,4-hexadiene: Evidence for Competing Conical Intersections. J. Am. Chem. Soc. 2004, 126, 1940–1941. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, F.; Jacobs, H.J.C.; Havinga, E.; van der Gen, A. The “over irradiation” products of previtamin D and tachysterol: Toxisterols. Recl. Trav. Chim. Pays Bas 1977, 96, 104–112. [Google Scholar] [CrossRef]
- Jacobs, H.J.C.; Boomsma, F.; Havinga, E.; van der Gen, A. The photochemistry of previtamin D and tachysterol. Recl. Trav. Chim. Pays Bas 1977, 96, 113–117. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Barton, D.H.R.; Russell, R.A.; Widdowson, D.A. Photochemical Transformations. Part 34. Structure of the Toxisterols. J. Chem. Soc. Perkin Trans. 1 1977, 631–643. [Google Scholar] [CrossRef]
- Hine, J. The principle of least nuclear motion. Adv. Phys. Org. Chem. 1977, 15, 1–61. [Google Scholar] [CrossRef]
- Reis, M.C.; Alajarin, M.; Marin-Luna, M. Violations to the principle of least motion: The shortest path is not always the fastest. Phys. Chem. Chem. Phys. 2022, 24, 8064–8075. [Google Scholar] [CrossRef]
