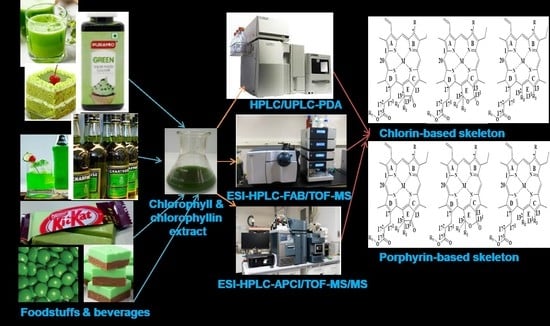
Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods
Abstract
1. Introduction
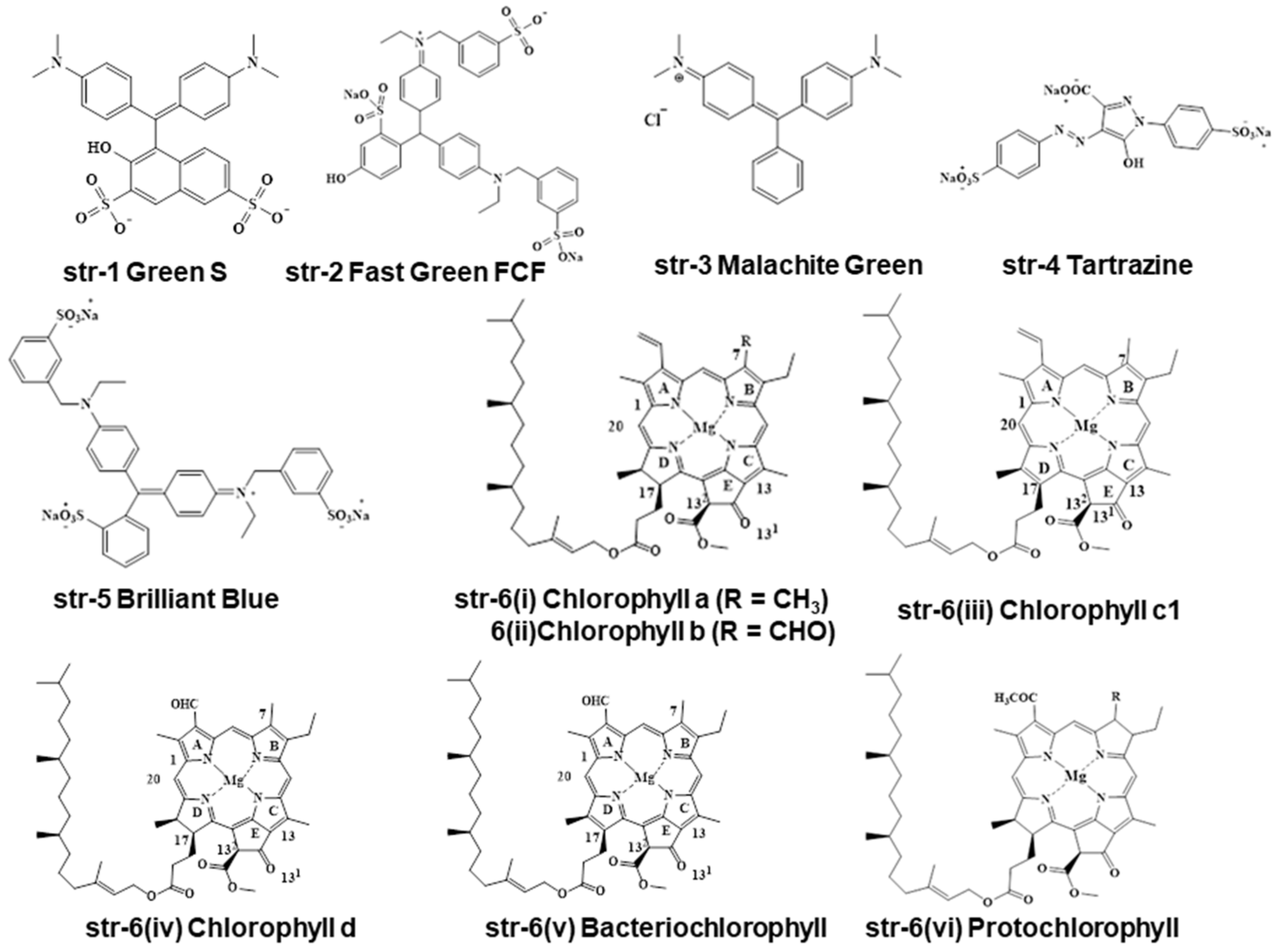
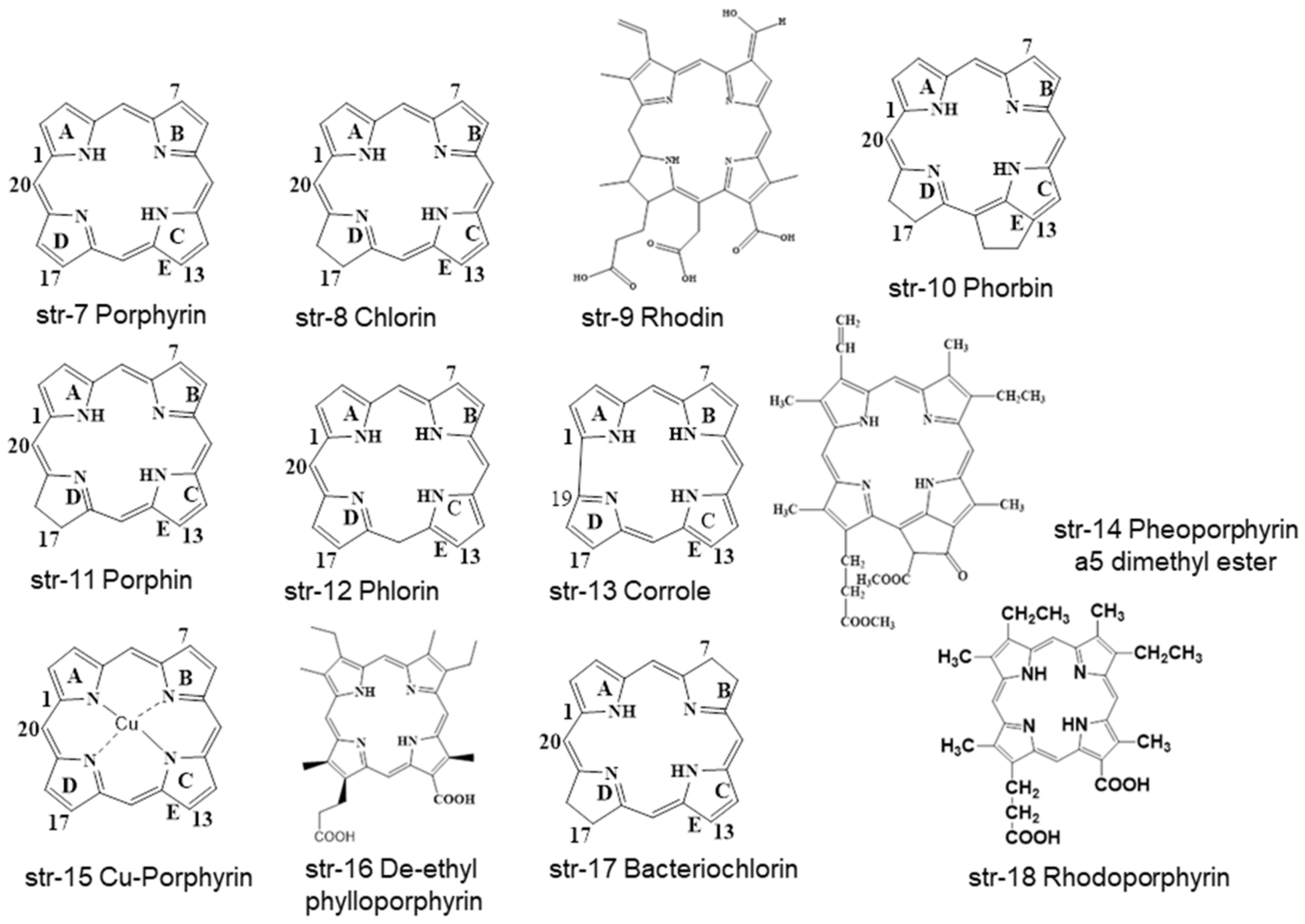
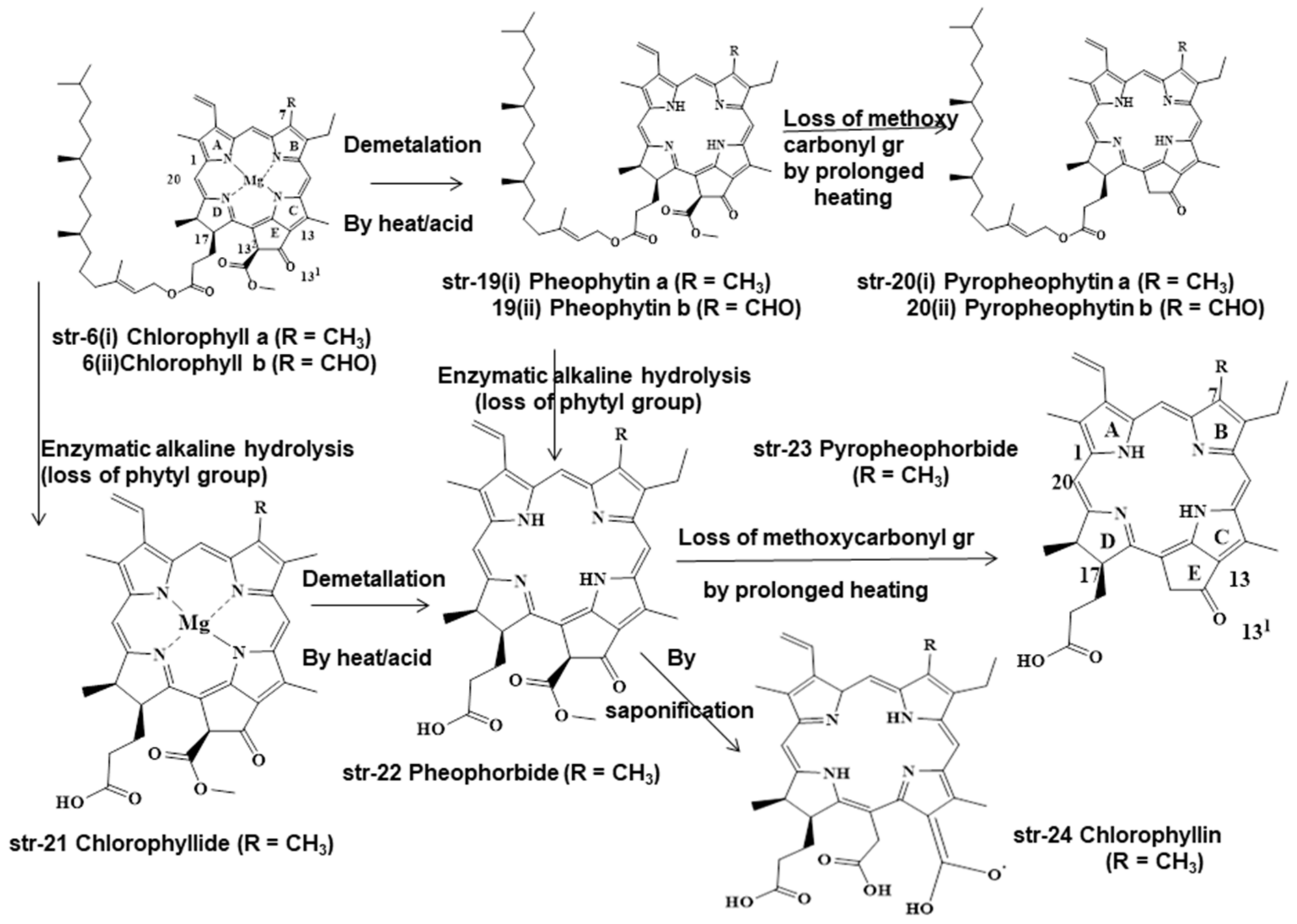
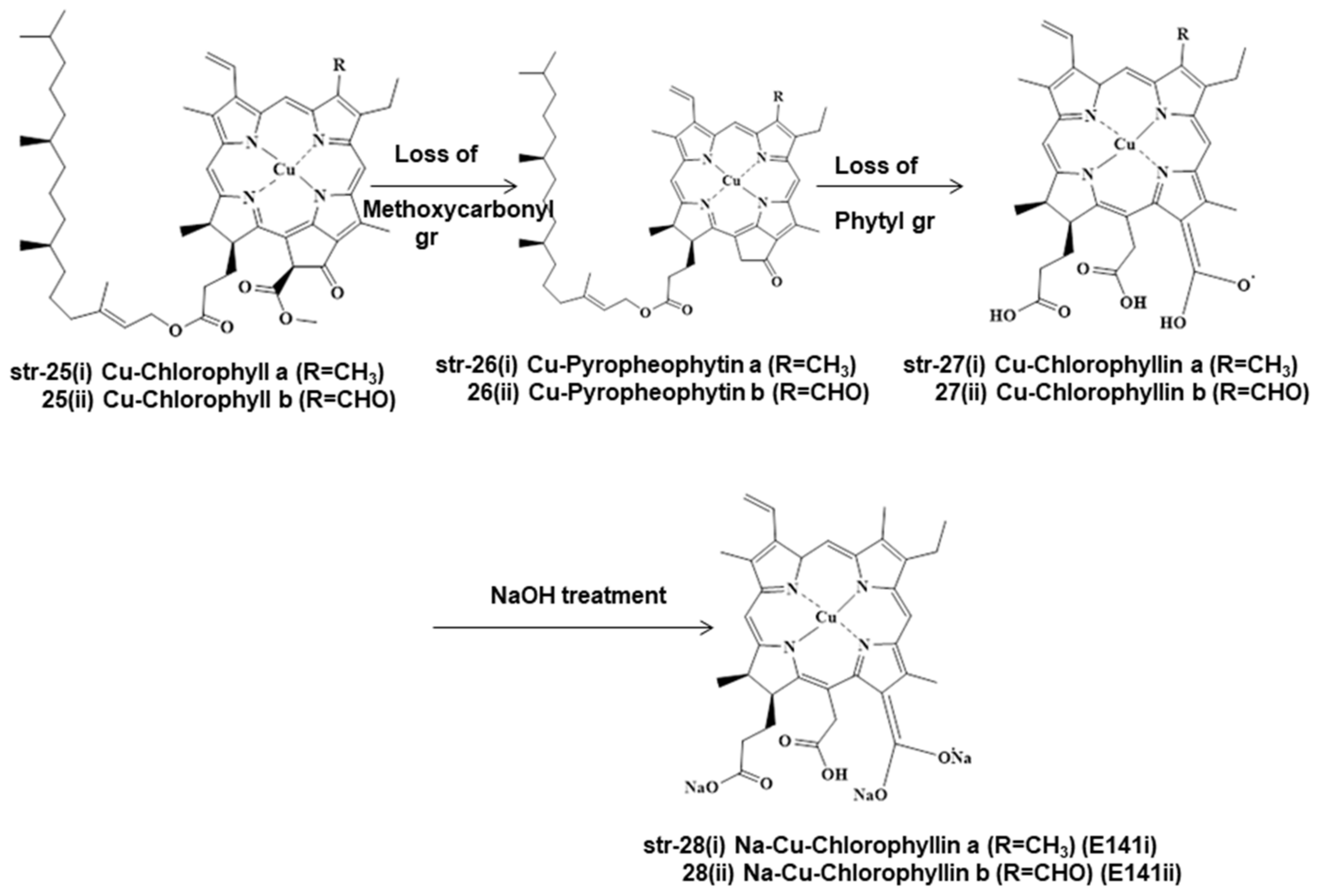
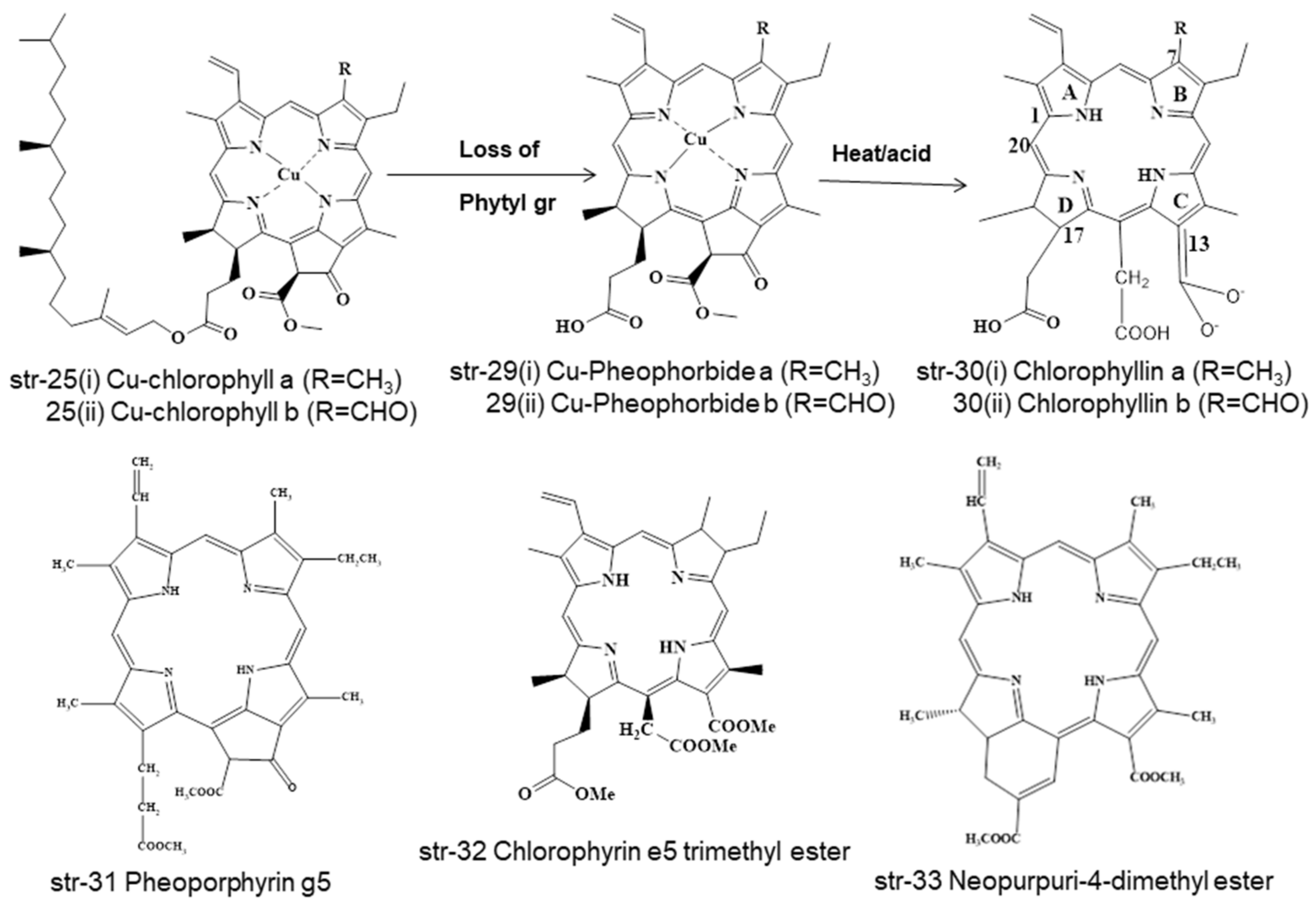
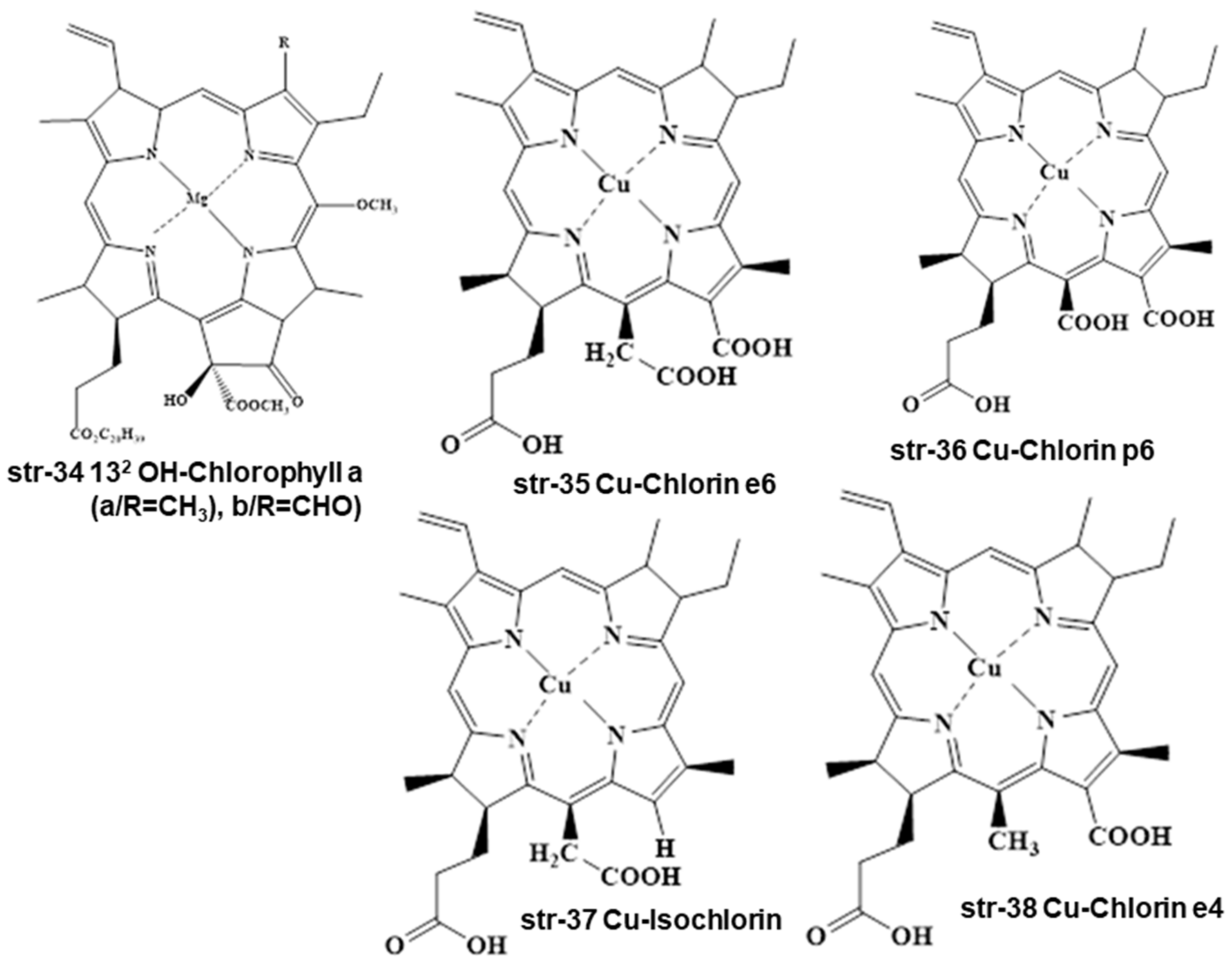
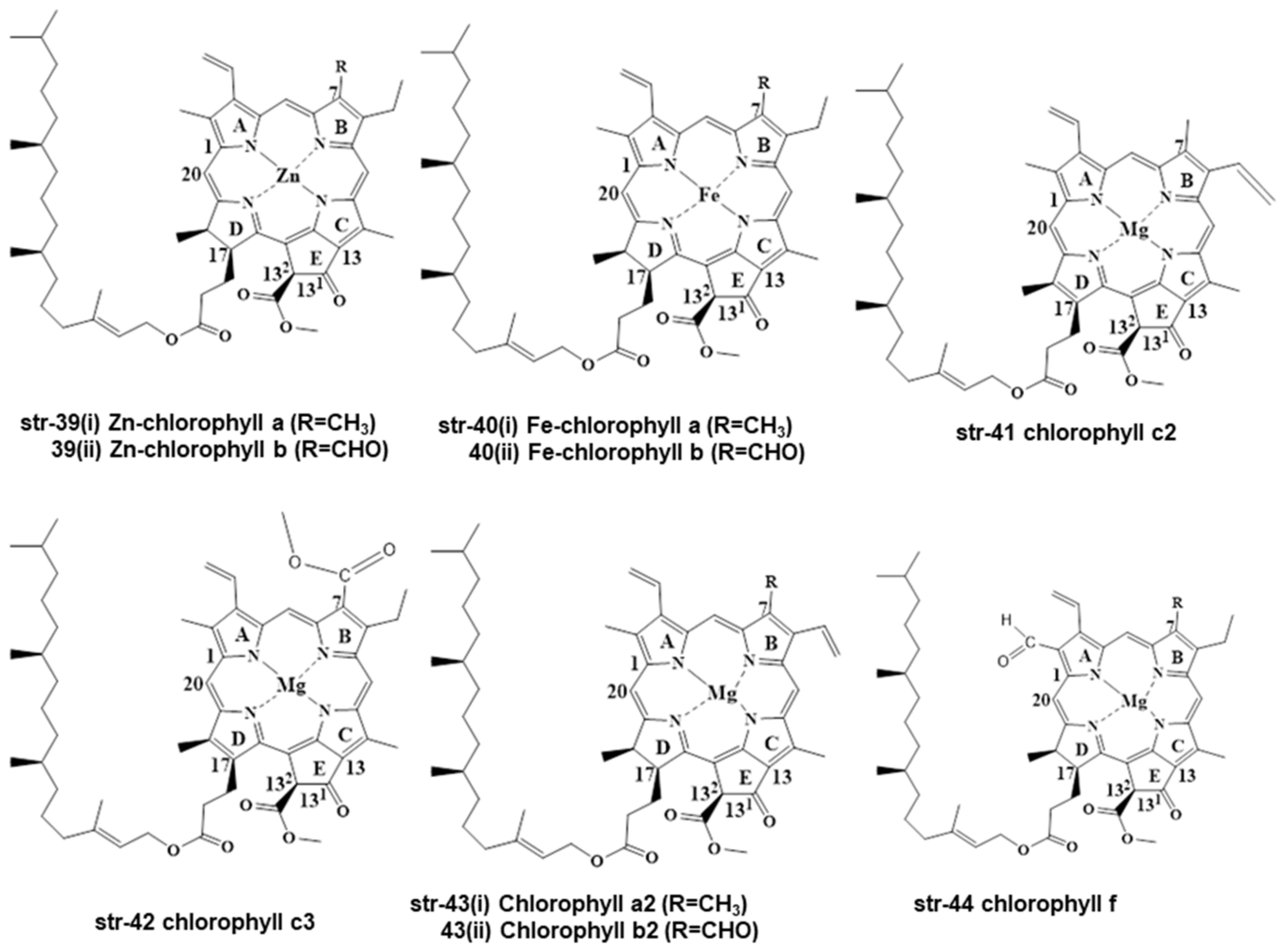
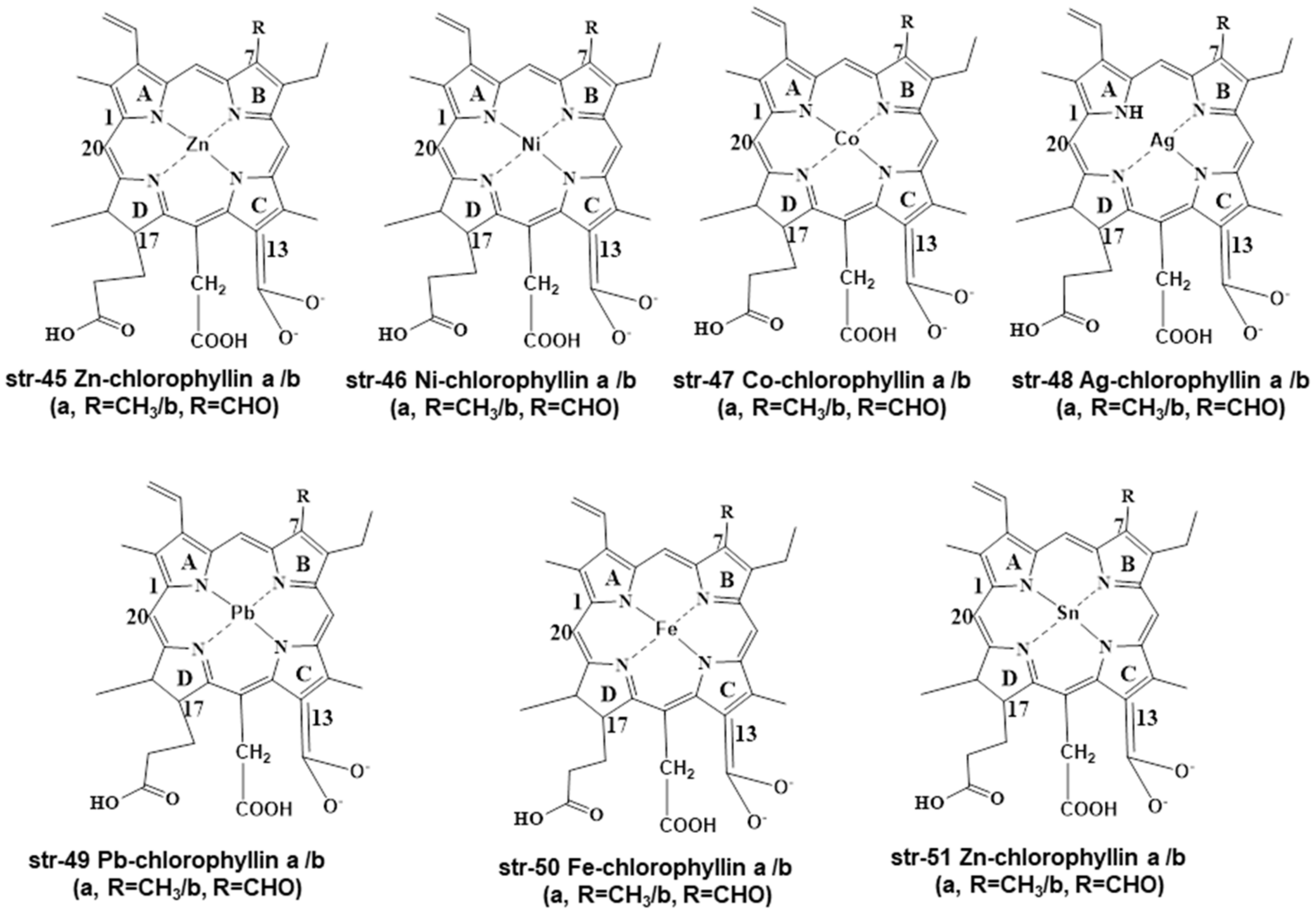
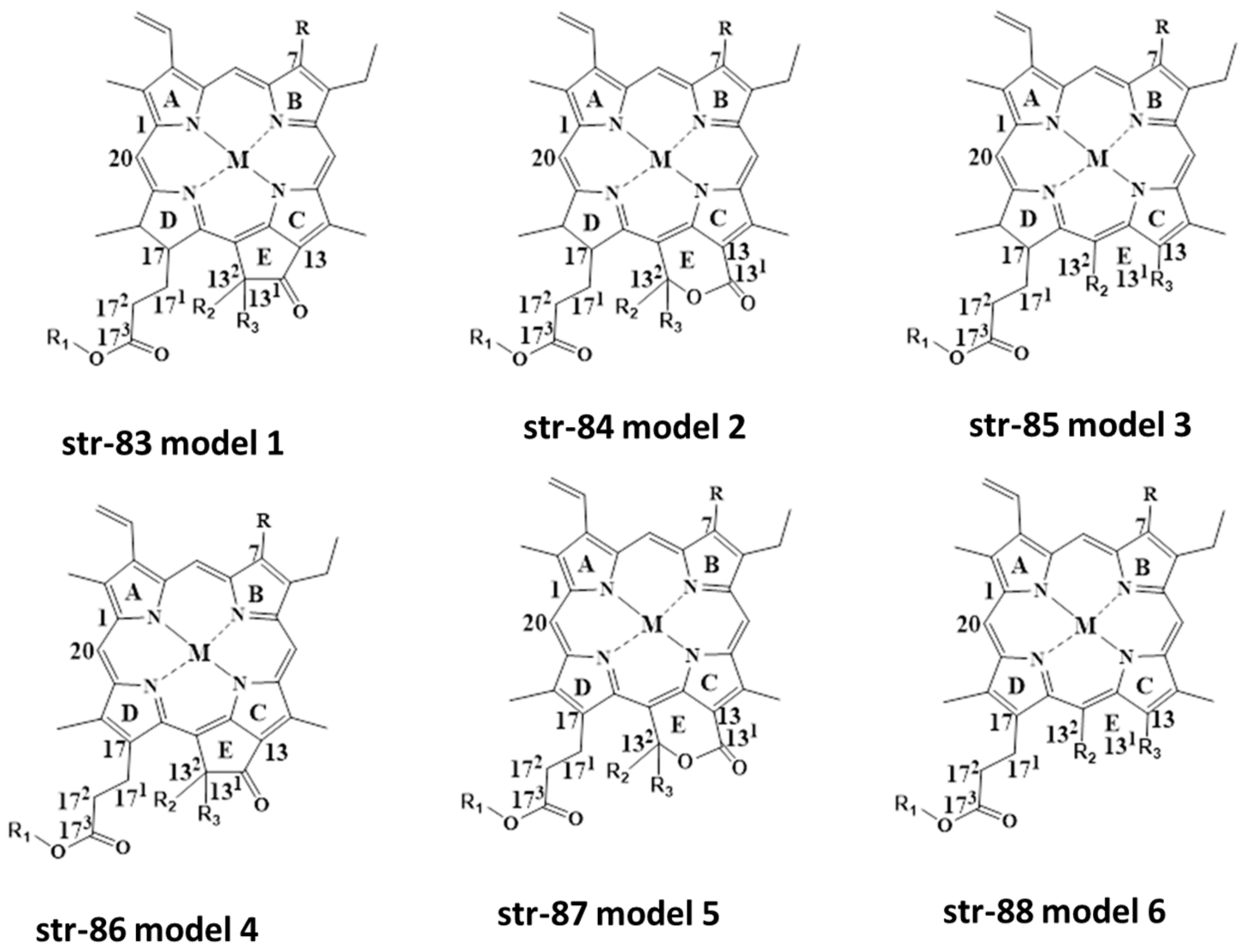
2. Chemistry and Stability of Chlorophylls
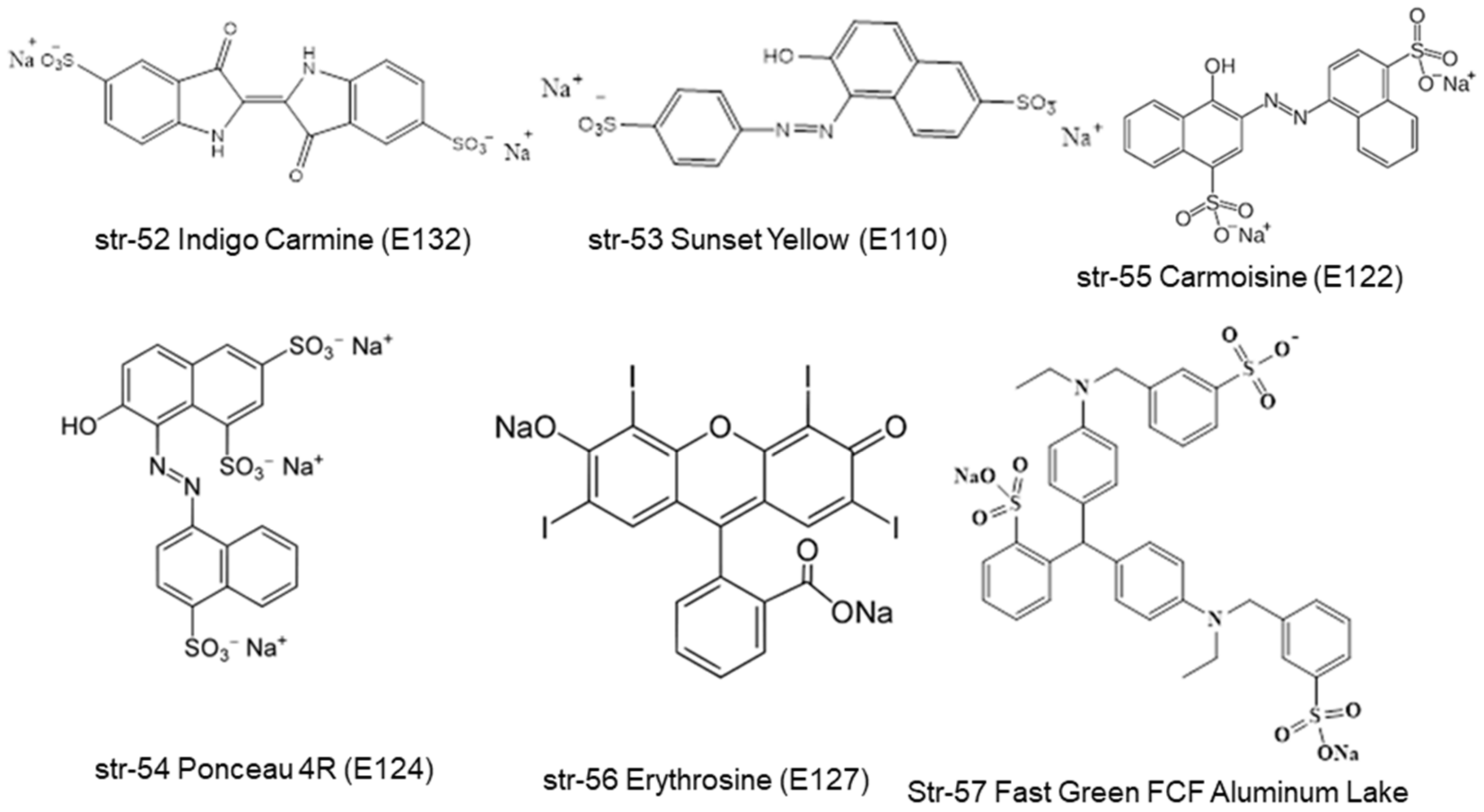
3. Legislations and Regulations
| Name of Country | Different Green Pigments | ||||||
|---|---|---|---|---|---|---|---|
| Chlorophyll | Chlorophyllin | Cu-Chlorophyll | Na-Cu-Chlorophyllin | Na-Fe-Chlorophyllin | Synthetic Colourants | Reference | |
| India (FSSAI) | 6 | E102, E110, E122, E127, E132, E133, E142, E143 | [42] | ||||
| Taiwan (TFDA) | E141i | E141ii | E143, Fast Green FCF Aluminum Lake | [43] | |||
| China | 08.153 | 08.009 | [44] | ||||
| USA | 73.125 | [36] | |||||
| Japan | 177 | 116 | 266 | 265 | 257 | [41] | |
| EU | E140i | E140ii | E141i | E141ii | [40] | ||
| Codex Alimentarius | INS 140 | INS 140 | INS 141i | INS 141ii | [37] | ||
4. Extraction of Chlorophylls and Chlorophyllins from Food Products
4.1. Extraction of Colorants from Fatty Food Products
4.2. Extraction of Colorants from Non-Fatty Food Products
5. Separation and Identification of Chlorophylls and Chlorophyllins in Food Products
5.1. Separation and Identification of Chlorophylls and Chlorophyllins in Food Products Using HPLC Methods
| S. No. | Sample Type | Instrument Used | Stationary Phase | Mobile Phase, Inj. Volume, Flow Rate (mL/min), Run Time (min) | Analyzed Colourants | Reference |
|---|---|---|---|---|---|---|
| 1 | Green table olives with E-141(ii) colourant | HPLC-PDA | C-18 stainless steel column (3 µm size × 20 cm length × 0.46 cm ID) | Mobile phases: (a) water/ion pair reagent/methanol (1/1/8, v/v/v) and (b) methanol/acetone (1/1, v/v). | Pheophorbide a Pyropheophorbide a 15-G-chlorophyll b 15-G-pheophytin b 15-G-chlorophyll a 15-G-pheophytin a Chlorophyll b Chlorophyll b’ 132-OH-chlorophyll b 15-F-chlorophyll b Chlorophyll a Chlorophyll a’ 132-OH-chlorophyll a 15-F-chlorophyll a Pheophytin b Pheophytin b’ Pheophytin a Pheophytin a’ Pyropheophytin a (note: G: glyoxylic acid, F: Formyl) | [35] |
| 2 | Food colour additives Cu-chlorophylls and Cu-chlorophyllins in foods and beverages | HPLC-PDA and HPLC-Fluorescence | Vydac 201TP54 C18 cokumn (5 µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) MeOH: 1.0 M ammomium acetate (80:20, v/v) and (b) MeOH:acetone (60: 40, v/v), 50 µL, 1 mL/min, 60 min | Chlorophyll, Cu-chlorin e6 | [62] |
| 3 | Adulterated green coloured olive oils with Cu-chlorophyll (E-141i) | HPLC-PDA | C18 stainless steel column (3 µm size × 20 cm length × 4.6 mm ID) | Mobile phases: (a) water/ion pair reagent/methanol (1/1/8, v/v/v) and (b) methanol/acetone (1:1, v/v); 1.25 mL/min, 40 min | Cu-pyropheophytin a, Pheophytin b/b’, Pheophytin a/a’, Pyro-pheophytin a, Cu-132-OH-pheophorbide a, Cu-pyro-pheophorbide a/b | [63] |
