Conformational Distributions of Phenyl β-D-Glucopyranoside and Gastrodin in Solution by Vibrational Optical Activity and Theoretical Calculations
Abstract
1. Introduction
2. Results and Discussion
2.1. Low-Energy Conformers of Phenyl β-D-Glucopyranoside and Gastrodin
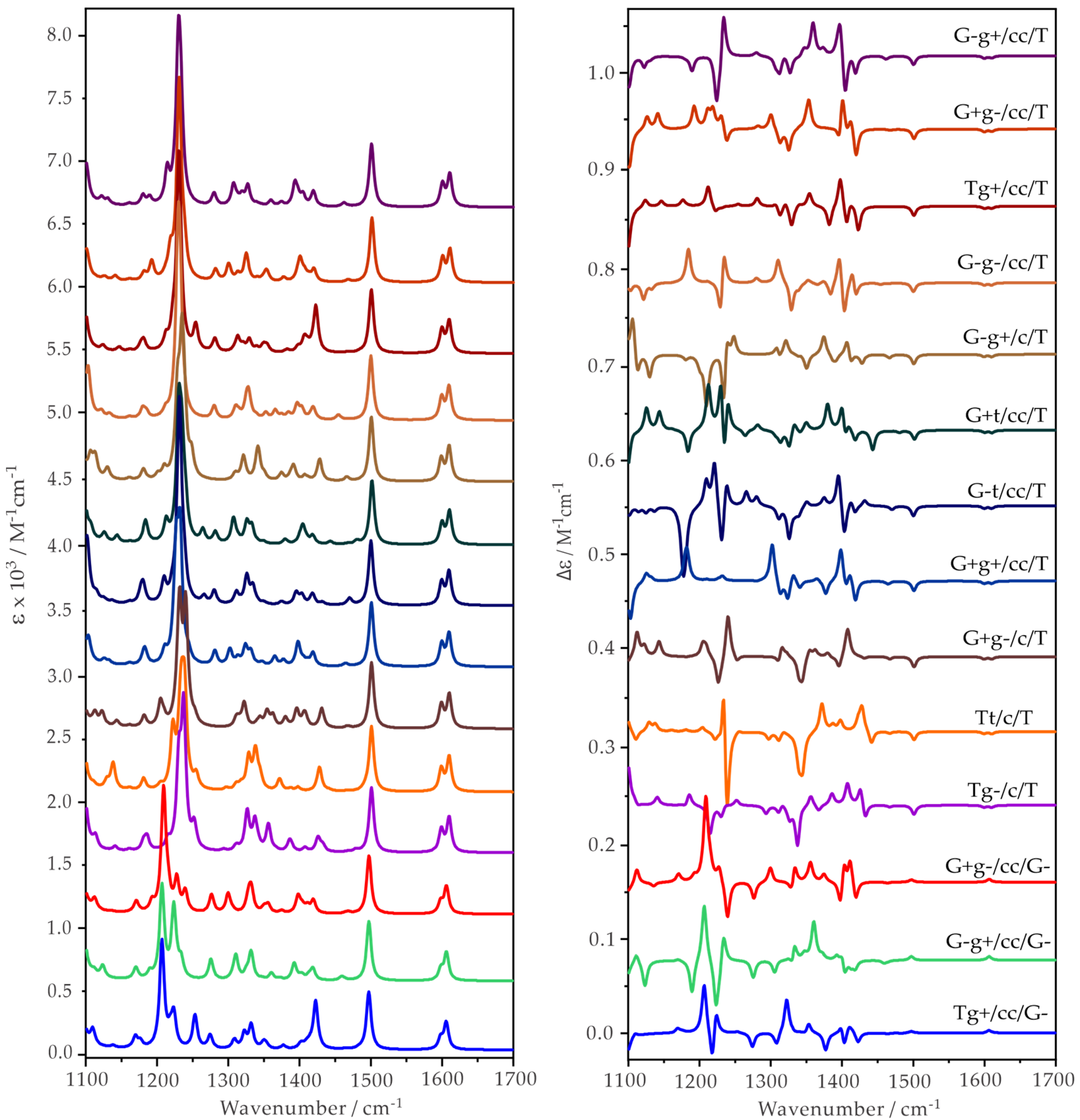
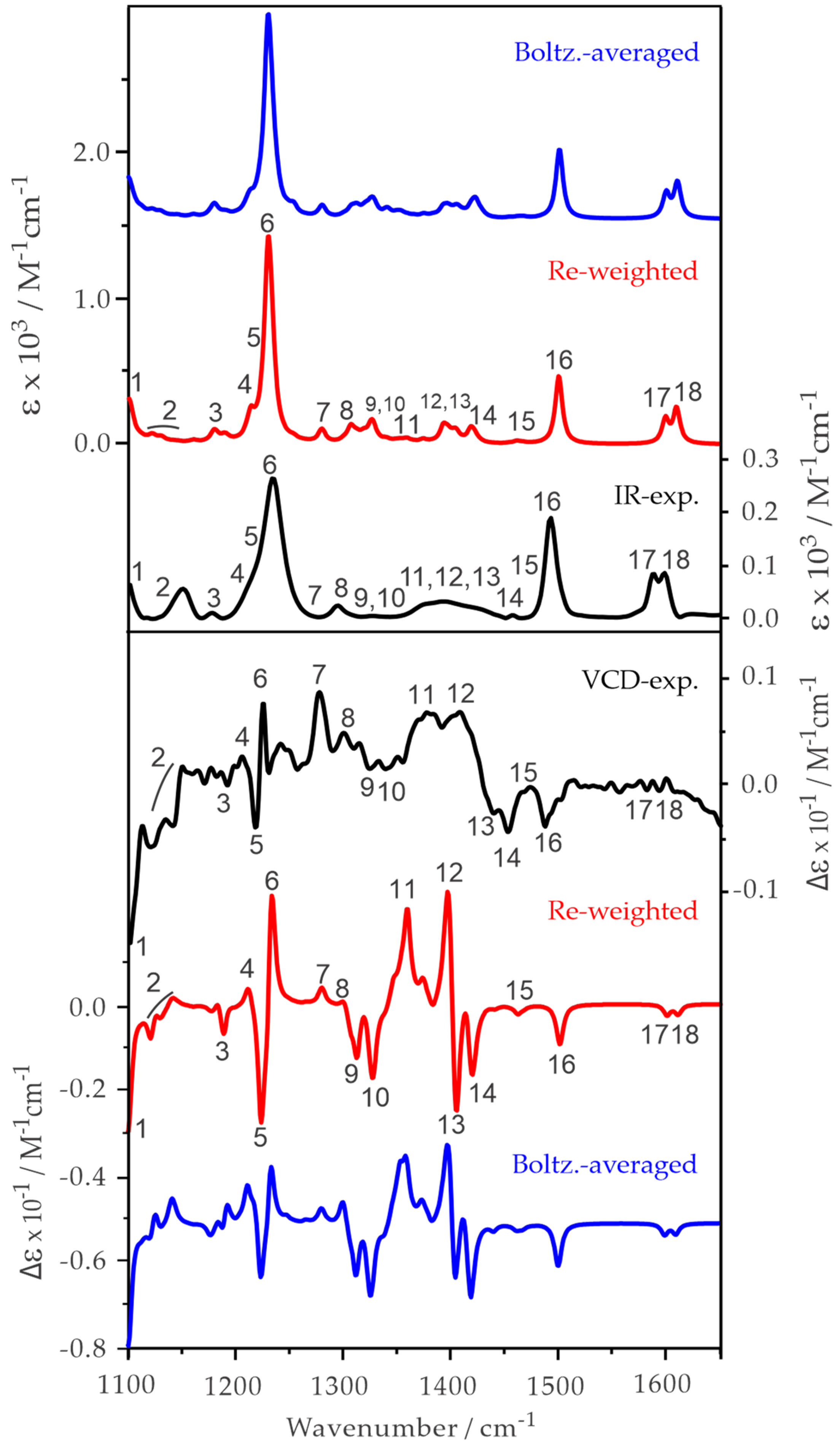
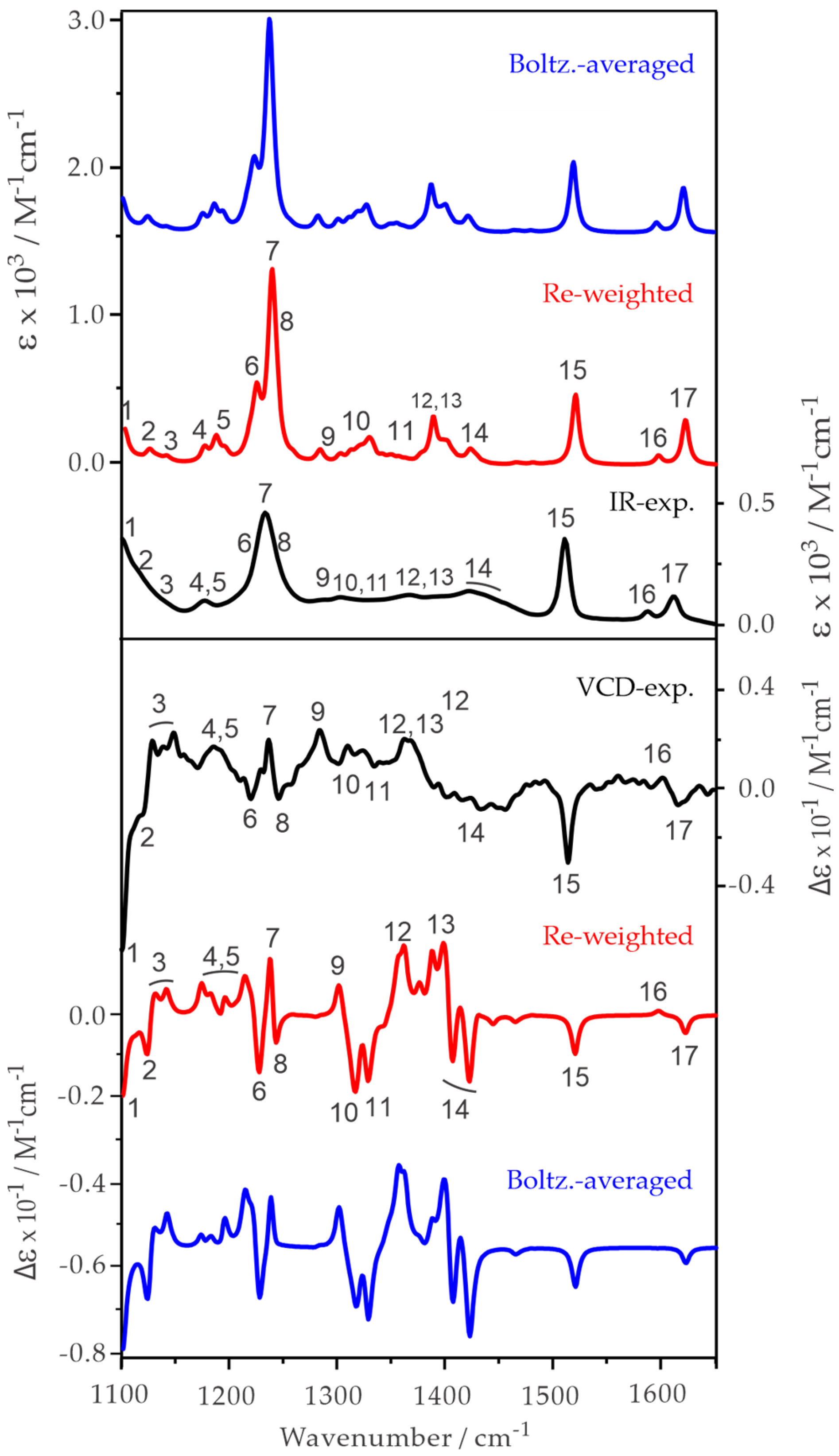
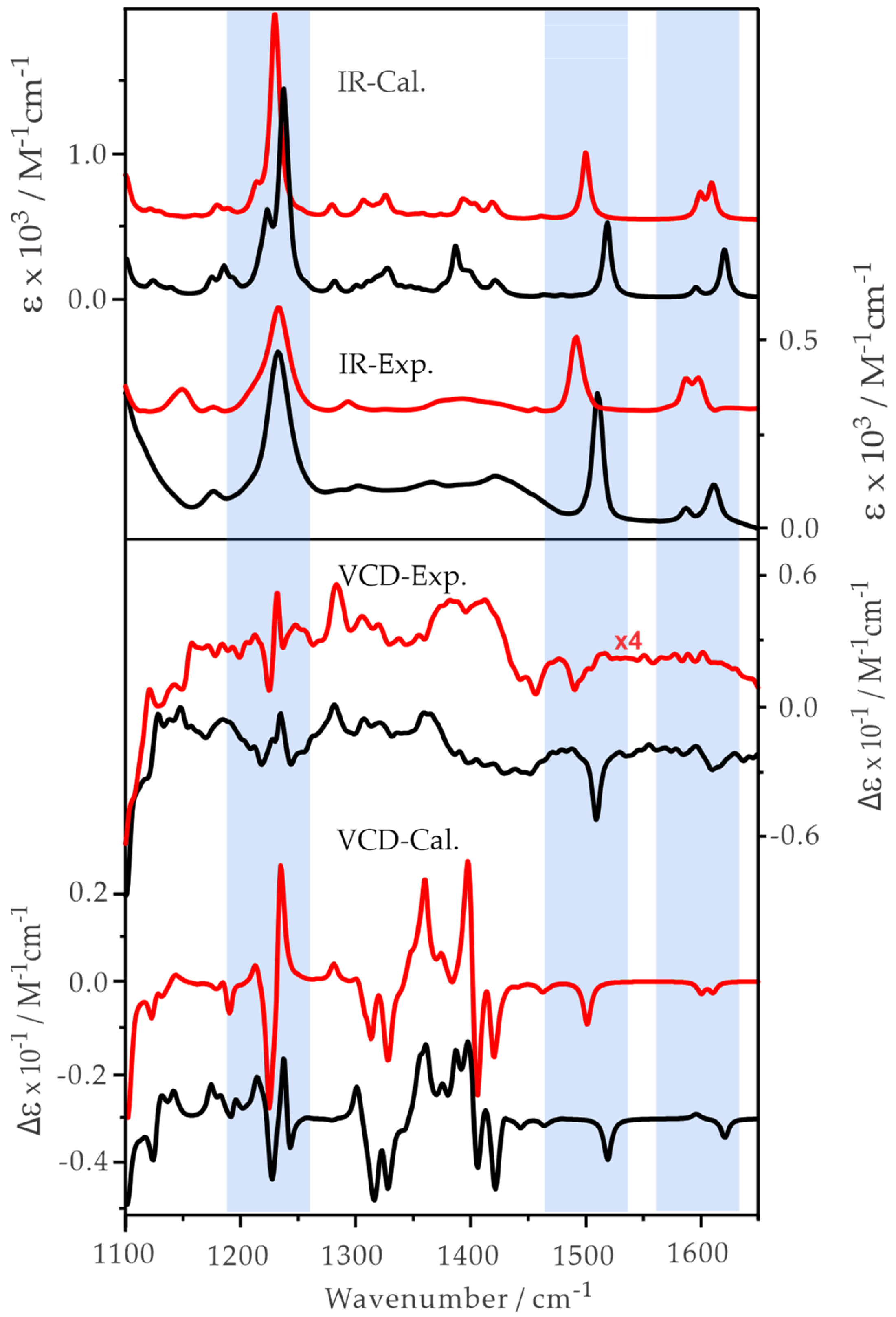
2.2. Experimental and Simulated IR and VCD Spectra of ph-β-Glu and Gastrodin
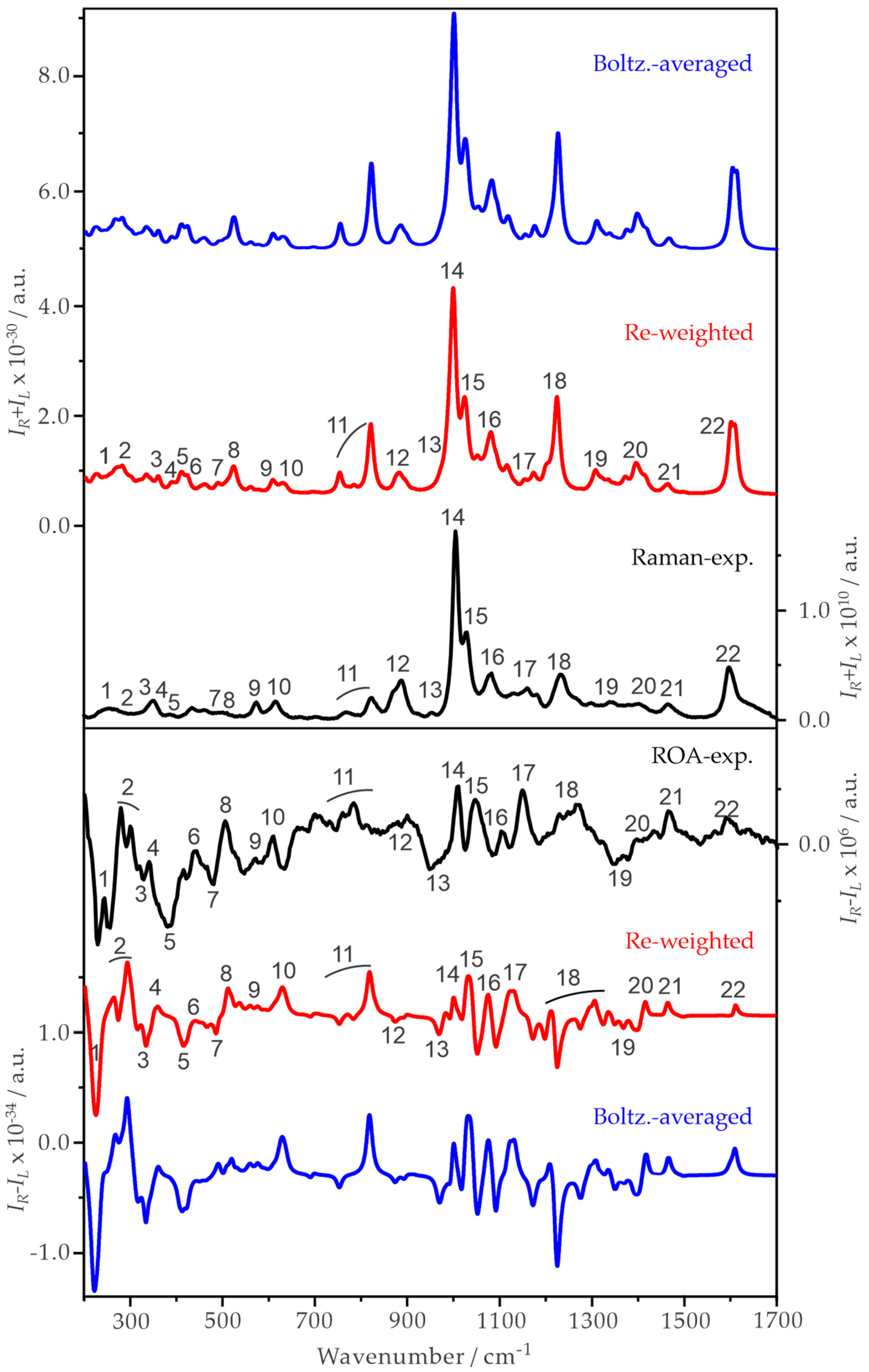
2.3. Experimental and Simulated Raman and ROA Spectra of ph-β-Glu and Gastrodin in Water
2.4. Some General Comments about the Conformational Distribution of ph-β-Glu and Gastrodin in DMSO and in Water
3. Materials and Methods
3.1. Experimental
3.2. Theoretical
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sosicka, P.; Ng, B.G.; Freeze, H.H. Therapeutic monosaccharides: Looking back, moving forward. Biochemistry 2020, 59, 3064–3077. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring carbohydrates for therapeutics: A review on future directions. Front. Pharmacol. 2021, 12, 756724. [Google Scholar] [CrossRef] [PubMed]
- Mayes, H.B.; Broadbelt, L.J.; Beckham, G.T. How sugars pucker: Electronic structure calculations map the kinetic landscape of five biologically paramount monosaccharides and their implications for enzymatic catalysis. J. Am. Chem. Soc. 2014, 136, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Chythra, J.N.; Mallajosyula, S.S. Impact of polarization on the ring puckering dynamics of hexose monosaccharides. J. Chem. Inf. Model. 2023, 63, 208–223. [Google Scholar]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, H.-J. Phenyl-β-d-glucopyranoside exhibits anti-inflammatory activity in lipopolysaccharide-activated RAW 264.7 cells. Inflammation 2015, 38, 1071–1079. [Google Scholar] [CrossRef]
