3.1. Chemistry
All chemicals were purchased from Sigma Aldrich (St. Louis, Missouri, USA), abcr GmbH (Karlsruhe, Germany), or Carl Roth (Karlsruhe, Germany) and used without any further purification.
Reaction progress was monitored by thin-layer chromatography (TLC) on 0.20 mm Polygram SIL G/UV254 (silica gel 60) TLC plates (Macherey-Nagel, Düren, Germany) with the chosen eluent mixture and/or analytical HPLC-MS (ESI detector, Agilent, Santa Clara, CA, USA) equipped with a Luna 5 µm C18 (2) 100 Å 50 × 2 mm column (Phenomenex, Torrance, California, USA) under the following gradient: 0–7.60 min (0% to 100% B), 7.60–8.80 (100% B), 8.80–9.30 min (100% to 0% B), 9.30–13.0 min (0% B); solvent A: 0.1% formic acid in H2O; solvent B: MeCN; 0.4 mL/min.
Purification was performed through automated flash chromatography on an Isolera 4 system (Biotage, Uppsala, Sweden).
1H and 13C NMR spectra were acquired on an Avance III AV 600 (1H: 600.13 MHz; 13C: 150.61 MHz) spectrometer (Bruker, Billerica, MA, USA). All chemical shifts (δ) are reported as parts per million (ppm) and referenced to residual solvent peaks (DMSO-d6: δH = 2.50, δC = 39.52).
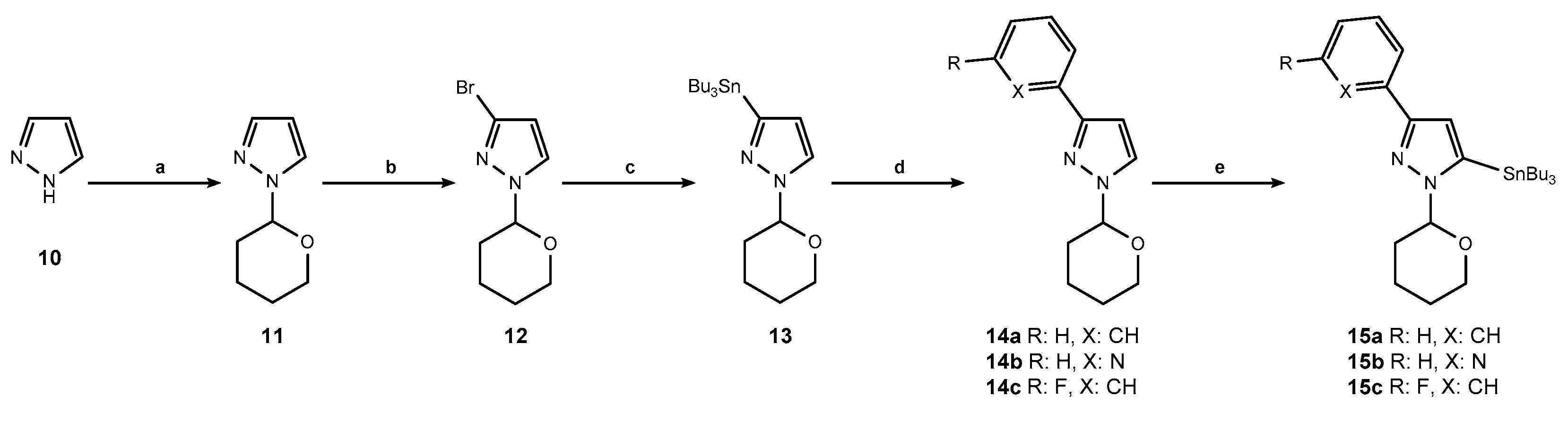
3.1.1. General Procedure A (Compounds 14a–14c)
To a solution of 13 (1.13 mmol) and the selected aryl bromide (2.27 mmol) in NMP (3.50 mL) under argon atmosphere was added Pd(PPh3)4 (5% mol). The mixture was stirred overnight at 100 °C. The crude product mixture was diluted in water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc).
3.1.2. General Procedure B (Compounds 15a–15c)
A solution of the selected 3-aryl-1-(tetrahydro-2H-pyran-2-yl)-pyrazole (0.63 mmol) in THF (2.50 mL) was cooled to −78 °C under a positive pressure of argon. A solution of n-BuLi 2.5M in hexane (0.76 mmol) was added dropwise, after which the mixture was stirred for 15 min. SnBu3Cl (0.76 mmol) was added dropwise, and the resulting mixture was stirred for 1 h, allowing it to reach room temperature. The reaction mixture was poured into water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc).
3.1.3. General Procedure C (Compounds 16a–17c)
To a solution of the selected 3-aryl-1-(tetrahydro-2H-pyran-2-yl)-5-(tributylstannyl)-pyrazole (0.56 mmol) and the selected 3-bromo-7-OR2-N-acetylphenothiazine (0.73 mmol) in NMP (8.50 mL) was added Pd(PPh3)4 (5% mol) under an argon atmosphere. The mixture was stirred overnight at 90 °C. The reaction mixture was diluted with water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc).
3.1.4. General Procedure D (Compounds 18a–18c)
To a solution of the selected 3-aryl-1-(tetrahydro-2H-pyran-2-yl)-5-(tributylstannyl)-pyrazole (0.31 mmol) and 9 (0.40 mmol) in DMF (5.00 mL) was added Pd(PPh3)4 (10% mol), under an argon atmosphere. The mixture was stirred overnight at 110 °C. The crude was diluted with water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc).
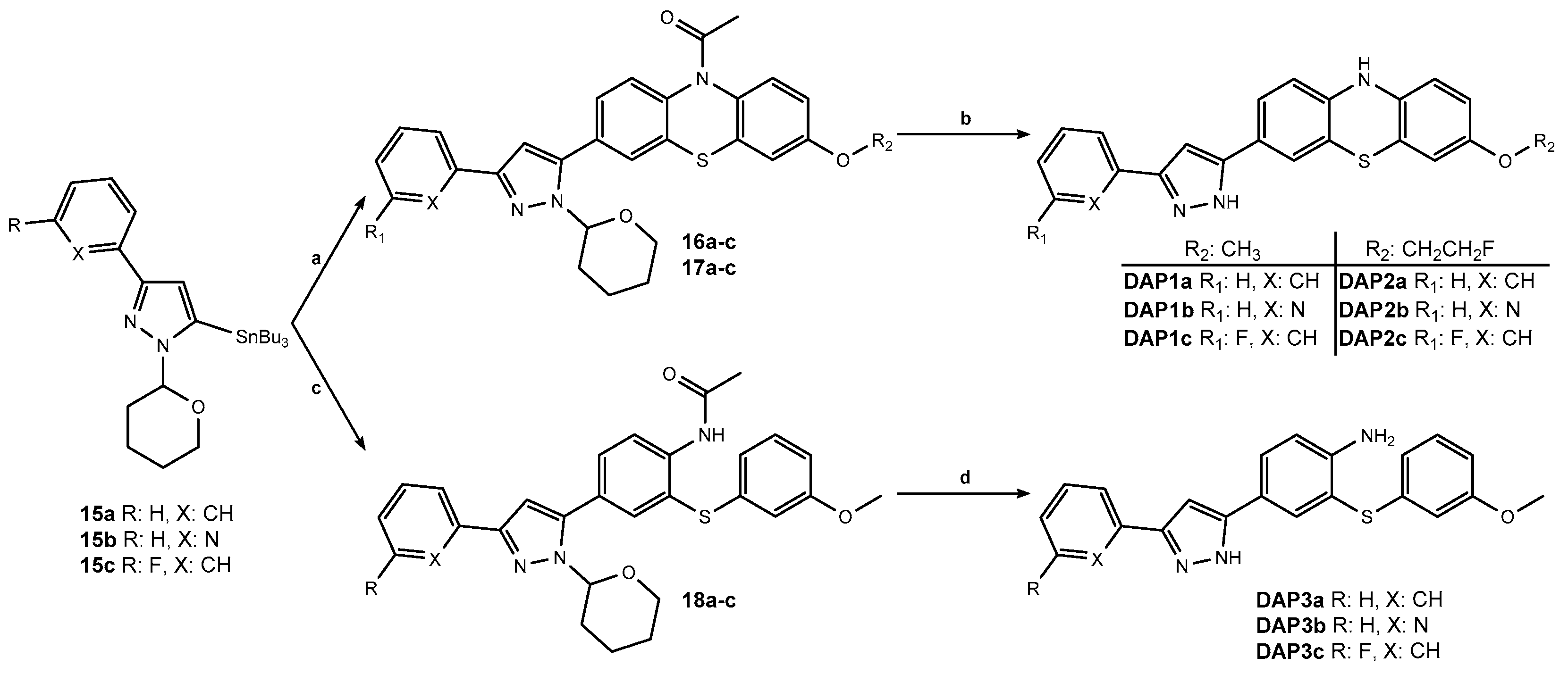
3.1.5. General Procedure E (DAP1a-DAP3c)
To a suspension of the selected protected DAP compound (0.26 mmol) in MeOH/H2O 1:1 v/v (13.0 mL, 13.0 mL), HCl 37% (1.30 mL) was added, and the reaction was stirred at 80 °C for 6 h. The mixture was poured into water, neutralized with NaOH aq. 18 M, and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc or DCM/MeOH).