| 4 | Fresh spinach (Spinacia oleracea), carrot (Daucus carota) and tomato (Lycopersicon esculentum), wastes of tomato paste and orange juice manufacturers | HPLC-PDA | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | MeOH:MeCN (50:50, v/v) with 0.1% (v/v) TEA and acetone | Fresh spinach (Spinacia oleracea), carrot (Daucus carota) and tomato (Lycopersicon esculentum), wastes of tomato paste and orange juice industries | [65] |
| 5 | Chlorophyll a and chlorophyll b | HPLC-UV-Vis | NA | CHCl3-MeOH (20:1, v/v) | Pheophytin a, Mesopurpurin-7 Trimethyl Ester, Purpurin- 18 Methyl Ester, Mesopurpurin- 18 Methyl Ester, Rhodoporphyrin-XV Dimethyl Ester, Chlorin-p6, Trimethyl Ester, Purpurin-7 Trimethyl Ester, Methyl mesopyrophaeophorbide-a, | [66] |
| 6 | 29 Edible oils (olive oil, grapeseed oil and blended oil) | UHPLC-PDA | InertSustain C18 column (2 µm size × 10 cm length × 2.1 mm ID) | Mobile phases: (a) 1 M ammonium acetate/MeOH (2/8, v/v) (b) MeCN (c) MeOH (d) H2O, 0.25 mL/min | Cu-pyropheophytin a, Cu-pheophytin a and a′, Cu-pyropheophytin b, Cu-152-Methyl-phytol-rhodin g7 ester (Cu-rhodin g7) | [67] |
| 7 | Synthesized and fortified sample with Pheophytinato a nickel(ll) and Pheophytinato b nickel(II) | HPLC-UV-Vis | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) | Mobile phase: Acetone-MeOH (50:50, v/v), 1.4 mL/min, at 20–30 °C and a λmax of 420 or 428 nm. | Pheophytinatonikel(II) | [68] |
| 8 | Fiber-rich vegetable puree, fat-rich virgin olive oil, and fruit juice | HPLC-UV-Vis | Mediterranea Sea18 column (3 µm size × 20 cm length × 4.6 mm ID) | Mobile phases: (a) H2O/0.05 M ammonium acetate/MeOH (1/1/8, v/v/v) and (b) MeOH/acetone (1/1, v/v). λ-range: 350 to 800 nm | Chlorins, Rhodins, Pheophorbides, Chlorophylls, Pheophytins, 132-OH-pheophorbides, 132-OH-chlorophylls, 132-OH-pheophytins, 151-OH-lactone-pheophorbides, 151-OH-lactone-pheophytins, Pyropheophytins | [69] |
| 9 | Rat plasma | HPLC-PDA | Luna C18 RP-HPLC column (100 Å 4.5 µm size × 25 cm length × 4.6 mm ID) | Mobile phase: MeOH:10 mM ammonium acetate (90:10, v/v), 20 µL, 1 mL/min, 20 min | Na-Cu-chlorophyllin | [70] |
| 10 | Processed foods (seaweed, pickled leaf, chewing gum, fried fish cake, white chocolate, mugwort-flavored rice cake) | HPLC-UV-Vis | Inertsil ODS-3V column (5 µm size × 15 cm length × 4.6 mm ID) | Mobile phases: (a) 1.0 mmol/L ammonium acetate:MeOH (20:80, v/v) and (b) MeOH:acetone (80:20, v/v), 10 µL, 1 mL/min, 30 min at 40 °C and 405 nm | Cu-chlorophylls, Na-Cu-chlorophylls | [71] |
| 11 | Na-Cu-chlorophyllin in water-soluble and fat-soluble food samples | HPLC-PDA | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) | Mobile phase: MeOH:H2O (97:3, v/v) including 1% acetic acid, 10 µL, 1 mL/min, 20 min at 35 °C and a λmax of 405 nm | Cu-isochlorin e4, Cu-chlorin p6, Cu-chlorin e6 | [72] |