- Hu, M.; Yan, H.; Fu, Y.; Jiang, Y.; Yao, W.; Yu, S.; Zhang, L.; Wu, Q.; Ding, A.; Shan, M. Optimal extraction study of gastrodin-type components from Gastrodia Elata tubers by response surface design with integrated phytochemical and bioactivity evaluation. Molecules 2019, 24, 547. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Lin, B.; Zhang, K. Gastrodin suppresses pyroptosis and exerts neuroprotective effect in traumatic brain injury model by inhibiting NLRP3 inflammasome signaling pathway. J. Integr. Neurosci. 2022, 21, 72. [Google Scholar] [CrossRef]
- Song, Z.; Luo, G.; Han, C.; Jia, G.; Zhang, B. Potential targets and action mechanism of gastrodin in the treatment of attention-deficit/hyperactivity disorder: Bioinformatics and network pharmacology analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 3607053. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Gui, L.; Deng, Y.; Wang, L.; Jiao, J.; Hu, Y.; Lan, X.; Hou, J.; Li, Y.; et al. Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis. Bioact. Mater. 2023, 19, 24–37. [Google Scholar] [CrossRef]
- Talbot, F.O.; Simons, J.P. Sugars in the gas phase: The spectroscopy and structure of jet-cooled phenyl β-D-glucopyranoside. Phys. Chem. Chem. Phys. 2002, 4, 3562–3565. [Google Scholar] [CrossRef]
- Simons, J.P.; Davis, B.G.; Cocinero, E.J.; Gamblin, D.P.; Stanca-Kaposta, E.C. Conformational change and selectivity in explicitly hydrated carbohydrates. Tetrahedron Asymmetry 2009, 20, 718–722. [Google Scholar] [CrossRef]
- Monde, K.; Taniguchi, T.; Miura, N.; Nishimura, S.I. Specific band observed in VCD predicts the anomeric configuration of carbohydrates. J. Am. Chem. Soc. 2004, 126, 9496–9497. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, G.; Poopari, M.R.; Bie, Z.; Xu, Y. Conformations of serine in aqueous solutions as revealed by vibrational circular dichroism. ChemPhysChem 2012, 13, 1272–1281. [Google Scholar] [CrossRef]