3.1.6. 1-(3-Methoxy-7-nitro-10H-phenothiazin-10-yl)ethan-1-one (1)
The three-step synthesis was carried out according to the literature procedure [
10], starting from 2-amino-6-methoxybenzo[d]thiazole and 1-chloro-2,4-dinitrobenzene. The product was afforded as an orange solid (13.1 g, 58%), with all analytical data corresponding to the published data. R
f: 0.43 (PE/EtOAc 1:1).
1H NMR (600 MHz, DMSO-
d6) δ 8.39 (d,
J = 2.6 Hz, 1H), 8.21 (dd,
J = 8.8, 2.6 Hz, 1H), 7.82 (d,
J = 8.8 Hz, 1H), 7.58 (d,
J = 8.8 Hz, 1H), 7.17 (d,
J = 2.8 Hz, 1H), 6.99 (dd,
J = 8.8, 2.8 Hz, 1H), 3.79 (s, 3H), 2.16 (s, 3H).
13C NMR (151 MHz, DMSO-
d6) δ 168.6, 158.0, 145.3, 144.5, 133.7, 132.2, 130.4, 128.2, 128.0, 122.8, 122.3, 114.1, 112.5, 55.7, 22.7. HPLC-MS (ESI):
m/
z calcd for C
15H
12N
2O
4S 316.05; [M + H]
+ found 317.10.
3.1.7. 1-(3-Amino-7-methoxy-10H-phenothiazin-10-yl)ethan-1-one (2)
To a solution of 1 (13.1 g, 41.4 mmol) in MeCN/H2O 10:1 v/v (260 mL, 26.0 mL) was added NiCl2∙6H2O (1.97 g, 8.28 mmol), and the mixture was stirred at room temperature for 5 min. NaBH4 (6.26 g, 165 mmol) was then added in portions, and the reaction was stirred at room temperature for a further 15 min. The reaction mixture was diluted with water and extracted with DCM. The organic phase was washed with water, dried over MgSO4, and evaporated to afford the product as a brown solid (11.0 g, 93%). Rf: 0.20 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 7.42 (d, J = 8.8 Hz, 1H), 7.19 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 2.8 Hz, 1H), 6.89 (dd, J = 8.8, 2.8 Hz, 1H), 6.66 (d, J = 2.4 Hz, 1H), 6.53 (dd, J = 8.5, 2.4 Hz, 1H), 5.30 (s, 2H), 3.76 (s, 3H), 2.04 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.9, 157.2, 147.4, 132.4, 127.8, 127.8, 127.4, 127.3, 127.3, 112.8, 112.5, 112.0, 111.4, 55.5, 22.4. HPLC-MS (ESI): m/z calcd for C15H14N2O2S 286.08; [M + H]+ found 287.10.
3.1.8. 1-(3-Bromo-7-methoxy-10H-phenothiazin-10-yl)ethan-1-one (3)
A solution of 2 (3.00 g, 10.5 mmol) in MeCN (1.00 L) was cooled to 0 °C under an argon atmosphere, and CuBr2 (3.51 g, 15.7 mmol) was added. t-BuONO (2.08 mL, 15.7 mmol) was added dropwise at 0 °C, and the mixture was stirred at room temperature for 3 h. The reaction was quenched with H3NSO3 aq. 48.5M (1.00 L) and extracted four times with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 5% to 45% B) to afford the product as a pink solid (1.07 g, 29%). Rf: 0.51 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 7.79 (d, J = 2.2 Hz, 1H), 7.56 (dd, J = 8.5, 2.2 Hz, 1H), 7.52 (d, J = 8.5 Hz, 2H), 7.13 (d, J = 2.8 Hz, 1H), 6.96 (dd, J = 8.8, 2.8 Hz, 1H), 3.78 (s, 3H), 2.10 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 157.7, 138.3, 138.3, 134.4, 131.2, 129.9, 129.9, 129.0, 128.0, 119.0, 113.7, 112.4, 55.7, 22.6. HPLC-MS (ESI): m/z calcd for C15H12BrNO2S 348.98; [M + H]+ found 349.95, 351.90.
3.1.9. 1-(3-Bromo-7-hydroxy-10H-phenothiazin-10-yl)ethan-1-one (4)
A solution of 3 (1.05 g, 3.01 mmol) in DCM (45.0 mL) was cooled to −78 °C under an argon atmosphere, and BBr3 1M in DCM (10.5 mL) was added. The reaction was stirred overnight at room temperature. The crude was poured into water and extracted with DCM. The organic phase was dried over MgSO4, evaporated, and further purified by flash chromatography (PE/EtOAc 8% to 66% B) to afford the product as a pink solid (632 mg, 63%). Rf: 0.37 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 9.90 (s, 1H), 7.77 (dd, J = 2.1, 1.1 Hz, 1H), 7.54 (dd, J = 8.3, 2.1 Hz, 1H), 7.50 (t, J = 8.3 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 6.89 (d, J = 2.7 Hz, 1H), 6.78 (dd, J = 8.7, 2.7 Hz, 1H), 2.09 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 156.1, 138.5, 138.4, 134.5, 132.4, 129.8, 129.8, 129.1, 128.0, 118.9, 114.6, 113.8, 22.6. HPLC-MS (ESI): m/z calcd for C14H10BrNO2S 334.96; [M + H]+ found 336.15, 337.95.