| 12 | Fortified candy samples with Na-Fe-chlorophyllin and Na-Cu-chlorophyllin | HPLC-PDA | Inertsil ODS-2 C18 column (5 µm size × 25 cm × 4.6 mm ID) | Mobile phase: MeOH:H2O (97:3 and 80:20, v/v) containing 1% acetic acid, 1 mL/min, 30 min at a λmax of 395 nm | Na-Fe-chlorophyllin, Na-Cu-chlorophyllin, Fe-Isochlorin e4, Cu-Isochlorin e4 | [73] |
| 13 | Grapes and Port wines | HPLC-DAD | Nova-Pak C18 RP HPLC column (60 Å 4 µm size × 30 cm length × 3.9 mm ID) | Mobile phase: (a) 100% ethyl acetate and (b) 90% MeCN in H2O (9:1, v/v), 20 µL, 1 mL/min, 45 min at a λmax of 447 nm | Chlorophyll b, Pheophytin a/b | [74] |
| 14 | Na-Cu-chlorophyllin and CuSO4 as additives in 16 table olives | HPLC-DAD | Alltech Prontosil C30 RP HPLC column (200 Å 5µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) Methanol:distilled water: Acetic acid (90:10:0.5 v/v/v) and (b) tert-butylmetyl ether:Methanol:Acetic acid (100:10:0.5 v/v), 1 mL/min, 45 min | Chlorin e6, Cu-rhodin g7, Cu-chlorin e6, Cu-chlorin p6, Pheophorbide a, Cu-isochlorin e4, Isochlorin e4, Cu-151-OH-lactone-pheophytin a, Pheophytin a/b, Cu-pyropheophorbide a, Chlorophyll a/b, Pheophorbide a, Cu-rhodochlorin | [35] |
5.2. Separation and Identification of Chlorophylls and Chlorophyllins in Food Products Using HPLC-MS Methods
| S. No. | Sample Type | Instrument Used | Stationary Phase | Mobile Phase, Flow Rate (mL/min), Run Time (min) | Analyzed Colourants | Reference |
|---|---|---|---|---|---|---|
| 1 | Five commercial Na-Cu-chlorophyllin samples | HPLC-PDA and HPLC-APCI/ESI-MS | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) MeOH:H2O:AcOH (90:10:0.5, v/v/v) and (b) tert-butyl methyl ether:MeOH:AcOH (100:10:0.5, v/v/v), 10µL (PDA)/100 µL (MS), 1.1 mL/min, 45 min | Cu-chlorin e6, Cu-chlorin p6, Cu-isochlorin e4, Chlorin e6, Cu-pyropheophorbide a, Cu-purpurin 7, Cu-rhodin g7, Rhodin, Cu-rhodin, Cu-rhodochlorin, Cu-porphyrin | [51] |
| 2 | Spinach-extracted chlorophyll a derived Fe-chlorophyllins | RP-HPLC-FAB-MS | Inertsil ODS C18 column (5 µm size × 25 cm length × 4.6 mm ID) | MeCN-phosphate buffer (pH 2) (60:40, v/v) containing tetramethyl ammonium chloride (0.01 M), | Fe(III)-pheophorbide a Fe(III)-chlorin e6 Fe(III)-chlorin e4 | [58] |
| 3 | Serum samples | HPLC, ESI/MS, and MS/MS | (a) Prodigy C18 column (5 µm size × 25 cm length × 4.6 mm ID) (b) Vydac C18 column | Mobile phases: (a) 0:20, v/v) with 1% (v/v) AcOH and (b) MeOH, 1 mL/min, | Chlorin e4 Ethyl Ester | [59] |
| 4 | 29 Edible oils (olive oil, grapeseed oil and blended oil) | UHPLC-APCI(-)-Q-Orbitrap-MS-MS | Halo C18 column (2.7 µm size × 10 cm length × 4.6 mm ID) | Mobile phases: (a) MeCN and (b) MeOH, 0.8 mL/min, at 30 °C | Cu-chloropheophytin a (m/z = 535) | [67] |
| 5 | Na-Cu-chlorophyllin in water-soluble and fat-soluble food samples | ESI-LC-TOF-MS | Acquity UPLC® BEH C-18 (1.7 μm size × 10 cm length × 2.1 mm ID) | Mobile phases: (a)Water and (b) MeCN (A:B = 62.5:37.5), 5 uL, 0.35 mL/min, 12 min at 35 °C | Cu-isochlorin e4, Cu-chlorin p6, Cu-chlorin e6 | [72] |
| 6 | Fortified food samples with Na-Fe-chlorophyllin | ESI-LC-TOF-MS | Acquity UPLC® BEH C-18 (1.7 μm size × 10 cm length × 2.1 mm ID) | A: Water and B: MeCN (A:B = 62.5:37.5), 5µL, 0.35 mL/min, 12 min at 35 °C. | Fe(III)-isochlorin e4, Fe(III)-chlorin e4 | [73] |