- Dobrowolski, J.; Lipinski, P.; Rode, J.; Sadlej, I. α-Amino Acids in Water: A Review of VCD and ROA Spectra. In Optical Spectroscopy and Computational Methods in Biology and Medicine: Challenges and Advances in Computational Chemistry and Physics; Baranska, M., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 14. [Google Scholar] [CrossRef]
- Merten, C.; Li, F.; Bravo-Rodriguez, K.; Sanchez-Garcia, E.; Xu, Y.; Sander, W. Solvent-induced conformational changes in cyclic peptides: A vibrational circular dichroism study. Phys. Chem. Chem. Phys. 2014, 16, 5627–5633. [Google Scholar] [CrossRef]
- Kessler, J.; Andrushchenko, V.; Kapitán, J.; Bouř, P. Insight into vibrational circular dichroism of proteins by density functional modeling. Phys. Chem. Chem. Phys. 2018, 20, 4926–4935. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Shaik, M.S.; Popelier, P.L.A.; Blanch, E.W. Calculation of Raman optical activity spectra of methyl-β-D-glucose incorporating a full molecular dynamics simulation of hydration effects. J. Am. Chem. Soc. 2011, 133, 4991–4997. [Google Scholar] [CrossRef]
- Palivec, V.; Kopecký, V., Jr.; Jungwirth, P.; Bouř, P.; Kaminský, J.; Martinez-Seara, H. Simulation of Raman and Raman optical activity of saccharides in solution. Phys. Chem. Chem. Phys. 2020, 22, 1983–1993. [Google Scholar] [CrossRef]
- Nafie, L.A. Vibrational Optical Activity: Principles and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Barron, L.D. Molecular Light Scattering and Optical Activity, 2nd ed.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Li, G.; Alshalalfeh, M.; Kapitán, J.; Bouř, P.; Xu, Y. Electronic circular dichroism-circularly polarized Raman (eCP-Raman): A new form of chiral Raman spectroscopy. Chem. Eur. J. 2022, 28, e202104302. [Google Scholar]