3.1.10. 1-(3-Bromo-7-(2-fluoroethoxy)-10H-phenothiazin-10-yl)ethan-1-one (5)
A solution of 4 (365 mg, 0.98 mmol) in dry DMF (24.0 mL) was cooled to 0 °C under an argon atmosphere, and a NaH 60% dispersion in mineral oil (58.6 mg, 1.47 mmol) was added. The mixture was stirred at 0 °C for 15 min. 1-Bromo-2-fluoroethane (0.11 mL, 1.47 mmol) was added dropwise, and the reaction was stirred overnight at room temperature. The reaction mixture was poured into water (220 mL) and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 8% to 66% B) to afford the product as a pink solid (301 mg, 91%). Rf: 0.18 (PE/EtOAc 2:1). 1H NMR (600 MHz, DMSO-d6) δ 7.79 (d, J = 2.1 Hz, 1H), 7.56 (dd, J = 8.4, 2.1 Hz, 1H), 7.55–7.47 (m, 2H), 7.18 (d, J = 2.8 Hz, 1H), 7.00 (dd, J = 8.8, 2.8 Hz, 1H), 4.73 (dt, J = 47.9, 3.9 Hz, 2H), 4.27 (dq, J = 30.4, 4.4 Hz, 2H), 2.10 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 156.6, 138.3, 138.3, 131.5, 130.0, 130.0, 129.9, 129.0, 128.1, 119.0, 114.2, 113.0, 82.0 (d, J = 166.6 Hz), 67.6 (d, J = 18.9 Hz), 22.6. HPLC-MS (ESI): m/z calcd for C16H13BrFNO2S 380.98; [M + H]+ found 382.05, 384.00.
3.1.11. 4-Bromo-2-iodoaniline (7)
NH4OAc 0.1% in MeOH (1.50 mL) was added to a solution of aniline (1.50 mL, 16.4 mmol) in MeCN (75.0 mL). N-Bromosuccinimide (2.92 g, 16.4 mmol) was added in portions, and the mixture was stirred at room temperature for 15 min. HPLC-MS confirmed that the mono-substitution of the aniline was achieved. N-Iodosuccinimide (4.07 g, 18.1 mmol) was added in portions, and the mixture was stirred at room temperature for 30 min. The solvent was concentrated under a vacuum and the crude was poured into water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 10% to 20% B) to afford the product (1.98 g, 40%). Rf: 0.64 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 7.65 (d, J = 2.3 Hz, 1H), 7.21 (dd, J = 8.6, 2.3 Hz, 1H), 6.70 (d, J = 8.6 Hz, 1H), 5.37 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 148.1, 139.3, 131.5, 115.5, 106.6, 83.4. HPLC-MS (ESI): m/z calcd for C6H5BrIN 296.87; found [M + H]+ 297.90, 299.85.
3.1.12. 4-Bromo-2-((3-methoxyphenyl)thio)aniline (8)
3-Methoxythiophenol (0.81 mL, 6.54 mmol) and potassium carbonate (1.09 g, 7.85 mmol) were added to a solution of 7 (1.95 g, 6.54 mmol) in NMP (20.0 mL) under argon atmosphere. CuI (62.3 mg, 5% mol) was added, and the mixture was stirred overnight at 100 °C. The reaction mixture was poured into water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 1% to 15% B) to afford the product (748 mg, 37%). Rf: 0.44 (PE/EtOAc 5:1). 1H NMR (600 MHz, DMSO-d6) δ 7.42 (d, J = 2.4 Hz, 1H), 7.30 (dd, J = 8.6, 2.4 Hz, 1H), 7.20 (t, J = 8.3 Hz, 1H), 6.78 (d, J = 8.6 Hz, 1H), 6.75 (ddd, J = 8.3, 2.4, 1.0 Hz, 1H), 6.66–6.62 (m, 2H), 5.59 (s, 2H), 3.69 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.7, 149.6, 137.9, 137.1, 133.5, 130.1, 119.0, 116.7, 114.1, 112.6, 111.2, 105.9, 55.1. HPLC-MS (ESI): m/z calcd for C13H12BrNOS 308.98; [M + H]+ found 309.90, 312.00.
3.1.13. N-(4-Bromo-2-((3-methoxyphenyl)thio)phenyl)acetamide (9)
Pyridine (3.5 mL) was added to a solution of 8 (735 mg, 2.37 mmol) in acetic anhydride (20.0 mL), and the mixture was stirred at room temperature for 3 h. The reaction mixture was poured into water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 1% to 20% B) to afford the product (708 mg, 85%). Rf: 0.28 (PE/EtOAc 5:1). 1H NMR (600 MHz, DMSO-d6) δ 9.43 (d, J = 22.9 Hz, 1H), 7.56 (d, J = 8.6 Hz, 1H), 7.49 (dd, J = 8.6, 2.4 Hz, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.31 (t, J = 8.2 Hz, 1H), 6.94–6.89 (m, 1H), 6.85 (d, J = 8.6 Hz, 1H), 6.85 (s, 1H), 3.73 (s, 3H), 2.00 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 159.8, 137.0, 134.8, 133.9, 130.9, 130.9, 130.5, 127.3, 122.8, 117.3, 116.1, 113.5, 55.2, 23.1. HPLC-MS (ESI): m/z calcd for C15H14BrNO2S 350.99; [M + H]+ found 352.05, 354.00.
3.1.14. 1-(Tetrahydro-2H-pyran-2-yl)-pyrazole (11)
To a solution of 1H-pyrazole (1.40 g, 21.0 mmol) in 3,4-dihydro-2H-pyran (2.57 mL, 27.3 mmol), TFA (0.50 mL, 6.21 mmol) was added. The mixture was refluxed for 45 min. The reaction mixture was diluted in water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 8% to 65% B) to afford the product as a colorless oil (2.97 g, 93%). Rf: 0.48 (PE/EtOAc 2:1). 1H NMR (600 MHz, DMSO-d6) δ 7.84 (dd, J = 2.4, 0.7 Hz, 1H), 7.47 (dd, J = 1.7, 0.7 Hz, 1H), 6.29 (dd, J = 2.4, 1.7 Hz, 1H), 5.40 (dd, J = 10.0, 2.5 Hz, 1H), 3.91 (dtd, J = 11.5, 3.8, 1.9 Hz, 1H), 3.67–3.57 (m, 1H), 2.15–2.02 (m, 1H), 1.97–1.86 (m, 2H), 1.71–1.62 (m, 1H), 1.57–1.50 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 138.7, 128.6, 105.7, 86.7, 66.8, 30.0, 24.7, 22.1. HPLC-MS (ESI): m/z calcd for C8H12N2O 152.09; [M + H]+ found 153.15.
3.1.15. 3-Bromo-1-(tetrahydro-2H-pyran-2-yl)-pyrazole (12)
A solution of 11 (2.97 g, 152 mmol) in THF (22.0 mL) was cooled to −78 °C under argon atmosphere. A solution of n-BuLi 2.5M in hexane (10.1 mL, 25.3 mmol) was added dropwise. Bromine (1.30 mL, 25.3 mmol) was added dropwise, and the mixture was stirred for 1 h. The reaction mixture was poured into water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and heated at 150 °C for a few seconds to allow conversion into the thermodynamically more stable 3-bromo regioisomer. It was purified by flash chromatography (PE/EtOAc 6% to 20% B) to afford the product as a light yellow oil (1.84 g, 41%). Rf: 0.33 (PE/EtOAc 2:1). 1H NMR (600 MHz, DMSO-d6) δ 7.89 (d, J = 2.5 Hz, 1H), 6.43 (d, J = 2.4 Hz, 1H), 5.37 (dd, J = 9.9, 2.4 Hz, 1H), 3.91 (dtd, J = 11.5, 4.0, 1.8 Hz, 1H), 3.66–3.56 (m, 1H), 2.10–2.00 (m, 1H), 1.96–1.85 (m, 2H), 1.70–1.59 (m, 1H), 1.56–1.49 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 131.8, 125.0, 108.5, 86.8, 66.8, 29.4, 24.5, 21.8. HPLC-MS (ESI): m/z calcd for C3H3BrN2 (THP cleaved by acidic conditions) 145.95; [M + H]+ found 147.00, 148.95.