| 7 | Grapes and Port wines | HPLC-DAD-MS (ESP+) | Waters YMC C30 column (5 µm size × 25 cm length × 4.6 mm ID) | Mobile phases: (a) H2O, (b) MeOH, and (c) tert-butyl methyl ether, 1 mL/min, acquisition of the mass data between m/z 100 and 700 | Pheophorbide b, Pheophytin a/b, Pheophytin a/b like compound, Unknown chlorophyll-derived compound | [74] |
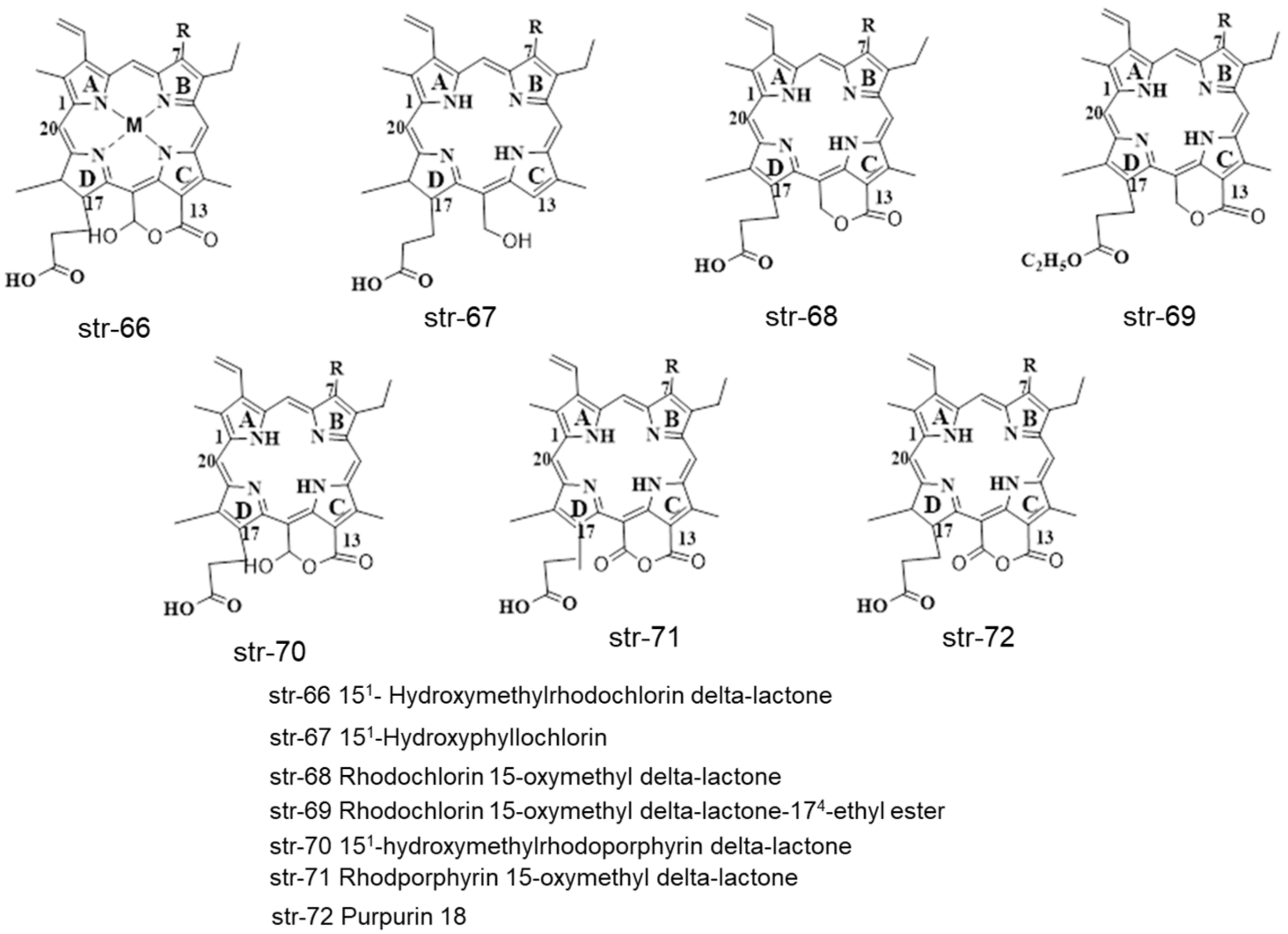
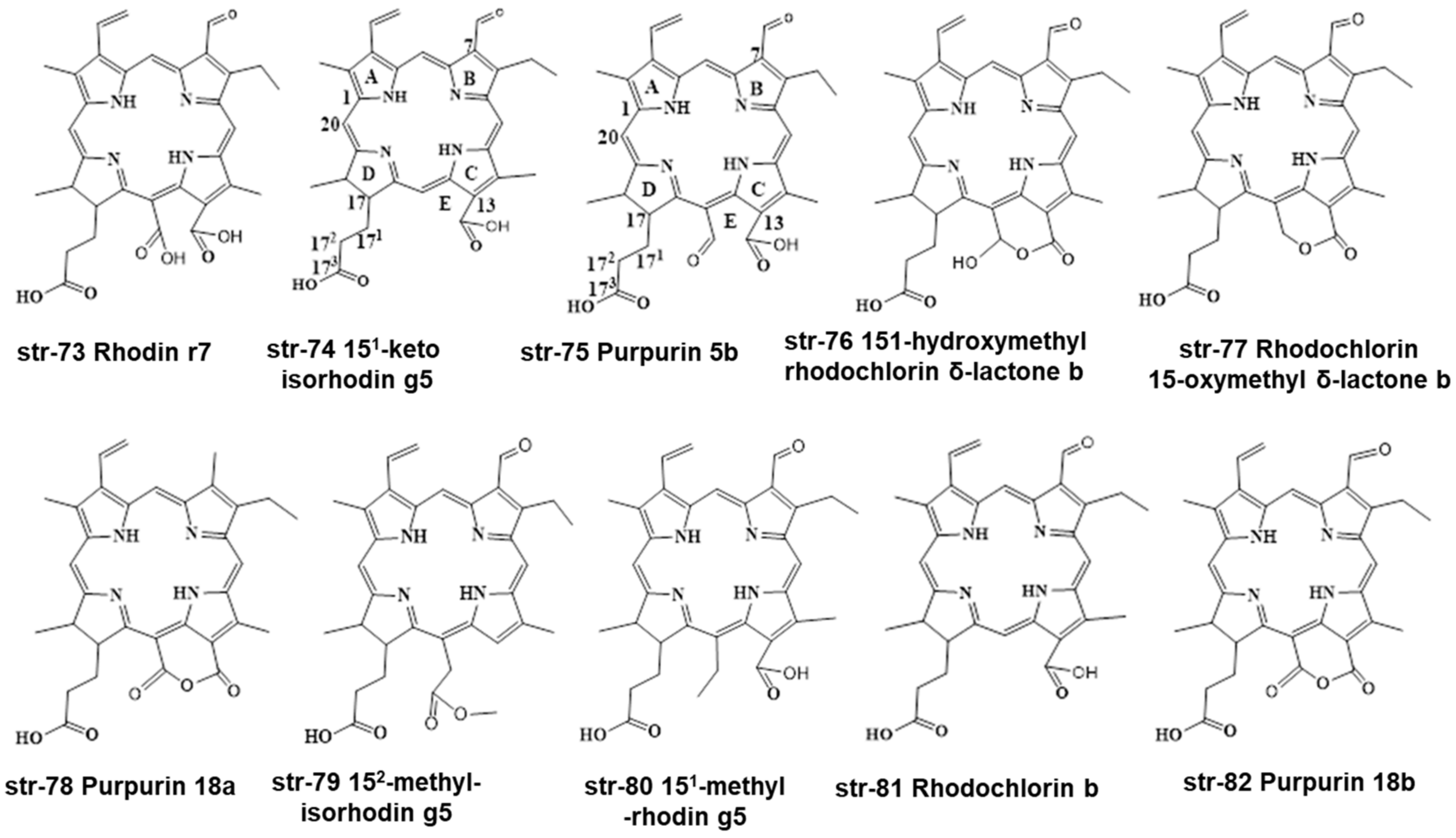
| 8 | Photolon formulation | HPLC-PDA-MS | C-18 RP-HPLC column (3.5 µm size × 15 cm length × 4.6 mm ID) and a semi-preparative column (5 µm size × 15 cm length × 10 mm ID) | Mobile phases: (a) (0.1% TFA in water) and (b) (MeCN), 10 µL, 1 mL/min, 30 min | chlorin e6 174-ethyl ester, chlorin e4, 15-hydroxyphyllochlorin, Rhodochlorin, 151-hydroxymethylrhodochlorin δ-lactone, Rhodochlorin-15-oxymethyl δ-lactone, Rhodochlorin-15-oxymethyl δ-lactone 174-ethyl ester, 151-hydroxymethylrhodoporphyrin δ-lactone, Rhodoporphyrin-15-oxymethyl δ-lactone, Purpurin 18 | [81] |
| 9 | Hot-air-dried and freeze-dried Chinese herb Rhinacanthus nasutus (L.) Kurz samples | HPLC-DAD-APCI-MS | Agilent Eclipse XDB C18 column (5 µm size × 15 cm length × 4.6 mm ID) | Mobile phases: (a) MeOH/N,N-dimethylformamide (97:3, v/v) and (b) MeCN under gradient elution, 1 mL/min, 2 min at a λmax of 600 nm | Chlorophyll a/a′, Hydroxychlorophyll a/b, 15-OH-lactone chlorophyll a, Chlorophyll b/b′, Pheophytin a/a′, Hydroxypheophytin a/a′, Pheophytin b | [78] |
6. Non-Targeted Analysis of Chlorophyll and Chlorophyllin-Related Compounds Using HPLC/MS-MS and HPLC/ICP-IDMS Methods
| Scheme | Sample Type | Instrument Used | Stationary Phase | Mobile Phase, Flow Rate (mL/min), Run Time (min) | Analyzed Colourants | Reference |
|---|---|---|---|---|---|---|
1 | Dephytylated chlorophyll standards derivatives | HPLC/UHPLC-APCI-hrTOF-MSMS | ODS-2 C18 LC column (3 µm size × 20 cm length × 0.46 cm ID) | Mobile phases: (a) H2O/ion pair reagent/MeOH (1:1:8, v/v/v) and (b) MeOH/acetone (1:1, v/v) with ion-pair reagent as 0.05 M tetrabutylammonium and 1 M ammonium acetate in water, 1 mL/min with scan range of m/z 50–1500 and mass resolving power of over 18,000 (m/∆m). | Chlorophyllide a/b, 132-OH-chlorophyllide a/b, 151-OH-lactone-chlorophyllide a/b, Pyrochlorophyllide a/b, Pheophorbide a/b, 132-OH-pheophorbide a/b, 151-OH-lactone-pheophorbide a/b, Pyropheophorbide b | [87] |
| 2 | Teas, processed vegetable foodstuffs | UHPLC-PDA-MS-MS | ACQUITY UPLCTM HSS T3 column (1.8 µm size × 10 cm length × 2.1 mm ID) | Mobile phases: (a) MeOH/iPrOH/MeCN (10/15/75, v/v/v)] and (b) MeOH/MeCN/H2O (25/25/50, v/v/v)], 5 µL, 1 mL/min, 6 min at 45 °C | (A) Tea samples: Chlorophyllide a/a’, Chlorophyllide b/b’, Pheophorbide a/a’, Pheophorbide b/b’, 132-OH-chlorophyll a/b, Chlorophyll a/b, Chlorophyll a’/b’, 151-OH-lactone-pheophytin a/b, Chlorophyll b’, 132-OH-pheophytin b/b’, Pheophytin b/b’, Pheophytin a/a’ 132-OH-pheophytin a/a’, Pyropheophytin a (B) Vegetable foodstauffs Pheophorbide a/a’, Pyropheophorbide a, Pyropheophytin a, Pheophytin b/b’, Pheophytin a/a’ | [13] |