- Wu, T.; Li, G.; Kapitán, J.; Kessler, J.; Xu, Y.; Bouř, P. Two spectroscopies in one: Interference of circular dichroism and Raman optical activity. Angew. Chem. Int. Ed. 2020, 59, 21895–21898. [Google Scholar] [CrossRef]
- Machalska, E.; Zajac, G.; Rode, J.E. Chirality transfer observed in Raman optical activity spectra. Spectrochim. Acta A Mol. Biomol. 2022, 281, 121604. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Seifert, N.A.; Heger, M.; Thomas, J.; Jäger, W.; Xu, Y. The rich conformational landscape of perillyl alcohol revealed by broadband rotational spectroscopy and theoretical modelling. Phys. Chem. Chem. Phys. 2019, 21, 15408–15416. [Google Scholar] [CrossRef] [PubMed]
- Seifert, N.A.; Thomas, J.; Jäger, W.; Xu, Y. Rotational spectra and theoretical study of tetramers and trimers of 2-fluoroethanol: Dramatic intermolecular compensation for intramolecular instability. Phys. Chem. Chem. Phys. 2018, 20, 27630–27637. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S.; Seifert, N.A.; Bohle, F.; Gawrilow, M.; Grimme, S.; Jäger, W.; Xu, Y.; Suhm, M.A. The chiral trimer and a metastable chiral dimer of achiral hexafluoroisopropanol: A multi-messenger study. Angew. Chem. Int. Ed. 2019, 58, 5080–5084. [Google Scholar] [CrossRef]
- Xie, F.; Fusè, M.; Hazrah, A.S.; Jäger, W.; Barone, V.; Xu, Y. Discovering the elusive global minimum in a ternary chiral cluster: Rotational spectra of propylene oxide trimer. Angew. Chem. Int. Ed. 2020, 59, 22427–22430. [Google Scholar] [CrossRef]
- Yang, Y.; Krin, A.; Cai, X.; Poopari, M.R.; Zhang, Y.; Cheeseman, J.R.; Xu, Y. Conformations of steroid hormones: Infrared and vibrational circular dichroism spectroscopy. Molecules 2023, 28, 771. [Google Scholar] [CrossRef]