3.1.16. 1-(Tetrahydro-2H-pyran-2-yl)-3-(tributylstannyl)-pyrazole (13)
To a solution of 12 (500 mg, 2.16 mmol) in toluene (6.00 mL), bis(tributyltin) (1.31 mL, 2.60 mmol) and Pd(PPh3)4 (250 mg, 0.22 mmol) were added under an argon atmosphere. The mixture was stirred overnight at 100 °C. The reaction mixture was diluted in water and extracted with EtOAc. The organic phase was dried over MgSO4, evaporated under reduced pressure, and purified by flash chromatography (PE/EtOAc 6% to 12% B) to afford the product as a colorless oil (359 mg, 38%). Rf: 0.46 (PE/EtOAc 9:1). 1H NMR (600 MHz, DMSO-d6) δ 7.86 (d, J = 2.3 Hz, 1H), 6.32 (d, J = 2.2 Hz, 1H), 5.45 (dd, J = 9.9, 2.6 Hz, 1H), 3.90 (dtd, J = 11.5, 3.8, 1.8 Hz, 1H), 3.67–3.56 (m, 1H), 2.07 (dddd, J = 12.9, 12.1, 9.8, 4.0 Hz, 1H), 1.95 (dtd, J = 13.3, 4.0, 1.8 Hz, 1H), 1.89 (dq, J = 12.9, 3.6 Hz, 1H), 1.72–1.64 (m, 1H), 1.65–1.55 (m, 2H), 1.55–1.51 (m, 6H), 1.29 (h, J = 7.8, 7.2 Hz, 6H), 1.01 (t, J = 7.9 Hz, 6H), 0.85 (t, J = 7.3 Hz, 9H). 13C NMR (151 MHz, DMSO-d6) δ 150.2, 128.1, 113.7, 86.4, 66.7, 30.1, 28.5, 26.6, 24.7, 22.1, 13.5, 9.4. HPLC-MS (ESI): m/z calcd for C20H38N2OSn 442.20; [M + H]+ found 443.25.
3.1.17. 3-Phenyl-1-(tetrahydro-2H-pyran-2-yl)-pyrazole (14a)
The synthesis was carried out according to general procedure A (149 mg, 58%). Rf: 0.51 (PE/EtOAc 3:1). 1H NMR (600 MHz, DMSO-d6) δ 7.92 (d, J = 2.5 Hz, 1H), 7.81 (dd, J = 8.2, 1.3 Hz, 2H), 7.40 (t, J = 7.7 Hz, 2H), 7.30 (tt, J = 7.4, 1.2 Hz, 1H), 6.77 (d, J = 2.4 Hz, 1H), 5.43 (dd, J = 10.1, 2.3 Hz, 1H), 3.95 (dtd, J = 13.1, 4.1, 1.8 Hz, 1H), 3.69–3.60 (m, 1H), 2.19–2.09 (m, 1H), 1.98–1.90 (m, 2H), 1.74–1.65 (m, 1H), 1.64–1.57 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 150.0, 133.2, 130.3, 128.6, 127.6, 125.2, 103.1, 86.9, 66.9, 29.9, 24.6, 22.1. HPLC-MS (ESI): m/z calcd for C9H8N2 144.07 and C14H16N2O 228.13; [M + H]+ found 145.20, 229.20.
3.1.18. 2-(1-(Tetrahydro-2H-pyran-2-yl)-1H-pyrazol-3-yl)pyridine (14b)
The synthesis was carried out according to general procedure A (171 mg, 66%). Rf: 0.23 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 8.57 (ddd, J = 4.8, 1.8, 1.0 Hz, 1H), 7.96 (t, J = 2.4 Hz, 1H), 7.94 (dq, J = 7.9, 1.2 Hz, 1H), 7.82 (td, J = 7.7, 1.8 Hz, 1H), 7.34–7.28 (m, 1H), 6.86 (t, J = 2.2 Hz, 1H), 5.46 (dt, J = 10.1, 2.1 Hz, 1H), 3.95 (dtd, J = 13.4, 4.2, 1.8 Hz, 1H), 3.69–3.62 (m, 1H), 2.19–2.10 (m, 1H), 1.98–1.92 (m, 2H), 1.74–1.63 (m, 1H), 1.58–1.51 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 151.7, 150.9, 149.3, 136.8, 130.5, 122.7, 119.3, 104.4, 87.0, 66.9, 29.8, 24.6, 22.0. HPLC-MS (ESI): m/z calcd for C8H7N3 145.06 and C13H15N3O 229.12; [M + H]+ found 146.10, 230.10.
3.1.19. 3-(3-Fluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-pyrazole (14c)
The synthesis was carried out according to general procedure A (386 mg, 69%). Rf: 0.61 (PE/EtOAc 2:1). 1H NMR (600 MHz, DMSO-d6) δ 7.94 (d, J = 2.5 Hz, 1H), 7.66 (dt, J = 7.7, 1.2 Hz, 1H), 7.59 (ddd, J = 10.6, 2.7, 1.6 Hz, 1H), 7.44 (td, J = 8.0, 6.2 Hz, 1H), 7.12 (tdd, J = 9.2, 2.7, 0.9 Hz, 1H), 6.83 (d, J = 2.5 Hz, 1H), 5.45 (dd, J = 10.1, 2.4 Hz, 1H), 3.95 (dtd, J = 11.5, 3.8, 1.8 Hz, 1H), 3.69–3.62 (m, 1H), 2.19–2.08 (m, 1H), 2.00–1.92 (m, 2H), 1.75–1.64 (m, 1H), 1.56 (tt, J = 7.8, 3.8 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 162.5 (d, J = 242.7 Hz), 148.8 (d, J = 3.0 Hz), 135.6 (d, J = 8.3 Hz), 130.5 (d, J = 8.6 Hz), 130.4, 121.1 (d, J = 2.8 Hz), 114.1 (d, J = 21.2 Hz), 111.6 (d, J = 22.4 Hz), 103.5, 86.8, 66.7, 29.7, 24.5, 21.9. HPLC-MS (ESI): m/z calcd for C9H7FN2 162.06 and C14H15FN2O 246.12; [M + H]+ found 163.05, 247.20.
3.1.20. 3-Phenyl-1-(tetrahydro-2H-pyran-2-yl)-5-(tributylstannyl)-pyrazole (15a)
The synthesis was carried out according to general procedure B (298 mg, 91%). Rf: 0.74 (PE/EtOAc 5:1). 1H NMR (600 MHz, DMSO-d6) δ 7.80 (dd, J = 8.3, 1.2 Hz, 2H), 7.38 (t, J = 7.7 Hz, 2H), 7.27 (tt, J = 7.4, 1.3 Hz, 1H), 6.74 (t, J = 3.9 Hz, 1H), 5.31–5.22 (m, 1H), 3.95 (dtd, J = 10.8, 3.6, 1.8 Hz, 1H), 3.68–3.58 (m, 1H), 2.12–2.04 (m, 2H), 2.03–1.95 (m, 1H), 1.72–1.64 (m, 1H), 1.64–1.56 (m, 2H), 1.56–1.47 (m, 6H), 1.31 (h, J = 7.3 Hz, 6H), 1.14–1.08 (m, 6H), 0.89–0.85 (m, 9H). 13C NMR (151 MHz, DMSO-d6) δ 149.8, 142.5, 133.5, 128.5, 127.3, 125.2, 112.1, 88.1, 66.9, 28.5, 27.7, 26.6, 26.2, 24.6, 13.5, 10.3. HPLC-MS (ESI): m/z calcd for C26H42N2OSn 518.23; [M + H]+ found 519.00.