- Nordlander, J.E.; Ownor, P.O.; Haky, J.F. Regiochemistry of the Addition of DCl to trans-1,3-Pentadiene. J. Am. Chem. Soc. 1979, 101, 1288–1289. [Google Scholar] [CrossRef]
- Smutzer, G.; Crawford, B.F.; Yeagle, P.L. Physical Properties of the Fluorescent Sterol Probe Dehydroergosterol. Biochim. Biophys. Acta 1986, 862, 361–371. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Morel, B.; Sauerheber, D. Organization and Interaction of Cholesterol and Phosphatidylcholine in Model Bilayer Membranes. Biochemistry 1990, 29, 1025–1038. [Google Scholar] [CrossRef]
- Arnold, D.R. (Department of Chemistry, Dalhousie University, Halifax, NS, Canada). Personal communication.
- Redmond, R.W.; Heihoff, K.; Braslavsky, S.E.; Truscott, T.G. Thermal-lensing and phosphorescence studies of the quantum yield and lifetime of singlet molecular oxygen (1Δg) sensitized by hematoporphyrin and related porphyrins in deuterated and non-deuterated ethanols. Photochem. Photobiol. 1987, 45, 209–213. [Google Scholar] [CrossRef]
- Rodgers, M.A.J. Solvent-Induced Deactivation of Singlet Oxygen: Additivity Relationships in Nonaromatic Solvents. J. Am. Chem. Soc. 1983, 105, 6201–6205. [Google Scholar] [CrossRef]
- Ponce, M.A.; Ramirez, J.A.; Galagovsky, L.R.; Gros, E.G.; Erra-Balsells, R. A new look into the reaction between ergosterol and singlet oxygen in vitro. Photochem. Photobiol. Sci. 2002, 1, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Albro, P.W.; Bilski, P.; Corbett, J.T.; Schroeder, J.L.; Chignell, C.F. Photochemical Reactions and Phototoxicity of Sterols: Novel Self-perpetuating Mechanism for Lipid Photooxidation. Photochem. Photobiol. 1997, 66, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.A.; Hamblett, I.; Rodgers, M.A.J. Ergosterol (Provitamin D2) Triplet State: An Efficient Sensitiser of Singlet Oxygen, O2 (1Δg), Formation. Photochem. Photobiol. 1987, 45, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Solokhiddinov, K.I.; Byteva, I.M.; Gurinovich, G.P. Lifetime of Singlet Oxygen in Various Solvents. Zhurnal Prikl. Spektrosk. 1981, 34, 892–897. [Google Scholar] [CrossRef]