| 3 | Fortified olive oil and processed vegetable samples | HPLC-ESI/APCI-HRMS | ODS-2 C18 LC column (3 µm size × 20 cm length × 0.46 mm ID) | Mobile phases: (a) water/ammonium acetate (1 M)/methanol (1/1/8, v/v/v) and (b) methanol/acetone (1/1, v/v), 1 mL/min, | Fortified with E-141i Cu-pyropheophorbide a, Cu-pheophytin b, Cu-132-OH-pheophytin a, Cu-132-OH-lactone-pheophytin a, Cu-pyropheophytin a/b, Cu-pheophytin a, Cu-pyropheophorbide a Fortified with E-141ii Cu-rhodin g7, Cu-chlorin e6/e4, Cu-chlorin p6, Cu-pyropheophorbide a | [90] |
| 4 | Various types of table olives sold on market | Agilent 1100 capillary-LC/Agilent 8800 ICP-MS | NA | NA | Lipophilic CDP/Cu-CDPs and Hydrophilic CDP/Cu-CDPs, (CDP: degradation products of chlorophyll) | [91] |
| 5 | Green colourant E141i via high-fat-containing foodstuffs | HPLC-ESI(+)/APCI(+)-hrTOF-MS2 | C18 RP-HPLC column (3 µm size × 20 cm length × 0.46 mm ID) | Mobile phases: (a) water/ion pair reagent/methanol (1:1:8, v/v/v) and (b) methanol/acetone (1:1, v/v). The ion pair reagent was 0.05 M tetrabutylammonium and 1 M ammonium acetate in water. 2 mL/min, 40 min | Chlorin p6, Cu-132-OH-pheophorbide a, Cu-pheophorbide a/b, Cu-pyropheophorbide a/b, 132-OH-pheophytin b Cu-151-OH-lactone-pheophytin, Pyropheophytin b, Cu-pheophytin a, Cu-pyropheophytin b, Cu-pheophytin a’, Cu-pyropheophytin a, Phytyl-chlorin p6 | [83] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mathiyalagan, S.; Mandal, B.K.; Ling, Y.-C. Determination of synthetic and natural colorants in selected green colored foodstuffs through reverse phase-high performance liquid chromatography. Food Chem. 2019, 278, 381–387. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO. Codex Alimentarius: General Standard for Food Additives, 2018th ed.; WHO/FAO: Rome, Italy, 2018. [Google Scholar]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.A.; Fawzia, S.E.-S. Toxicological and safety assessment of tartrazine as a synthetic food additive on health biomarkers: A review. Afr. J. Biotechnol. 2018, 17, 139–149. [Google Scholar] [CrossRef]
- Leo, L.; Loong, C.; Ho, X.L.; Raman, M.F.B.; Suan, M.Y.T.; Loke, W.M. Occurrence of azo food dyes and their effects on cellular inflammatory responses. Nutrition 2018, 46, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bianca, R.; Albuquerque, M.; Beatriz, P.P.; Oliveira, L.B.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2021, 61, 805–835. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Sentanin, M.A.; Souza, D. Analytical methods in food additives determination: Compounds with functional applications. Food Chem. 2019, 272, 732–750. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, Y.; Kim, Y.J.; Chang, Y.K. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Lafeuille, J.L.; Lefèvre, S.; Lebuhotel, J. Quantitation of chlorophylls and 22 of their colored degradation products in culinary aromatic herbs by HPLC-DAD-MS and correlation with color changes during the dehydration process. J. Agric. Food. Chem. 2014, 62, 1926–1935. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–30. [Google Scholar] [CrossRef]