3.1.21. 2-(1-(Tetrahydro-2H-pyran-2-yl)-5-(tributylstannyl)-1H-pyrazol-3-yl)pyridine (15b)
The synthesis was carried out according to general procedure B (245 mg, 68%). Rf: 0.25 (PE/EtOAc 5:1). 1H NMR (600 MHz, DMSO-d6) δ 8.56 (ddd, J = 4.8, 1.8, 0.9 Hz, 1H), 7.90 (dt, J = 8.0, 1.1 Hz, 1H), 7.80 (td, J = 7.7, 1.8 Hz, 1H), 7.28 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 6.88 (t, J = 4.0 Hz, 1H), 5.34–5.27 (m, 1H), 3.96 (dtd, J = 10.8, 4.2, 1.8 Hz, 1H), 3.69–3.60 (m, 1H), 2.09 (ddd, J = 11.0, 8.6, 4.2 Hz, 2H), 2.02–1.97 (m, 1H), 1.75–1.65 (m, 1H), 1.60–1.56 (m, 2H), 1.56–1.48 (m, 6H), 1.31 (h, J = 7.3 Hz, 6H), 1.14–1.08 (m, 6H), 0.86 (t, J = 7.3 Hz, 9H). 13C NMR (151 MHz, DMSO-d6) δ 152.0, 150.6, 149.2, 142.7, 136.7, 122.4, 119.4, 113.4, 88.2, 66.9, 30.7, 28.5, 26.6, 24.6, 21.7, 13.5, 10.4. HPLC-MS (ESI): m/z calcd for C25H41N3OSn 519.23; [M + H]+ found 520.35.
3.1.22. 3-(3-Fluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-5-(tributylstannyl)-pyrazole (15c)
The synthesis was carried out according to general procedure B (516 mg, 63%). Rf: 0.74 (PE/EtOAc 5:1). 1H NMR (600 MHz, DMSO-d6) δ 7.65 (dt, J = 7.7, 1.2 Hz, 1H), 7.59 (ddd, J = 10.5, 2.7, 1.5 Hz, 1H), 7.42 (td, J = 8.0, 6.2 Hz, 1H), 7.09 (tdd, J = 9.1, 2.6, 0.9 Hz, 1H), 6.82 (t, J = 4.1 Hz, 1H), 5.30–5.23 (m, 1H), 3.94 (dtd, J = 11.2, 3.9, 1.8 Hz, 1H), 3.67–3.58 (m, 1H), 2.10–2.04 (m, 2H), 1.97 (dtd, J = 13.2, 4.0, 1.6 Hz, 1H), 1.72–1.64 (m, 1H), 1.59–1.55 (m, 2H), 1.56–1.47 (m, 6H), 1.30 (h, J = 7.3 Hz, 6H), 1.13–1.07 (m, 6H), 0.86 (t, J = 7.3 Hz, 9H). 13C NMR (151 MHz, DMSO-d6) δ 162.6 (d, J = 242.3 Hz), 148.8 (d, J = 2.7 Hz), 142.9, 136.0 (d, J = 8.3 Hz), 130.6 (d, J = 8.5 Hz), 121.3 (d, J = 2.7 Hz), 113.9 (d, J = 20.9 Hz), 112.6, 111.7 (d, J = 22.5 Hz), 88.2, 66.9, 30.7, 28.5, 26.6, 24.6, 21.7, 13.5, 10.3. HPLC-MS (ESI): m/z calcd for C21H33FN2Sn (THP cleaved by acidic conditions) 452.16; [M+NH4]+ found 470.40.
3.1.23. 1-(3-Methoxy-7-(3-phenyl-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-10H-phenothiazin-10-yl)ethan-1-one (16a)
The synthesis was carried out according to general procedure C (149 mg, 53%). Rf: 0.35 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 7.87 (d, J = 7.3 Hz, 2H), 7.75 (d, J = 7.6 Hz, 2H), 7.60 (d, J = 8.1 Hz, 1H), 7.57 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 7.7 Hz, 2H), 7.33 (t, J = 7.4 Hz, 1H), 7.17 (d, J = 2.8 Hz, 1H), 7.02 (s, 1H), 6.97 (dd, J = 8.8, 2.8 Hz, 1H), 5.29 (d, J = 9.6 Hz, 1H), 4.03 (d, J = 7.3 Hz, 1H), 3.79 (s, 3H), 3.59 (t, J = 11.3 Hz, 1H), 2.50–2.43 (m, 1H), 2.17 (s, 3H), 1.97 (s, 1H), 1.85 (d, J = 13.0 Hz, 1H), 1.66–1.57 (m, 2H), 1.55–1.50 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 157.7, 149.6, 143.7, 139.3, 133.0, 132.8, 132.5, 131.2, 128.7, 128.1, 128.0, 127.9, 127.7, 127.4, 127.1, 125.3, 113.6, 112.4, 104.4, 83.8, 66.5, 55.7, 29.0, 24.5, 22.7, 22.0. HPLC-MS (ESI): m/z calcd for C24H19N3O2S (THP cleaved by acidic conditions) 413.12; [M + H]+ found 414.15.
3.1.24. 1-(3-Methoxy-7-(3-(pyridin-2-yl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-10H-phenothiazin-10-yl)ethan-1-one (16b)
The synthesis was carried out according to general procedure C (43.0 mg, 30%). Rf: 0.18 (PE/EtOAc 1:3). 1H NMR (600 MHz, DMSO-d6) δ 8.60 (dt, J = 4.8, 1.4 Hz, 1H), 8.00 (dd, J = 7.7, 1.2 Hz, 1H), 7.86 (td, J = 7.7, 1.9 Hz, 1H), 7.77 (s, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.63–7.60 (m, 1H), 7.57 (d, J = 8.9 Hz, 1H), 7.34 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 7.18 (d, J = 2.9 Hz, 1H), 7.08 (s, 1H), 6.97 (dd, J = 8.9, 2.9 Hz, 1H), 5.32 (dd, J = 11.6, 2.3 Hz, 1H), 4.08–4.01 (m, 1H), 3.79 (s, 3H), 3.65–3.56 (m, 1H), 2.49–2.40 (m, 1H), 2.17 (s, 3H), 2.05–1.95 (m, 1H), 1.87 (d, J = 12.8 Hz, 1H), 1.66–1.58 (m, 2H), 1.56–1.51 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 157.7, 151.4, 150.4, 149.3, 143.8, 139.4, 137.0, 136.9, 131.2, 128.8, 128.7, 128.0, 127.7, 127.5, 127.2, 123.0, 119.4, 113.6, 112.4, 105.3, 83.9, 66.5, 55.7, 29.0, 24.5, 22.7, 22.0. HPLC-MS (ESI): m/z calcd for C23H18N4O2S (THP cleaved by acidic conditions) 414.12; [M + H]+ found 415.05.