- Hurst, J.R.; McDonald, J.D.; Schuster, G.B. Lifetime of Singlet Oxygen in Solution Directly Determined by Laser Spectroscopy. J. Am. Chem. Soc. 1982, 104, 2065–2067. [Google Scholar] [CrossRef]
- Sagadevan, A.; Huang, K.C.; Su, M.-D. Singlet Oxygen-Mediated Selective C-H Bond Hydroperoxidation of Ethereal Hydrocarbons. Nat. Commun. 2017, 8, 1812. [Google Scholar] [CrossRef]
- Juaristi, E.; dos Passos Gomes, G.; Terent’ev, A.O.; Notario, R.; Alabugin, I.V. Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular α-Effect. J. Am. Chem. Soc. 2017, 139, 10799–10813. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent’ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Medvedev, M.G.; Krivoshchapov, N.V.; Vil’, V.A.; Yaremenko, I.A.; Mehaffy, P.; Yarie, M.; Terent’ev, A.O.; Zolfigol, M.A. Stereoelectronic Power of Oxygen in Control of Chemical Reactivity: The Anomeric Effect is not Alone. Chem. Soc. Rev. 2021, 50, 10253–10345. [Google Scholar] [CrossRef]

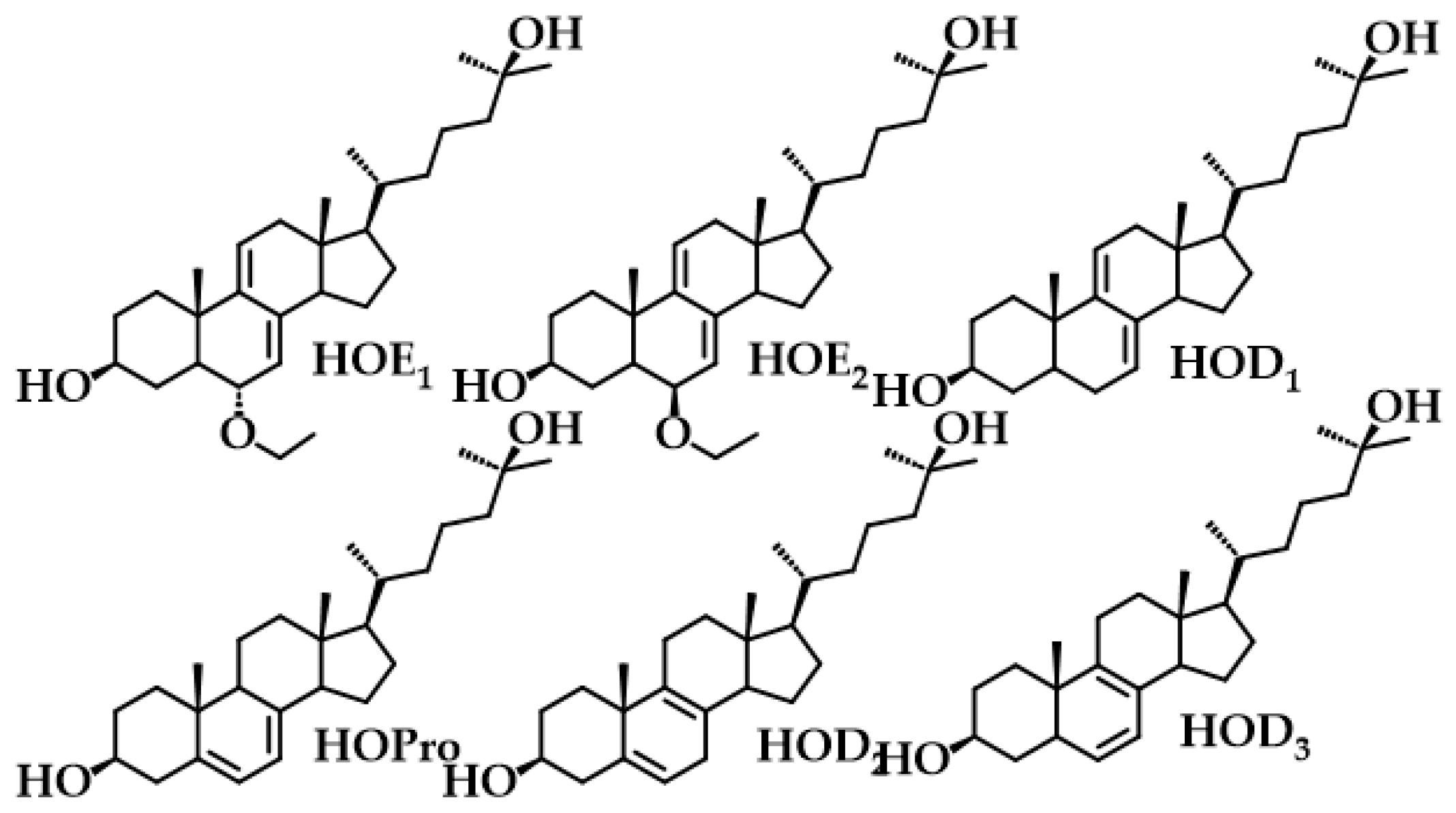



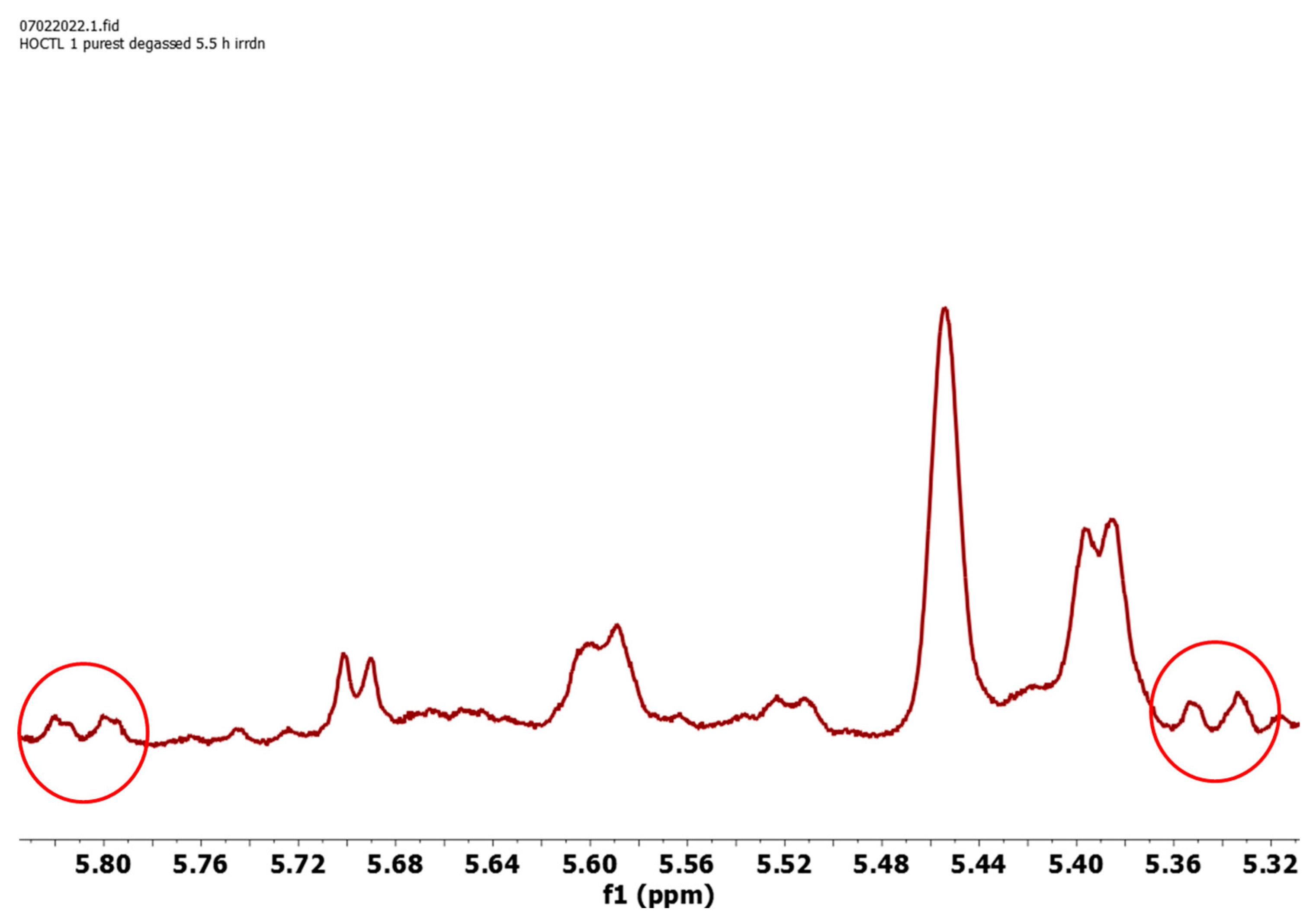
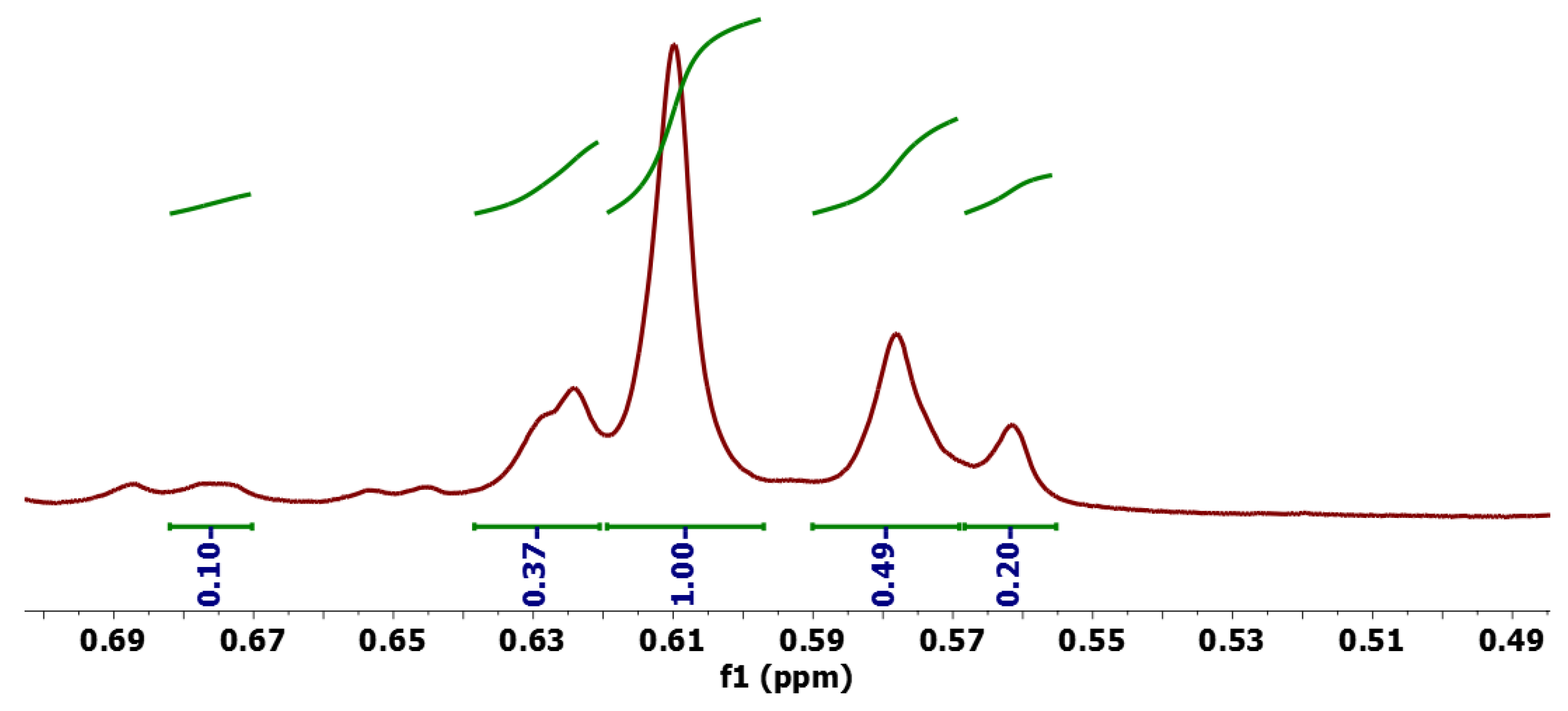
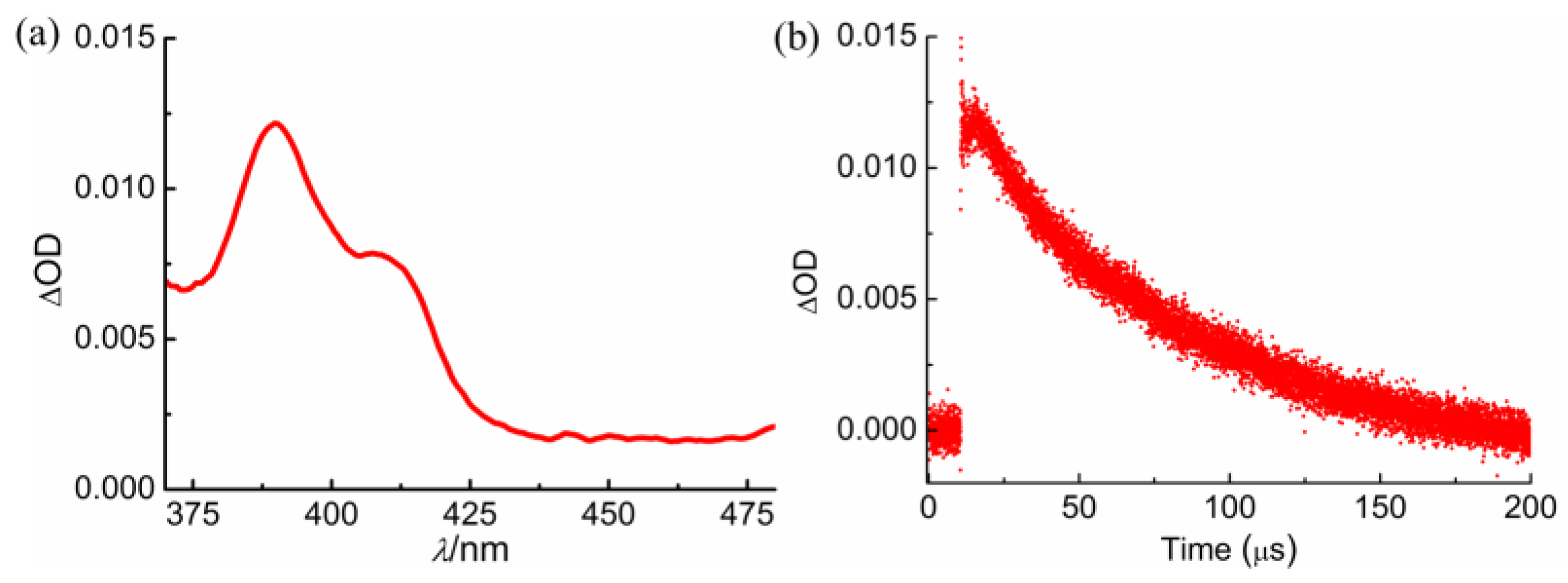

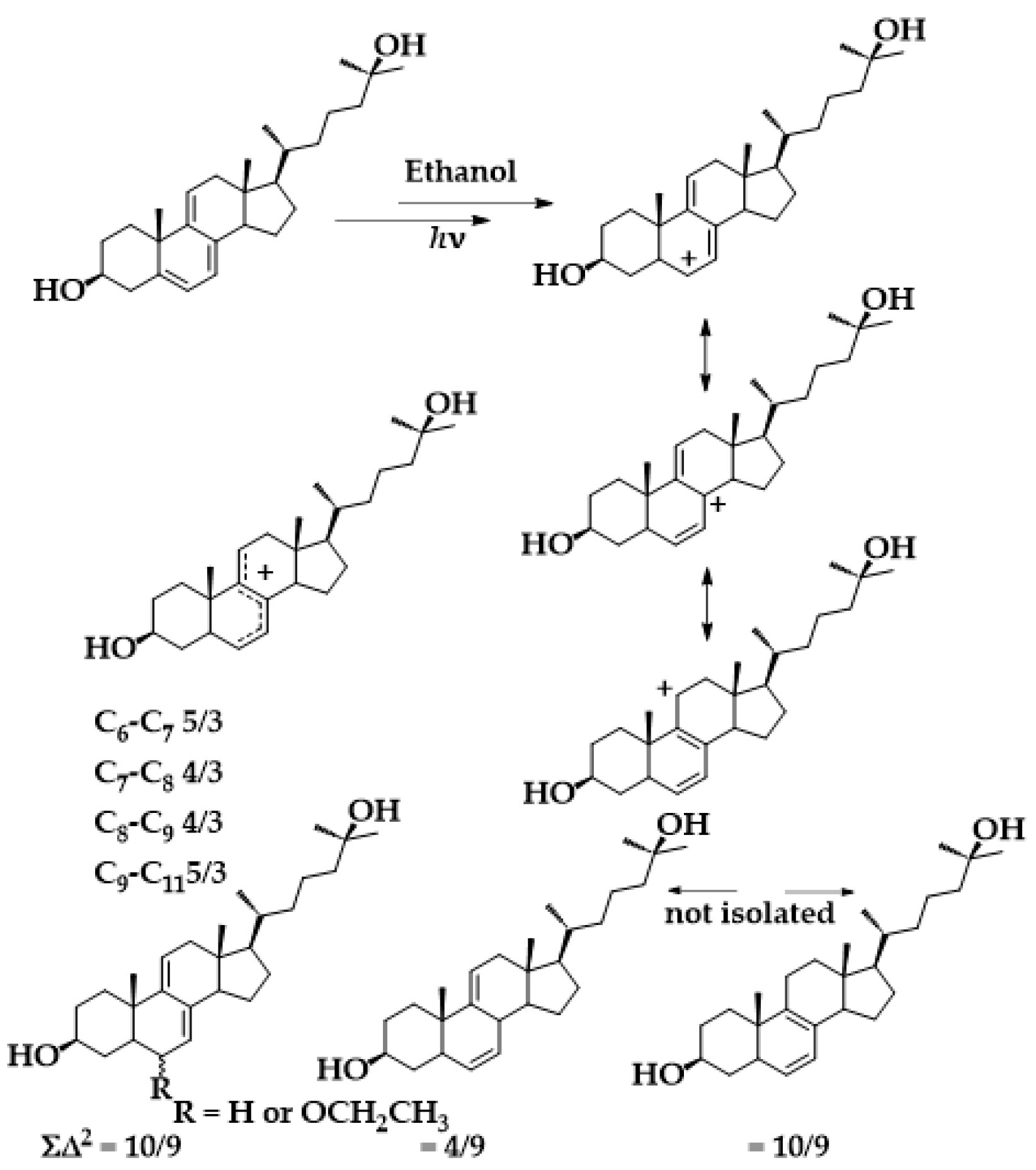

| Compound | C18 Me/δ | Vinyl H/δ | % b | ϕP |
|---|---|---|---|---|
| HOPro | 0.657 | 5.57 C6 | 3.0 (2) | 0.0019 |
| HORP1 | 0.590 | 5.45/5.38 c | 37.0 (2) | 0.0240 |
| HOD1 | 0.542 | 5.52 C11 | 9.1 (2) | 0.0059 |
| HOD2 | 0.627 | 5.45 C6 | 0.7 (1) | 0.00045 |
| HOD3 | 5.82 C7 | 8.2 (2) | 0.0053 | |
| HOE1 | 0.558 | 5.60 C11 | 24.5 (2) | 0.0159 |
| HOE2 | 0.609 | 5.69 C11 | 13.4 (2) | 0.0087 |
| 103[HOCTL]/M−1 | ϕPer | τΔ/μs |
|---|---|---|
| 1.34 | 0.055 | 14.8 |
| 2.00 | 0.071 | 14.6 |
| 4.01 | 0.093 | 14.0 |
| [O2]/M | ϕf 25 °C | ϕf 20 °C | τf/ns | χ2 | 10−8 ϕf/τf | |
|---|---|---|---|---|---|---|
| Ar | 0 | 0.0281 | 0.0353 | 0.206 | 1.9 | 1.71 |
| Air | 0.00194 | 0.0277 | 0.0345 | 0.200 | 1.7 | 1.72 |
| O2 | 0.00924 | 0.0245 | 0.0322 | 0.188 | 1.5 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltiel, J.; Krishnan, S.B.; Gupta, S.; Chakraborty, A.; Hilinski, E.F.; Lin, X. Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol in Ethanol. Molecules 2023, 28, 4086. https://doi.org/10.3390/molecules28104086
Saltiel J, Krishnan SB, Gupta S, Chakraborty A, Hilinski EF, Lin X. Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol in Ethanol. Molecules. 2023; 28(10):4086. https://doi.org/10.3390/molecules28104086
Chicago/Turabian StyleSaltiel, Jack, Sumesh B. Krishnan, Shipra Gupta, Anjan Chakraborty, Edwin F. Hilinski, and Xinsong Lin. 2023. "Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol in Ethanol" Molecules 28, no. 10: 4086. https://doi.org/10.3390/molecules28104086
APA StyleSaltiel, J., Krishnan, S. B., Gupta, S., Chakraborty, A., Hilinski, E. F., & Lin, X. (2023). Photochemistry and Photophysics of Cholesta-5,7,9(11)-trien-3β-ol in Ethanol. Molecules, 28(10), 4086. https://doi.org/10.3390/molecules28104086