- Pérez-gálvez, A.; Viera, I.; Benito, I.; Roca, M. HPLC-hrTOF-MS study of copper chlorophylls: Composition of food colorants and biochemistry after ingestion. Food Chem. 2020, 321, 126721. [Google Scholar] [CrossRef]
- Delpino-Rius, A.; Cosovanu, D.; Eras, J.; Vilaró, F.; Balcells, M.; Canela-Garayoa, R. A fast and reliable ultrahigh-performance liquid chromatography method to assess the fate of chlorophylls in teas and processed vegetable foodstuff. J. Chromatogr. A 2018, 1568, 69–79. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M. Stability of virgin olive oil. 2. Photo-oxidation studies. J. Agric. Food Chem. 2002, 50, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108. [Google Scholar] [CrossRef]
- Tabet, J.-C.; Jablonski, M.; Cotter, R.J.; Hunt, J.E. Time-resolved laser desorption. III. The metastable decomposition of chlorophyll-a and some derivatives. Int. J. Mass Spectrom. Ion Process. 1985, 65, 105–117. [Google Scholar] [CrossRef]
- Suzuki, T.; Midonoya, H.; Shioi, Y. Analysis of chlorophylls andtheirderivativesby matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry. Anal. Biochem. 2009, 390, 57–62. [Google Scholar] [CrossRef]
- Wei, J.; Li, H.H.; Barrow, M.P.; O’Connor, P.B. Structural characterization of chlorophyll-a by high resolution tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 753–760. [Google Scholar] [CrossRef]
- Grese, R.P.; Cerny, R.L.; Gross, M.L.; Senge, M. Determination of structure and properties of modified chlorophylls by using fast atom bombardment combined with tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1990, 1, 72–84. [Google Scholar] [CrossRef]
- Van Breenmen, R.B.; Canjura, F.L.; Schwartz, S.J. Identification of chlorophyll derivatives by mass spectrometry. J. Agric. Food Chem. 1991, 39, 1452–1456. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Hyvärinen, K. Tracing the allomerization pathways of chlorophylls by 18O-labeling and mass spectrometry. J. Org. Chem. 2002, 67, 4055–4061. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Viera, I.; Herrera, M.; Roca, M. Influence of food composition on chlorophyll bioaccessibility. Food Chem. 2022, 386, 132805. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition of commercial green food colorants and coloring foodstuffs by HPLC-ESI-QTOF-MS/MS: Chlorophyllins. Food Chem. 2023, 415, 135746. [Google Scholar] [CrossRef] [PubMed]
- Porraud, S.; Pranee, A. Microencapsulation of Zn-chlorophyll pigment from Pandan leaf. Int. Food Res. 2010, 17, 1031–1042. [Google Scholar]
- Bilek, S.E.; Özkan, G. Encapsulation of zinc-chlorophyll derivatives in whey protein matrix by emulsion/cold-set gelation. J. Food 2018, 43, 174–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Ma, H.; Zeng, X. Effect of inlet air drying temperatures on the physicochemical properties and antioxidant activity of whey protein isolate-kale leaves chlorophyll (WPI-CH) microcapsules. J. Food Eng. 2019, 245, 149–156. [Google Scholar] [CrossRef]