3.1.25. 1-(3-(3-(3-Fluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-7-methoxy-10H-phenothiazin-10-yl)ethan-1-one (16c)
The synthesis was carried out according to general procedure C (134 mg, 56%). Rf: 0.37 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 7.80–7.73 (m, 2H), 7.72 (dd, J = 7.8, 1.2 Hz, 1H), 7.65 (dt, J = 10.5, 2.0 Hz, 1H), 7.63–7.58 (m, 1H), 7.57 (d, J = 9.0 Hz, 1H), 7.48 (dq, J = 8.2, 1.8 Hz, 1H), 7.20–7.14 (m, 2H), 7.11 (s, 1H), 6.98 (dt, J = 8.9, 2.6 Hz, 1H), 5.30 (t, J = 6.4 Hz, 1H), 4.03 (dt, J = 9.9, 3.5 Hz, 1H), 3.79 (s, 3H), 3.60 (s, 1H), 2.46 (t, J = 11.3 Hz, 1H), 2.17 (s, 3H), 1.98–1.93 (m, 1H), 1.89–1.81 (m, 1H), 1.62 (h, J = 11.6 Hz, 2H), 1.53 (d, J = 12.2 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 162.6 (d, J = 242.6 Hz), 157.7, 148.5 (d, J = 2.3 Hz), 144.0, 139.4, 135.3 (d, J = 8.4 Hz), 132.6, 131.2, 130.8 (d, J = 8.3 Hz), 128.1, 128.0, 128.0, 127.7, 127.4, 127.2, 121.3 (d, J = 1.8 Hz), 114.6 (d, J = 21.0 Hz), 113.7, 112.4, 111.8 (d, J = 22.7 Hz), 104.8, 83.9, 66.6, 55.7, 29.0, 24.5, 22.7, 22.0. HPLC-MS (ESI): m/z calcd for C24H18FN3O2S (THP cleaved by acidic conditions) 431.11; [M + H]+ found 432.15.
3.1.26. 1-(3-(2-Fluoroethoxy)-7-(3-phenyl-1H-pyrazol-5-yl)-10H-phenothiazin-10-yl)ethan-1-one (17a)
The synthesis was carried out according to general procedure C (51.0 mg, 20%). Rf: 0.28 (PE/EtOAc 1:2). 1H NMR (600 MHz, DMSO-d6) δ 7.86 (dt, J = 7.0, 1.2 Hz, 2H), 7.76 (br s, 2H), 7.61 (d, J = 9.0 Hz, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.43 (t, J = 7.7 Hz, 2H), 7.34 (tt, J = 7.4, 1.2 Hz, 1H), 7.23 (d, J = 2.8 Hz, 1H), 7.03 (s, 1H), 7.02 (dd, J = 8.8, 2.8 Hz, 1H), 5.29 (d, J = 9.6 Hz, 1H), 4.74 (dt, J = 47.8, 3.9 Hz, 2H), 4.29 (d, J = 31.7 Hz, 2H), 4.03 (dp, J = 11.3, 2.1 Hz, 1H), 3.60 (br s, 1H), 2.49–2.43 (m, 1H), 2.17 (s, 3H), 2.01–1.95 (m, 1H), 1.85 (d, J = 12.9 Hz, 1H), 1.67–1.57 (m, 2H), 1.56–1.50 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 156.6, 149.6, 143.7, 139.2, 133.0, 132.9, 132.5, 131.5, 128.7, 128.2, 128.1, 127.9, 127.7, 127.4, 127.2, 125.3, 114.2, 113.1, 104.4, 83.9, 82.0 (d, J = 166.6 Hz), 67.6 (d, J = 18.8 Hz), 66.5, 29.1, 24.5, 22.7, 22.1. HPLC-MS (ESI): m/z calcd for C25H20FN3O2S (THP cleaved by acidic conditions) 445.13; [M + H]+ found 446.10.
3.1.27. 1-(3-(2-Fluoroethoxy)-7-(3-(pyridin-2-yl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-10H-phenothiazin-10-yl)ethan-1-one (17b)
The synthesis was carried out according to general procedure C (66.0 mg, 29%). Rf: 0.23 (PE/EtOAc 1:5). 1H NMR (600 MHz, DMSO-d6) δ 8.61 (ddd, J = 4.9, 1.8, 1.0 Hz, 1H), 8.00 (dt, J = 7.9, 1.1 Hz, 1H), 7.86 (td, J = 7.7, 1.8 Hz, 1H), 7.78 (s, 1H), 7.75 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.35 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 7.23 (d, J = 2.8 Hz, 1H), 7.08 (s, 1H), 7.02 (dd, J = 8.8, 2.8 Hz, 1H), 5.32 (d, J = 9.6 Hz, 1H), 4.74 (dt, J = 47.8, 3.9 Hz, 2H), 4.29 (d, J = 29.9 Hz, 2H), 4.04 (d, J = 11.4 Hz, 1H), 3.61 (s, 1H), 2.49–2.43 (m, 1H), 2.17 (s, 3H), 1.98 (br s, 1H), 1.85 (br s, 1H), 1.68–1.57 (m, 2H), 1.56–1.52 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 156.6, 151.4, 150.4, 149.3, 143.8, 139.3, 139.3, 136.9, 131.5, 128.8, 128.7, 128.1, 128.0, 127.7, 127.3, 123.0, 119.5, 114.2, 113.1, 105.3, 83.9, 82.0 (d, J = 166.8 Hz), 67.6 (d, J = 18.8 Hz), 66.6, 29.0, 24.5, 22.7, 22.0. HPLC-MS (ESI): m/z calcd for C24H19FN4O2S (THP cleaved by acidic conditions) 446.12; [M + H]+ found 447.15.
3.1.28. 1-(3-(2-Fluoroethoxy)-7-(3-(3-fluorophenyl)-1H-pyrazol-5-yl)-10H-phenothiazin-10-yl)ethan-1-one (17c)
The synthesis was carried out according to general procedure C (109 mg, 43%). Rf: 0.31 (PE/EtOAc 1:2). 1H NMR (600 MHz, DMSO-d6) δ 13.55 (d, J = 10.2 Hz, 1H), 7.99 (d, J = 1.8 Hz, 1H), 7.84 (dd, J = 39.6, 6.3 Hz, 1H), 7.73–7.66 (m, 2H), 7.66–7.60 (m, 2H), 7.57–7.51 (m, 2H), 7.36 (s, 1H), 7.21 (s, 1H), 7.00 (dd, J = 9.3, 2.6 Hz, 1H), 4.74 (dt, J = 47.8, 3.9 Hz, 2H), 4.29 (dt, J = 29.8, 5.0 Hz, 2H), 2.15 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 169.2, 163.7 (d, J = 243.3 Hz), 157.0, 150.8 (d, J = 13.8 Hz), 142.9, 133.5, 132.8, 132.5, 132.5, 132.0 (d, J = 9.9 Hz), 131.6, 129.2 (d, J = 11.7 Hz), 128.6, 128.6, 124.5, 124.4, 121.6, 115.3 (d, J = 13.6 Hz), 114.5, 113.5, 112.2 (d, J = 8.9 Hz), 101.3, 82.5 (d, J = 166.7 Hz), 68.1 (d, J = 18.9 Hz), 23.1. HPLC-MS (ESI): m/z calcd for C25H19F2N3O2S 463.12; [M + H]+ found 464.40.
3.1.29. N-(2-((3-Methoxyphenyl)thio)-4-(3-phenyl-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)phenyl)acetamide (18a)
The synthesis was carried out according to general procedure D (45.0 mg, 29%). Rf: 0.56 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 9.55 (s, 1H), 7.88–7.82 (m, 3H), 7.61–7.55 (m, 2H), 7.42 (t, J = 7.7 Hz, 2H), 7.33 (tt, J = 7.4, 1.1 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 6.93 (s, 1H), 6.88–6.84 (m, 2H), 6.82 (t, J = 2.1 Hz, 1H), 5.11 (dd, J = 10.0, 2.5 Hz, 1H), 3.85–3.80 (m, 1H), 3.71 (s, 3H), 3.19 (td, J = 11.3, 2.6 Hz, 1H), 2.42 (tdd, J = 13.5, 9.9, 4.2 Hz, 1H), 2.06 (s, 3H), 1.97–1.92 (m, 1H), 1.82–1.76 (m, 1H), 1.55–1.48 (m, 2H), 1.45–1.40 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 159.8, 149.6, 143.8, 138.9, 137.4, 136.3, 133.0, 132.8, 130.4, 129.0, 128.5, 127.7, 126.9, 125.3, 125.2, 121.9, 115.3, 112.6, 103.8, 83.8, 66.3, 55.1, 29.0, 24.4, 23.3, 22.0. HPLC-MS (ESI): m/z calcd for C24H21N3O2S (THP cleaved by acidic conditions) 415.14; [M + H]+ found 416.25.