- Eikås, K.D.R.; Beerepoot, M.T.P.; Ruud, K. A computational protocol for vibrational circular dichroism spectra of cyclic oligopeptides. J. Phys. Chem. A 2022, 126, 5458–5471. [Google Scholar] [CrossRef]
- Alonso, J.L.; Lozoya, M.; Peña, I.; Lόpez, J.C.; Cabezas, C.; Mata, S.; Blanco, S. The conformational behaviour of free D-glucose—At last. Chem. Sci. 2014, 5, 515–522. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Case, D.A. Generalized Born implicit solvent models for biomolecules. Annu. Rev. Biophys. 2019, 48, 275–296. [Google Scholar] [CrossRef]
- Wang, H.; Heger, M.; Al-Jabiri, M.H.; Xu, Y. Vibrational spectroscopy of homo- and heterochiral amino acid dimers: Conformational landscapes. Molecules 2022, 27, 38. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange–energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised damping parameters for the D3 dispersion correction to density functional theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, A.D. A density-functional model of the dispersion interaction. J. Chem. Phys. 2005, 123, 154101. [Google Scholar] [CrossRef]
- Xu, B.; Unione, L.; Sardinha, J.; Wu, S.; Ethève-Quelquejeu, M.; Rauter, A.P.; Blériot, Y.; Zhang, Y.; Martín-Santamaría, S.; Díaz, D.; et al. Gem-difluorocarbadisaccharides: Restoring the exo-anomeric effect. Angew. Chem. Int. Ed. 2014, 53, 9597–9602. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Krivoshchapov, N.V.; Mehaffy, P.; Medvedev, M.G. Anomeric effect, hyperconjugation and electrostatics: Lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 2021, 50, 10212–10252. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Pavia, A.A.; Martin, J.C.; Watanabe, K.A. Solvation effects on conformational equilibria. Studies related to th conformational properties of 2-methoxytetrahydropyran and related methyl glycopyranosides. Can. J. Chem. 1969, 47, 4427–4439. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Marquez, M. The energy components of the anomeric effect for 2-methoxytetrahydropyran. an experimental comparison of the gas phase and solutions. J. Am. Chem. Soc. 1994, 116, 2197–2198. [Google Scholar] [CrossRef]