- Fischer, H.; Gottschaldt, W.; Klebs, G. Organic chemistry. Ann. Chem. 1932, 498, 194. [Google Scholar] [CrossRef]
- Fischer, H.; Breitner, S. Über Chlorophyll b. Eur. J. Org. Chem. 1933, 511, 183–202. [Google Scholar] [CrossRef]
- Hynninen, P.H. Chlorophylls IV-preparation and purification of some derivatives of chlorophylls a and b. Acta Chem. Scand. 1973, 27, 1771–1780. [Google Scholar] [CrossRef]
- Woodward, R.B.; Ayer, W.A.; Beaton, J.M.; Bickelhaupt, F.; Bonnett, R.; Buchschacher, P.; Closs, G.L.; Dutler, H.; Hannah, J.; Hauck, F.P.; et al. The total synthesis of chlorophyll a. Tetrahedron 1990, 46, 7599–7659. [Google Scholar] [CrossRef]
- Strain, H.H.; Manning, W.M. Isomerization of chlorophylls a and b. J. Biol. Chem. 1942, 146, 275. [Google Scholar] [CrossRef]
- Mathiyalagan, S.; Mandal, B.K.; Sinha, M.; Ling, Y.-C. Synthesis of different metallochlorophyllins and quantification in food samples by reversed phase—High performance liquid chromatography. Nat. Prod. Res. 2019, 33, 3120–3126. [Google Scholar] [CrossRef]
- Viera, I.; Perez-Galvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Detection of the color adulteration of green table olives with copper chlorophyllin complexes (E-141ii colorant). LWT Food Sci. Technol. 2012, 1, 311–318. [Google Scholar] [CrossRef]
- US FDA Electronic Code of Federal Regulations (eCFR) Listing of Color Additives Exempt from Certification Title 21, Chapter I, Subchapter A, Part 73. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-73 (accessed on 30 January 2023).
- The Codex Alimentarius General Standard of Food Additives. Available online: https://www.fao.org/gsfaonline/additives/index.html?lang=en (accessed on 30 January 2023).
- FDA Electronic Code of Federal Regulations, §73.125 Sodium Copper Chlorophyllin. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=301866fcce8fbe4833e785ac56479d0e&mc=true&node=se21.1.73_1125&rgn=div8, (accessed on 13 February 2020).
- EFSA. Scientific opinion on re-evaluation of chlorophyllins (E 140 (II)) as food additives. EFSA J. 2015, 13, 4085. [Google Scholar] [CrossRef]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (OJ L 354 31.12.2008, pp. 16–33). Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/2016-05-25 (accessed on 30 January 2023).
- Japanese Food Additives Regulations. Available online: https://www.jetro.go.jp/ext_images/en/reports/regulations/pdf/foodext2010e.pdf (accessed on 30 January 2023).
- The Indian Food Safety and Standards Regulations. Available online: https://www.fssai.gov.in/home/fsslegislation/fss-regulations.html (accessed on 25 September 2018).
- Taiwan Standards for Specification, Scope, Application and Limitation of Food Additives. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0040084 (accessed on 30 January 2023).
- China Food Additive Regulation. GB 2760-2014. Available online: http://nehrc.nhri.org.tw/foodsafety/ref/GB27602014.pdf (accessed on 30 January 2023).