3.1.30. N-(2-((3-Methoxyphenyl)thio)-4-(3-(pyridin-2-yl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)phenyl)acetamide (18b)
The synthesis was carried out according to general procedure D (65.0 mg, 21%). Rf: 0.17 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 9.63 (s, 1H), 8.59 (dt, J = 4.8, 1.9, 0.9 Hz, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.84 (td, J = 7.9, 1.9 Hz, 1H), 7.82 (d, J = 8.3 Hz, 1H), 7.58 (dd, J = 8.3, 2.2 Hz, 1H), 7.56 (d, J = 2.2 Hz, 1H), 7.33 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 6.98 (s, 1H), 6.88–6.84 (m, 3H), 5.12 (dd, J = 10.0, 2.4 Hz, 1H), 3.82 (dt, J = 12.1, 4.2 Hz, 1H), 3.71 (s, 3H), 3.16 (td, J = 11.4, 2.5 Hz, 1H), 2.46–2.40 (m, 1H), 2.06 (s, 3H), 1.93 (dd, J = 10.1, 3.7 Hz, 1H), 1.80 (dd, J = 13.7, 3.1 Hz, 1H), 1.60 (p, J = 7.7 Hz, 2H), 1.51 (dd, J = 10.1, 2.7 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.8, 159.9, 151.4, 150.4, 149.3, 144.0, 138.8, 136.9, 136.3, 131.4, 130.5, 129.0, 128.8, 128.7, 125.6, 123.0, 122.3, 119.4, 115.6, 112.7, 104.8, 84.0, 66.5, 55.2, 29.0, 24.4, 23.3, 22.1. HPLC-MS (ESI): m/z calcd for C23H20N4O2S (THP cleaved by acidic conditions) 416.13; [M + H]+ found 417.15.
3.1.31. N-(4-(3-(3-Fluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)-2-((3-methoxyphenyl)thio)phenyl)acetamide (18c)
The synthesis was carried out according to general procedure D (29.0 mg, 15%). Rf: 0.55 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 9.55 (s, 1H), 7.87 (d, J = 8.3 Hz, 1H), 7.69 (dt, J = 7.8, 1.2 Hz, 1H), 7.62 (ddd, J = 10.4, 2.7, 1.5 Hz, 1H), 7.59 (d, J = 2.0 Hz, 1H), 7.57 (dd, J = 8.3, 2.1 Hz, 1H), 7.46 (td, J = 8.0, 6.1 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.15 (tdd, J = 9.0, 2.6, 0.9 Hz, 1H), 7.01 (s, 1H), 6.87–6.84 (m, 2H), 6.82 (t, J = 2.1 Hz, 1H), 5.12 (dd, J = 10.0, 2.4 Hz, 1H), 3.84–3.80 (m, 1H), 3.71 (s, 3H), 3.19 (td, J = 11.3, 2.5 Hz, 1H), 2.42 (qd, J = 9.9, 6.6 Hz, 1H), 2.06 (s, 3H), 1.96–1.93 (m, 1H), 1.79 (dd, J = 13.0, 3.5 Hz, 1H), 1.55–1.49 (m, 2H), 1.44–1.40 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 162.5 (d, J = 242.8 Hz), 159.8, 148.4 (d, J = 2.9 Hz), 144.0, 139.0, 136.3, 135.3 (d, J = 8.2 Hz), 133.0, 130.6 (d, J = 8.4 Hz), 130.4, 129.0, 126.7, 125.3, 124.5, 121.9, 121.2 (d, J = 2.3 Hz), 115.3, 114.4 (d, J = 21.4 Hz), 112.6, 111.7 (d, J = 22.5 Hz), 104.3, 83.9, 66.4, 55.1, 28.9, 24.3, 23.3, 22.0. HPLC-MS (ESI): m/z calcd for C24H20FN3O2S (THP cleaved by acidic conditions) 433.13; [M + H]+ found 434.10.
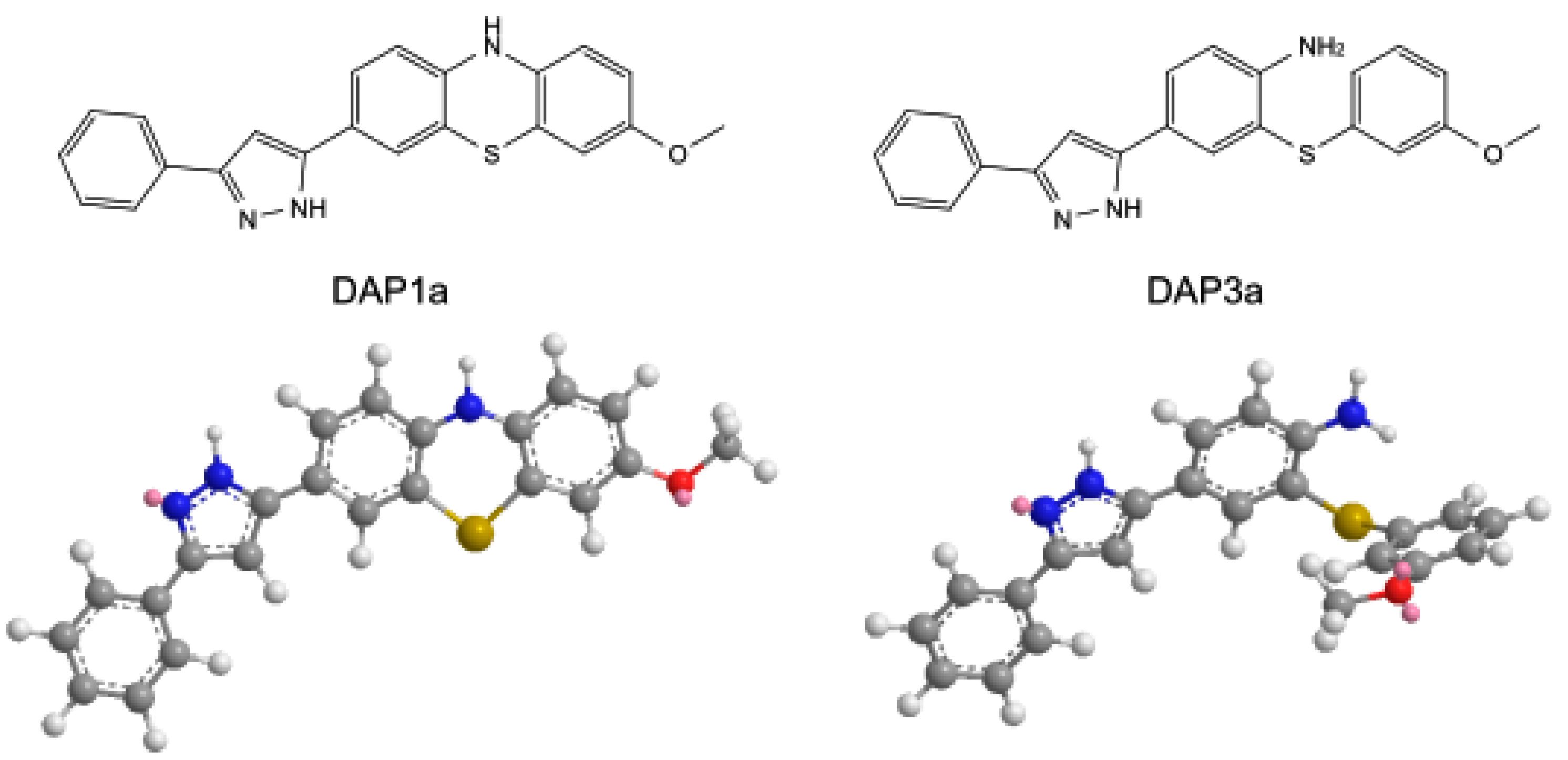
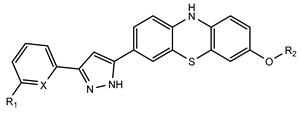
3.1.32. 3-Methoxy-7-(3-phenyl-1H-pyrazol-5-yl)-10H-phenothiazine (DAP1a)
The synthesis was carried out according to general procedure E (43.0 mg, 44%). Rf: 0.55 (PE/EtOAc 1:3). 1H NMR (600 MHz, DMSO-d6) δ 13.16 (d, J = 71.1 Hz, 1H), 8.52 (d, J = 59.0 Hz, 1H), 7.81 (dd, J = 38.5, 7.2 Hz, 2H), 7.51–7.37 (m, 4H), 7.32 (d, J = 27.8 Hz, 1H), 7.03 (d, J = 13.3 Hz, 1H), 6.72 (d, J = 8.2 Hz, 1H), 6.66 (d, J = 8.4 Hz, 1H), 6.62 (dd, J = 11.3, 2.3 Hz, 1H), 6.61 (d, J = 7.6 Hz, 1H), 3.67 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.7, 151.1, 142.8, 142.3, 134.8, 133.7, 129.0, 128.6, 127.3, 125.0, 124.6, 122.7, 117.0, 116.3, 115.2, 114.2, 113.2, 111.6, 98.5, 55.4. HPLC-MS (ESI): m/z calcd for C22H17N3OS 371.11; [M + H]+ found 372.15.