- Perera, A.S.; Carlson, C.D.; Cheramy, J.; Xu, Y. IR and VCD spectra of methyl-β-D-glucopyranose in water: The application of the quantum cluster growth and clusters-in-a-liquid solvation models. Chirality 2023. [Google Scholar] [CrossRef]
- Spicher, S.; Plett, C.; Pracht, P.; Hansen, A.; Grimme, S. Automated molecular cluster growing for explicit solvation by efficient force field and tight binding methods. J. Chem. Theory Comput. 2022, 18, 3174–3189. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Kirchner, B. Classical magnetic dipole moments for the simulation of vibrational circular dichroism by ab Initio molecular dynamics. J. Phys. Chem. Lett. 2016, 7, 509–513. [Google Scholar] [CrossRef]
- Jähnigen, S.; Sebastiani, D.; Vuilleumier, R. The important role of non-covalent interactions for the vibrational circular dichroism of lactic acid in aqueous solution. Phys. Chem. Chem. Phys. 2021, 23, 17232–17241. [Google Scholar] [CrossRef]
- Brehm, M.; Thomas, M. Computing bulk phase Raman optical activity spectra from ab initio molecular dynamics simulations. J. Phys. Chem. Lett. 2017, 8, 3409–3414. [Google Scholar] [CrossRef]
- Yang, Y.; Cheramy, J.; Brehm, M.; Xu, Y. Raman optical activity of N-acetyl-L-cysteine in water and in methanol: The “clusters-in-a-liquid” model and ab initio molecular dynamics simulations. ChemPhysChem 2022, 23, e202200161. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general-purpose quantum chemistry program package. Wires Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.03; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Mennucci, B.; Tomasi, J.; Cammi, R.; Cheeseman, J.R.; Frisch, M.J.; Devlin, F.J.; Gabriel, S.; Stephens, P.J. Polarizable continuum model (PCM) calculations of solvent effects on optical rotations of chiral molecules. J. Phys. Chem. A 2002, 106, 6102–6113. [Google Scholar] [CrossRef]
- Katari, M.; Nicol, E.; Steinmetz, V.; van der Rest, G.; Carmichael, D.; Frison, G. Improved infrared spectra prediction by DFT from a new experimental database. Chem. Eur. J. 2017, 23, 8414–8423. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Alshalalfeh, M.; Cheramy, J.; Zhang, H.; Xu, Y. Stereochemical properties of two Schiff-base transition metal complexes and their ligand by using multiple chiroptical spectroscopic tools and DFT calculations. Molecules 2023, 28, 2571. [Google Scholar] [CrossRef]
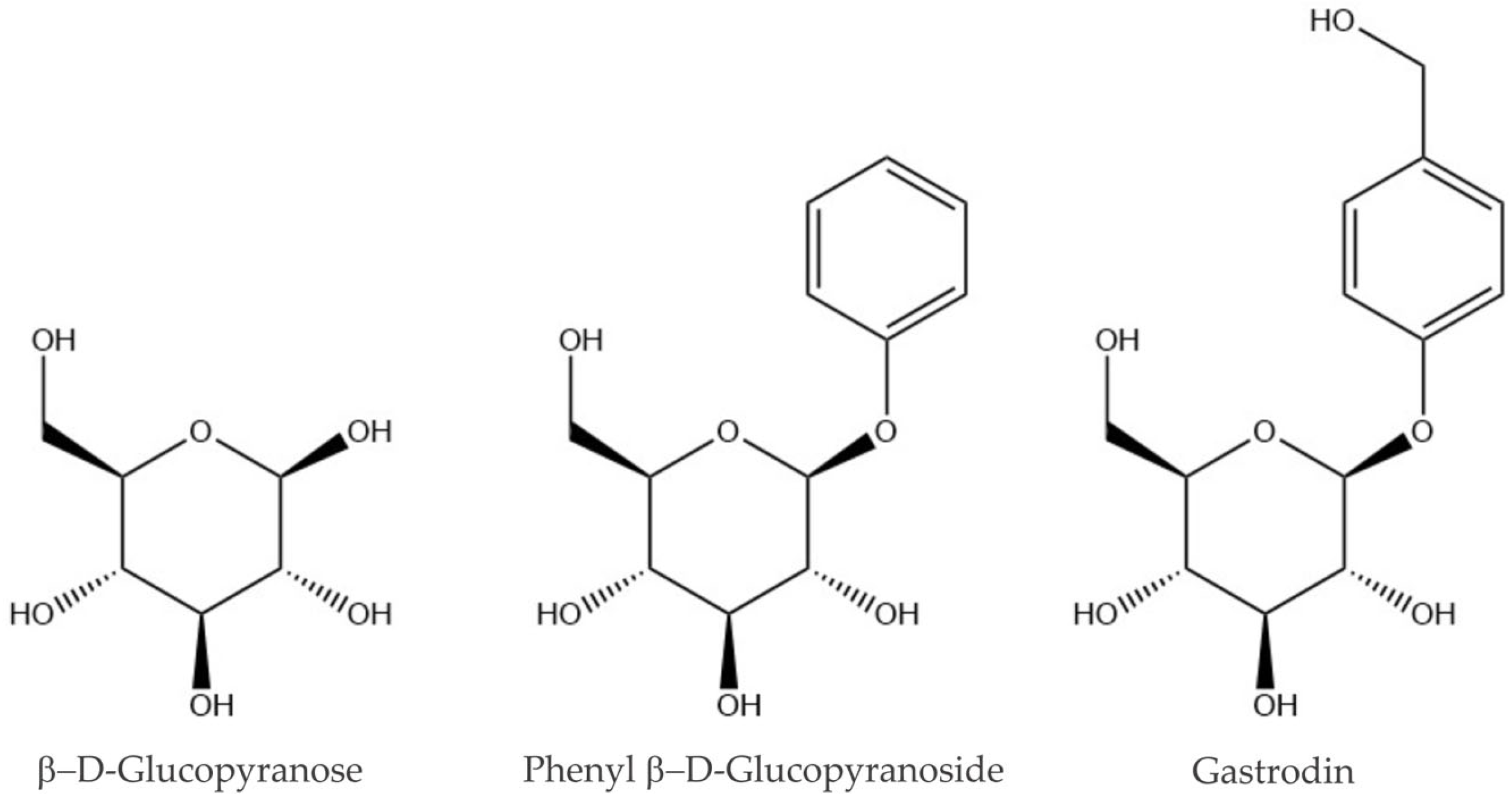
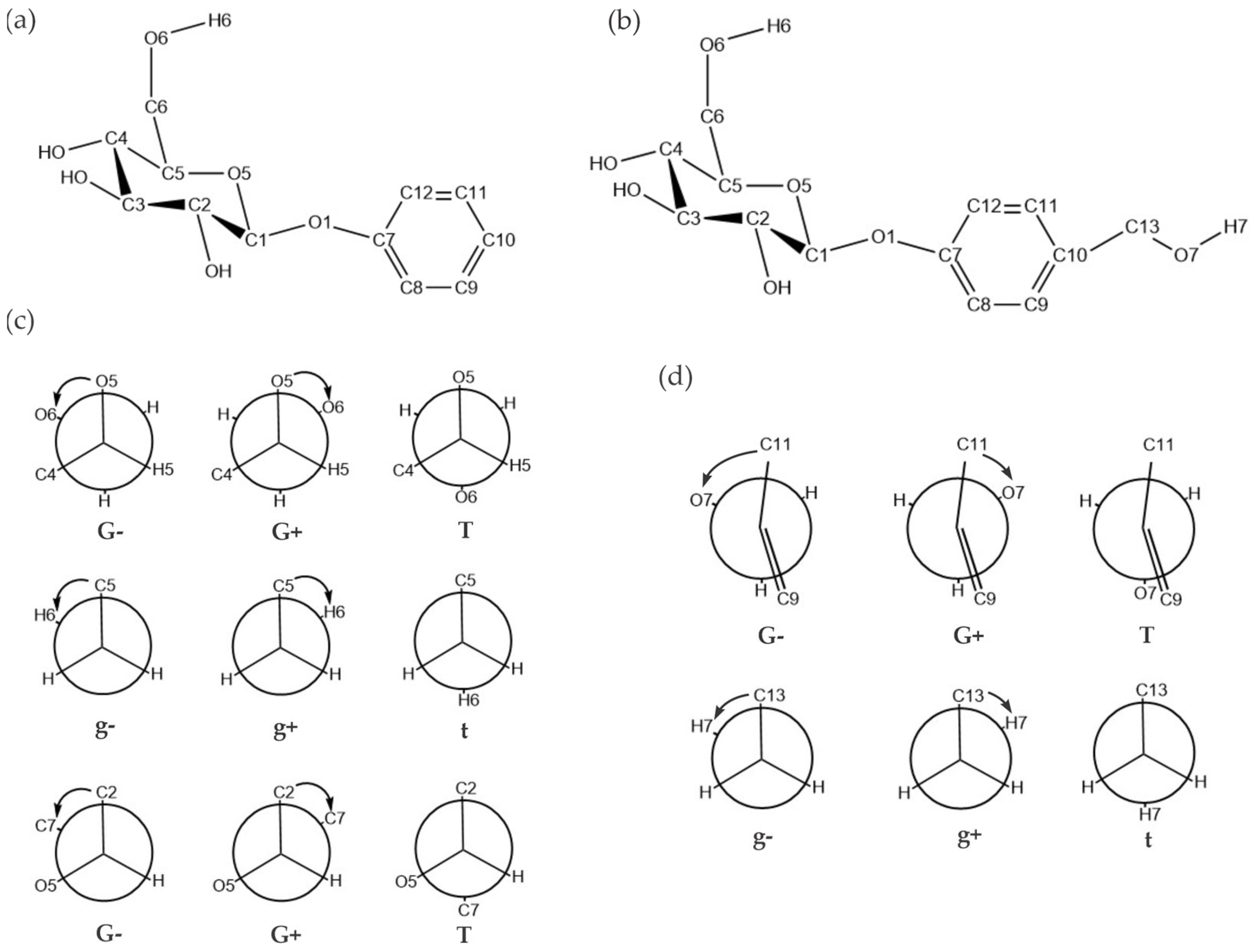
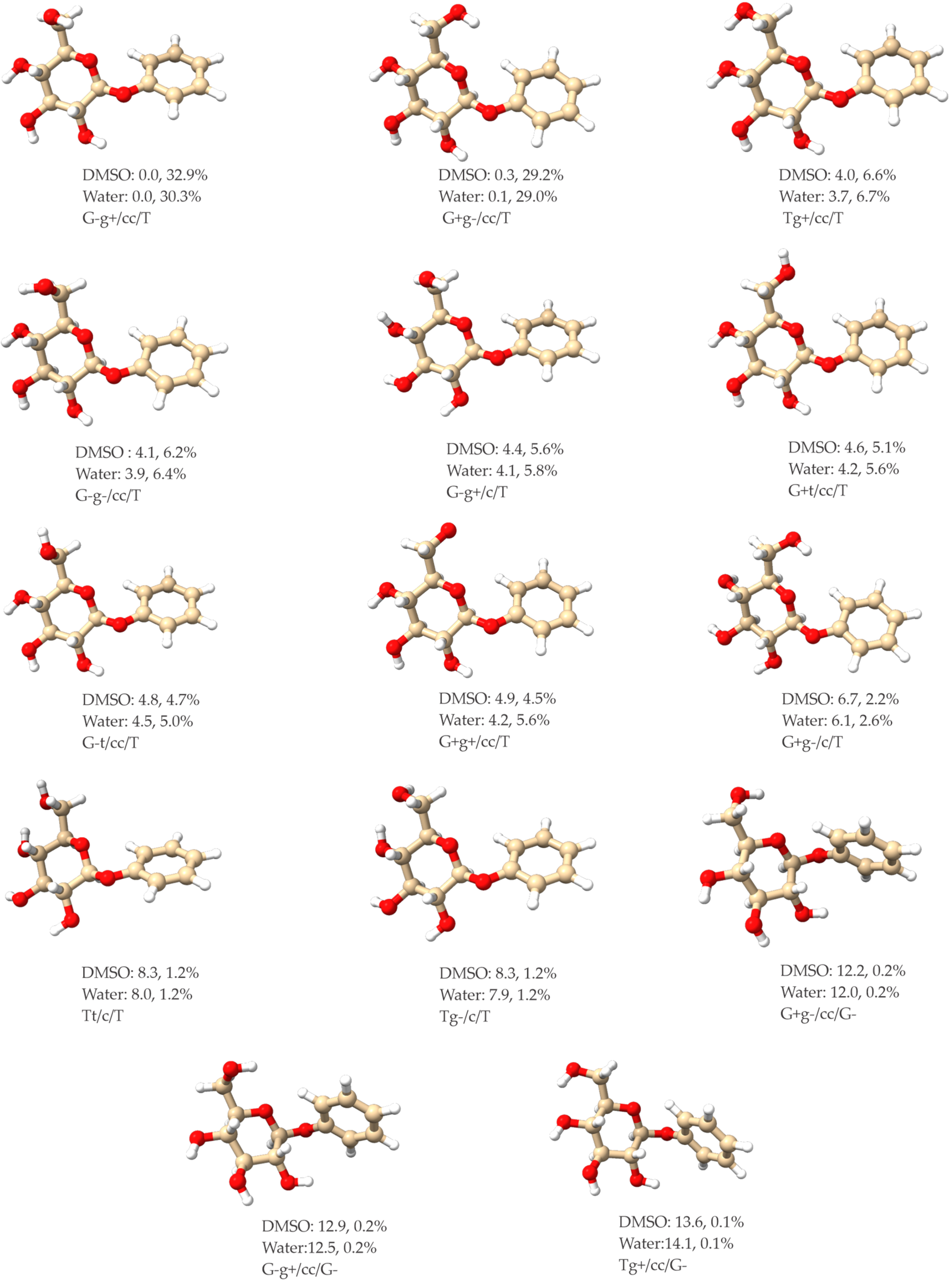
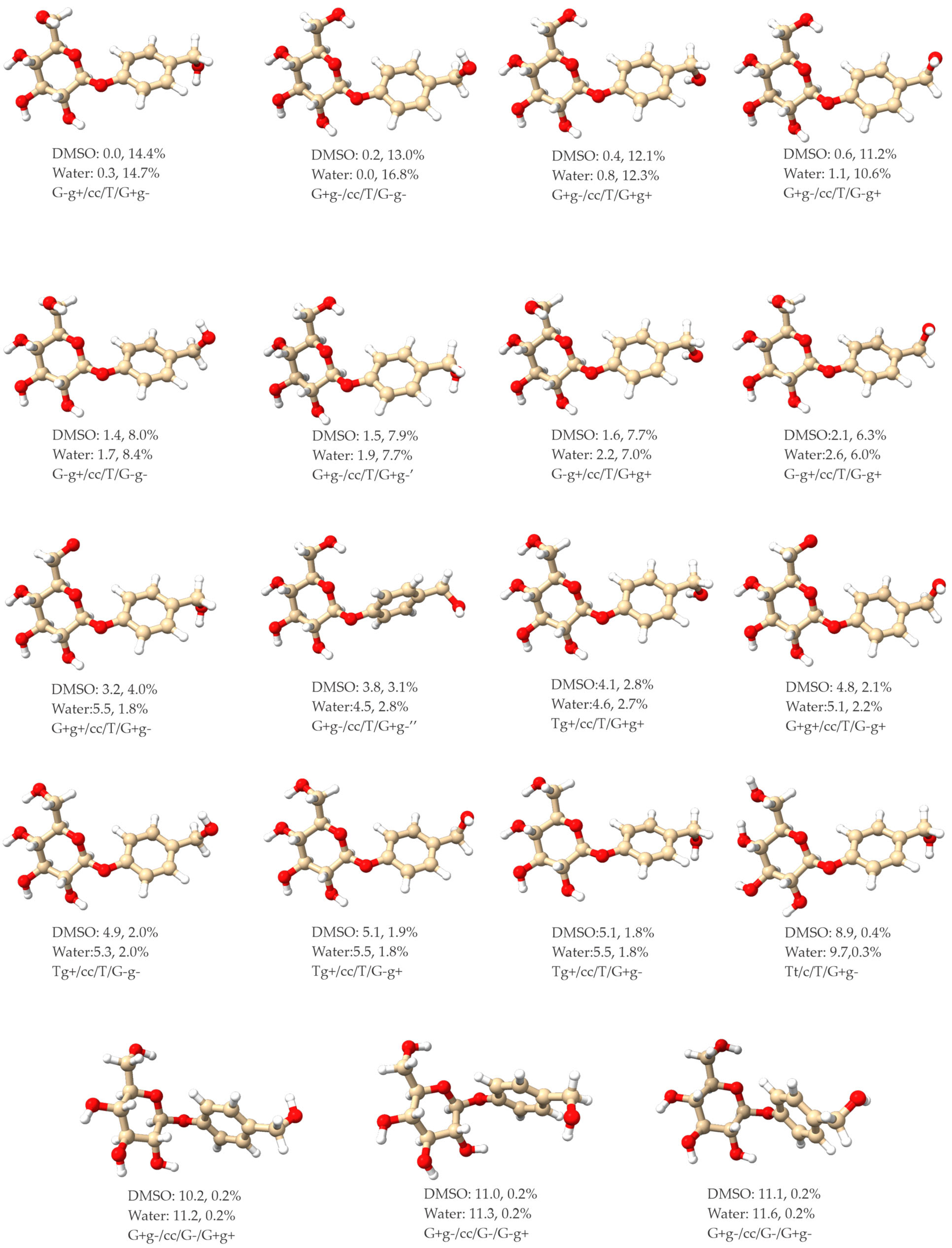

| Structural Parameters | G-g+/cc/T | G+g-/cc/T | Tg+/cc/T | G-g-/cc/T |
|---|---|---|---|---|
| r(H2-O1)/Å | 2.583 | 2.590 | 2.583 | 2.582 |
| r(H3-O2)/Å | 2.528 | 2.540 | 2.551 | 2.542 |
| r(H4-O3)/Å | 2.426 | 2.420 | 2.471 | 2.419 |
| r(H6-O4/O5)/Å | 2.423 | 2.407 | 1.994 | 3.060 |
| θ(C2-C1-O1-C7)/° | 160.1 | 161.2 | 160.0 | 160.1 |
| θ(C3-C2-O2-H2)/° | −172.4 | −172.3 | −172.2 | −172.5 |
| θ(C4-C3-O3-H3)/° | 176.8 | 175.8 | 176.3 | 176.6 |
| θ(C5-C4-O4-H4)/° | 173.3 | 171.9 | 179.3 | 172.5 |
| θ(O5-C5-C6-O6)/° | −60.2 | 61.6 | 169.7 | −66.5 |
| θ(C5-C6-O6-H6)/° | 57.2 | −56.6 | 48.2 | −82.1 |
| θ(C1-O1-C7-C12)/° | 14.8 | 12.6 | 14.8 | 14.4 |
| Structural Parameters | G-g+/cc/T/G+g- | G+g-/cc/T/G-g- | G+g-/cc/T/G+g+ | G+g-/cc/T/G-g+ |
|---|---|---|---|---|
| r(H2-O1)/Å | 2.596 | 2.595 | 2.596 | 2.593 |
| r(H3-O2)/Å | 2.531 | 2.529 | 2.532 | 2.531 |