- Simon, J.E.; Decker, E.A.; Ferruzzi, M.G.; Giusti, M.; Mejia, C.D.; Goldschmidt, M.; Talcott, S.T. Establishing standards on color from natural sources. J. Food Sci. 2017, 82, 2539–2553. [Google Scholar] [CrossRef]
- Mathiyalagan, S.; Mandal, B.K. A review on assessment of acceptable daily intake for food additives. Biointerf. Res. Appl. Chem. 2020, 10, 6033–6038. [Google Scholar] [CrossRef]
- Mathiyalagan, S.; Mandal, B.K. A review on analytical methods for the determination of natural colorants (green) in food commodities and beverages. Biointerf. Res. Appl. Chem. 2020, 10, 7993–8010. [Google Scholar] [CrossRef]
- Karanikolopoulos, G.; Gerakis, A.; Papadopoulou, K.; Mastrantoni, I. Determination of synthetic food colorants in fish products by an HPLC-DAD method. Food Chem. 2015, 177, 197–203. [Google Scholar] [CrossRef]
- Dixit, S.; Khanna, S.K.; Das, M. All India survey for analyzes of colors in sweets and savories: Exposure risk in Indian population. J. Food Sci. 2013, 78, T642–T647. [Google Scholar] [CrossRef]
- Dixit, S.; Purshottam, S.; Khanna, S.; Das, M. Usage pattern of synthetic food colors in different states of India and exposure assessment through commodities preferentially consumed by children. Food Addit. Contam. Part A 2011, 28, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Geppel, A. HPLC-MS analysis of the green food colorant sodium copper chlorophyllin. IFSET 2007, 8, 419–425. [Google Scholar] [CrossRef]
- Inoue, H.; Yamashita, H.; Furuya, K.; Nonomura, Y.; Yoshioka, N.; Lib, S. Determination of copper (II) chlorophyllin by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1994, 679, 99–104. [Google Scholar] [CrossRef]
- Chernomorsky, S.; Rancourt, R.; Sahai, D.; Poretz, R. Evaluation of commercial chlorophyllin copper complex preparations by liquid chromatography with photodiode array detection. J. AOAC Int. 1997, 80, 433–435. [Google Scholar] [CrossRef]
- Almela, L.; Fernández-López, J.A.; Roca, M.A.J. High-performance liquid chromatographic screening of chlorophyll derivatives produced during fruit storage. J. Chromatogr. A 2000, 870, 483–489. [Google Scholar] [CrossRef]
- Cano, M.P. HPLC Separation of Chlorophyll and Carotenoid Pigments of Four Kiwi Fruit Cultivars. J. Agric. Food. Chem. 1991, 39, 1786–1791. [Google Scholar] [CrossRef]
- Yasuda, K.; Tadano, K.; Ushiyama, H.; Ogawa, H.; Kawai, Y.; Nishima, T. Investigation to find an indicator substance for the analysis of sodium copper chlorophyllin in foods. J. Food Hyg. Soc. Jpn. 1995, 36, 710–716. [Google Scholar] [CrossRef]
- Ushiyama, H.; Nishijima, M.; Yasuda, K.; Kamimura, H.; Tabata, S.; Nishima, T. Determination of sodium copper chlorophyllin in foods. J. Food Hyg. Soc. Jpn. 1986, 27, 417–420. [Google Scholar] [CrossRef]
- Nonomura, Y.; Yamaguchi, Y.; Hara, K.; Furuya, K.; Yoshioka, N.; Inoue, H. High-performance liquid chromatographic separation of iron(III) chlorophyllin. J. Chromatogr. A 1996, 721, 350–354. [Google Scholar] [CrossRef]
- Egner, P.A.; Stansbury, K.H.; Snyder, E.P.; Rogers, M.E.; Hintz, P.A.; Kensler, T.W. Identification and characterization of chlorin e4 ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000, 13, 900–906. [Google Scholar] [CrossRef]
- Wang, L.F.; Park, S.C.; Chung, J.O.; Baik, J.H.; Park, S.K. The Compounds Contributing to the Greenness of Green Tea. J. Food Sci. 2004, 69, S301–S305. [Google Scholar] [CrossRef]
- Bohn, T.; Walczyk, T. Determination of chlorophyll in plant samples by liquid chromatography using zinc–phthalocyanine as an internal standard. J. Chromatogr. A 2004, 1024, 123–128. [Google Scholar] [CrossRef]
- Scotter, M.J.; Castle, L.; Roberts, D. Method development and HPLC analysis of retail foods and beverages for copper chlorophyll (E141 [i]) and chlorophyllin (E141 [ii]) food coloring materials. Food Addit. Contam. 2005, 22, 1163–1175. [Google Scholar] [CrossRef]
- Roca, M.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I.; Gandul Rojas, B.B. Control of Olive Oil Adulteration with Copper-Chlorophyll Derivatives. J. Agric. Food. Chem. 2010, 58, 51–56. [Google Scholar] [CrossRef]
- Loranty, A.; Rembiałkowska, E.; Rosa, E.A.; Bennett, R.N. Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas. J. Food Compos. Anal. 2010, 23, 432–441. [Google Scholar] [CrossRef]
- Baskan, K.S.; Tutem, E.; Ozer, N.; Apak, R. Spectrophotometric and Chromatographic Assessment of Contributions of Carotenoids and Chlorophylls to the Total Antioxidant Capacities of Plant Foods. J. Agric. Food Chem. 2013, 61, 11371–11381. [Google Scholar] [CrossRef]
- Kenner, G.W.; McCombie, S.W.; Smith, K.M. Pyrroles and Related Compounds. Part XX1V.l Separation and Oxidative Degradation of Chlorophyll Derivatives. J. Chem. Soc. Perkin Trans. 1973, 1, 2517–2523. [Google Scholar] [CrossRef]
- Fang, M.; Tsai, C.-F.; Wu, G.-Y.; Tseng, S.H.; Cheng, H.-F.; Kuo, C.-H.; Hsua, C.-L.; Kao, Y.M.; Shih, D.Y.C.; Chiang, Y.-M. Identification and quantification of Cu-chlorophyll adulteration of edible oils. Food Addit. Contam. Part B Surveill. 2015, 8, 157–162. [Google Scholar] [CrossRef]
- Furuya, K.; Ohki, N.; Inoue, H.; Shirai, T. Determination of Pheophytinatonickel (II) by Reversed-Phase HighPerformance Liquid Chromatography. Chromatographia 1988, 25, 319–323. [Google Scholar] [CrossRef]
- Viera, I.; Herrera, M.; Roca, M. In Vitro Bioaccessibility Protocol for Chlorophylls. J. Agri. Food Chem. 2021, 69, 8777–8786. [Google Scholar] [CrossRef]
- Laddha, A.P.; Nalawade, V.V.; Gharpure, M.; Kulkarni, Y.A. Development and Validation of HPLC Method for Determination of Sodium Copper Chlorophyllin—A Food Colorant and Its Application in Pharmacokinetic Study. Chem. Biodivers. 2020, 17, e2000223. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Kubota, H.; Terami, S.; Hara, T.; Hirakawa, Y.; Iizuka, T.; Tatebe, C.; Ohtsuki, T.; Yano, T.; Sato, K.; et al. Development of an analytical method for copper chlorophyll and sodium copper chlorophyllin in processed foods. Jpn. J. Food Chem. Saf. 2016, 23, 55–62. [Google Scholar] [CrossRef]
- Chong, H.S.; Park, H.I.; Cho, S.R.; Yamaguchi, T.; Lee, O.K.; Park, J.-H.; Lee, C.; Lee, G.-Y.; Yun, S.S.; Lim, H.-S.; et al. Establishment and Validation of an Optimized Analytical Method for Sodium Copper Chlorophyllin in Food Using HPLC and LC/MS. J. Korean Soc. Food Sci. Nutr. 2018, 47, 550–558. [Google Scholar] [CrossRef]
- Chong, H.S.; Park, Y.J.; Kim, E.G.; Park, Y.L.; Kim, J.M.; Yamaguchi, T.; Lee, C.; Suh, H.-J. The Optimization and Verification of an Analytical Method for Sodium Iron Chlorophyllin in Foods Using HPLC and LC/MS. J. Food Hyg. Saf. 2019, 34, 148–157. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Ferreira, A.N.C.S.S.; Caris-Veyrat, C.; Pinto, P.G.D. Carotenoid, Chlorophyll, and Chlorophyll-Derived Compounds in Grapes and Port Wines. J. Agric. Food Chem. 2005, 53, 10034–10041. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Garrido-Fernández, J. Chlorophyll and carotenoid presence in olive fruit (Olea europaea, L.). J. Agri. Food Chem. 1989, 37, 1–7. [Google Scholar] [CrossRef]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef]
- Huang, S.; Hung, C.; Wu, W.; Chen, B. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 105–112. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal Degradation Kinetics of Chlorophyll Pigments in Virgin Olive Oils. 1. Compounds of Series a. J. Agric. Food. Chem. 2010, 58, 6200–6208. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, C.J.; Chen, B.H. An improved high performance liquid chromatography–photodiode array detection–atmospheric pressure chemical ionization–mass spectrometry method for determination of chlorophylls and their derivatives in freeze-dried and hot-air-dried Rhinacanthus nasutus (L.) Kurz. Talanta 2011, 8, 349–355. [Google Scholar] [CrossRef]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Q.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef]
- Isakau, H.A.; Trukhacheva, T.V.; Petrov, P.T. Isolation and identification of impurities in chlorin e6. J. Pharm. Biomed. Anal. 2007, 45, 20–29. [Google Scholar] [CrossRef]
- Loh, C.H.; Inbaraj, B.S.; Liu, M.H.; Chen, B.H. Determination of Chlorophylls in Taraxacum formosanum by High-Performance Liquid Chromatography–Diode Array Detection–Mass Spectrometry and Preparation by Column Chromatography. J. Agric. Food. Chem. 2012, 60, 6108–6115. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Development of an accurate and direct method for the green food colorants detection. Food Res. Int. 2020, 136, 109484. [Google Scholar] [CrossRef]
- Scheer, H. An overview of chlorophylls and bacteriochlorophylls: Biochemistry, biophysics, functions and applications. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–26. [Google Scholar]
- Ngo, T.; Zhao, Y. Formation of zinc-chlorophyll-derivative complexes in thermally processed green pears (Pyrus communis L.). J. Food Sci. 2007, 72, C397–C404. [Google Scholar] [CrossRef]
- Milman, B.L.; Zhurkovich, I.K. Present-Day Practice of Non-Target Chemical Analysis. J. Anal. Chem. 2022, 77, 537–549. [Google Scholar] [CrossRef]
- Chen, K.; Rios, J.J.; Roca, M.; Perez-Galvez, A. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (II) Dephytylated derivatives. J. Chromatogr. A 2015, 1412, 90–99. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Ríos, J.J.; Roca, M. A new probe for tracking the presence of E141i food colorant. Food Control. 2015, 51, 240–243. [Google Scholar] [CrossRef]
- Negro, C.; De Bellis, L.; Sabella, E.; Nutricati, E.; Luvisi, A.; Miceli, A. Detection of not allowed food-coloring additives (copper chlorophyllin, copper-sulphate) in green table olives sold on the Italian market. Adv. Hortic. Sci. 2017, 31, 225–234. [Google Scholar]
- Chong, H.S.; Sim, S.; Yamaguchi, T.; Park, J.; Lee, C.; Kim, M.; Lee, G.; Yun, S.S.; Lim, H.S.; Suh, H.J. Simultaneous determination of sodium iron chlorophyllin and sodium copper chlorophyllin in food using high-performance liquid chromatography and ultra-performance liquid chromatography-mass spectrometry. Food Chem. 2019, 276, 390–396. [Google Scholar] [CrossRef]
- Harp, B.P.; Scholl, P.F.; Gray, P.J.; Delmonte, P. Quantitation of copper chlorophylls in green table olives by ultra-high-performance liquid chromatography with inductively coupled plasma isotope dilution mass spectrometry. J. Chromatogr. A 2020, 1620, 461008. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Viera, I.; Roca, M. HPLC–MS2 Analysis of Chlorophylls in Green Teas Establishes Differences among Varieties. Molecules 2022, 27, 6171. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, B.K.; Ling, Y.-C. Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods. Molecules 2023, 28, 4012. https://doi.org/10.3390/molecules28104012
Mandal BK, Ling Y-C. Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods. Molecules. 2023; 28(10):4012. https://doi.org/10.3390/molecules28104012
Chicago/Turabian StyleMandal, Badal Kumar, and Yong-Chien Ling. 2023. "Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods" Molecules 28, no. 10: 4012. https://doi.org/10.3390/molecules28104012
APA StyleMandal, B. K., & Ling, Y.-C. (2023). Analysis of Chlorophylls/Chlorophyllins in Food Products Using HPLC and HPLC-MS Methods. Molecules, 28(10), 4012. https://doi.org/10.3390/molecules28104012