| r(H4-O3)/Å | 2.409 | 2.412 | 2.409 | 2.411 |
| r(H6-O5)/Å | 2.405 | 2.400 | 2.403 | 2.401 |
| r(H12-O5)/Å | 2.553 | 2.558 | 2.556 | 2.550 |
| θ(C2-C1-O1-C7)/° | 161.6 | 161.5 | 161.6 | 161.1 |
| θ(C3-C2-O2-H2)/° | −171.6 | −171.6 | −171.6 | −171.7 |
| θ(C4-C3-O3-H3)/° | 176.2 | 176.4 | 176.2 | 176.2 |
| θ(C5-C4-O4-H4)/° | 171.2 | 171.4 | 171.2 | 171.4 |
| θ(O5-C5-C6-O6)/° | −60.2 | 61.4 | 61.4 | 61.4 |
| θ(C5-C6-O6-H6)/° | 57.4 | −57.1 | −57.3 | −57.2 |
| θ(C1-O1-C7-C12)/° | 12.5 | 12.7 | 12.5 | 13.3 |
| θ(C9-C10-C13-O7)/° | 101.2 | −73.4 | 74.6 | −100.9 |
| θ(C10-C13-O7-H7)/° | −56.4 | −56.4 | 56.4 | 56.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshalalfeh, M.; Sun, N.; Moraes, A.H.; Utani, A.P.A.; Xu, Y. Conformational Distributions of Phenyl β-D-Glucopyranoside and Gastrodin in Solution by Vibrational Optical Activity and Theoretical Calculations. Molecules 2023, 28, 4013. https://doi.org/10.3390/molecules28104013
Alshalalfeh M, Sun N, Moraes AH, Utani APA, Xu Y. Conformational Distributions of Phenyl β-D-Glucopyranoside and Gastrodin in Solution by Vibrational Optical Activity and Theoretical Calculations. Molecules. 2023; 28(10):4013. https://doi.org/10.3390/molecules28104013
Chicago/Turabian StyleAlshalalfeh, Mutasem, Ningjie Sun, Amanda Hanashiro Moraes, Alexandra Paola Aponte Utani, and Yunjie Xu. 2023. "Conformational Distributions of Phenyl β-D-Glucopyranoside and Gastrodin in Solution by Vibrational Optical Activity and Theoretical Calculations" Molecules 28, no. 10: 4013. https://doi.org/10.3390/molecules28104013
APA StyleAlshalalfeh, M., Sun, N., Moraes, A. H., Utani, A. P. A., & Xu, Y. (2023). Conformational Distributions of Phenyl β-D-Glucopyranoside and Gastrodin in Solution by Vibrational Optical Activity and Theoretical Calculations. Molecules, 28(10), 4013. https://doi.org/10.3390/molecules28104013