3.1.33. 3-Methoxy-7-(3-(pyridin-2-yl)-1H-pyrazol-5-yl)-10H-phenothiazine (DAP1b)
The synthesis was carried out according to general procedure E (9.00 mg, 34%). Rf: 0.35 (PE/EtOAc 1:5). 1H NMR (600 MHz, DMSO-d6) δ 13.32 (d, J = 117.9 Hz, 1H), 8.60 (s, 1H), 8.52 (d, J = 53.3 Hz, 1H), 7.93 (d, J = 44.3 Hz, 1H), 7.84 (s, 1H), 7.53–7.28 (m, 3H), 7.16 (d, J = 69.4 Hz, 1H), 6.70 (d, J = 8.2 Hz, 1H), 6.68–6.45 (m, 3H), 3.67 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.7, 149.8, 149.4, 149.3, 139.7, 137.2, 134.9, 134.0, 129.4, 124.7, 122.7, 122.6, 119.3, 117.2, 117.1, 115.1, 114.3, 113.2, 111.6, 99.9, 55.4. HPLC-MS (ESI): m/z calcd for C21H16N4OS 372.10; [M + H]+ found 373.05.
3.1.34. 3-(3-(3-Fluorophenyl)-1H-pyrazol-5-yl)-7-methoxy-10H-phenothiazine (DAP1c)
The synthesis was carried out according to general procedure E (26.0 mg, 29%). Rf: 0.34 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 13.24 (d, J = 50.7 Hz, 1H), 8.61 (d, J = 62.1 Hz, 1H), 7.66 (br s, 1H), 7.62 (d, J = 10.4 Hz, 1H), 7.44 (d, J = 31.7 Hz, 2H), 7.38 (s, 1H), 7.13 (br s, 1H), 7.11 (s, 1H), 6.72 (d, J = 8.2 Hz, 1H), 6.65 (d, J = 8.5 Hz, 1H), 6.62 (dd, J = 8.8, 2.3 Hz, 1H), 6.60 (d, J = 2.7 Hz, 1H), 3.67 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 162.6 (d, J = 242.7 Hz), 154.7, 150.3, 142.9, 142.4, 134.8, 133.4, 131.0, 130.7 (d, J = 8.9 Hz), 124.6, 122.8, 121.1, 117.0, 116.3, 115.2, 114.3, 114.0 (d, J = 23.7 Hz), 113.2, 111.6, 111.5, 99.0, 55.4. HPLC-MS (ESI): m/z calcd for C22H16FN3OS 389.10; [M + H]+ found 390.15.
3.1.35. 3-(2-Fluoroethoxy)-7-(3-phenyl-1H-pyrazol-5-yl)-10H-phenothiazine (DAP2a)
The synthesis was carried out according to general procedure E (14.0 mg, 40%). Rf: 0.50 (PE/EtOAc 1:2). 1H NMR (600 MHz, DMSO-d6) δ 13.10 (s, 1H), 8.56 (s, 1H), 7.80 (d, J = 7.6 Hz, 2H), 7.49–7.36 (m, 4H), 7.32 (t, J = 7.6 Hz, 1H), 7.03 (s, 1H), 6.80–6.56 (m, 4H), 4.68 (dt, J = 47.7, 3.9 Hz, 2H), 4.13 (d, J = 30.2 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 153.5, 146.9, 143.1, 142.1, 135.4, 132.1, 128.8, 127.7, 127.6, 125.0, 124.6, 122.8, 117.2, 116.2, 115.2, 114.3, 114.1, 112.5, 98.7, 82.2 (d, J = 166.8 Hz), 67.6 (d, J = 18.8 Hz). HPLC-MS (ESI): m/z calcd for C23H18FN3OS 403.12; [M + Na]+ found 446.10.
3.1.36. 3-(2-Fluoroethoxy)-7-(3-(pyridin-2-yl)-1H-pyrazol-5-yl)-10H-phenothiazine (DAP2b)
The synthesis was carried out according to general procedure E (12.0 mg, 26%). Rf: 0.44 (PE/EtOAc 1:5). 1H NMR (600 MHz, DMSO-d6) δ 13.33 (d, J = 118.9 Hz, 1H), 8.61 (d, J = 18.9 Hz, 1H), 8.54 (d, J = 49.4 Hz, 1H), 7.93 (dt, J = 47.1, 7.7 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 7.65–7.27 (m, 3H), 7.16 (d, J = 76.3 Hz, 1H), 6.68 (d, J = 38.2 Hz, 4H), 4.68 (d, J = 47.9 Hz, 2H), 4.14 (d, J = 30.1 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 153.5 (d, J = 24.5 Hz), 152.1 (d, J = 28.5 Hz), 150.6, 149.3 (d, J = 38.2 Hz), 142.3, 137.0 (d, J = 97.0 Hz), 135.6, 135.2, 127.1, 124.7 (d, J = 45.7 Hz), 122.9, 122.7 (d, J = 12.8 Hz), 119.5 (d, J = 114.6 Hz), 117.2 (d, J = 34.0 Hz), 116.1 (d, J = 62.7 Hz), 115.2, 114.3, 114.1, 112.5, 99.9 (d, J = 65.7 Hz), 82.2 (d, J = 167.0 Hz), 67.5 (d, J = 19.2 Hz). HPLC-MS (ESI): m/z calcd for C22H17FN4OS 404.11; [M + H]+ found 405.15.
3.1.37. 3-(2-Fluoroethoxy)-7-(3-(3-fluorophenyl)-1H-pyrazol-5-yl)-10H-phenothiazine (DAP2c)
The synthesis was carried out according to general procedure E (11.0 mg, 13%). Rf: 0.57 (PE/EtOAc 1:2). 1H NMR (600 MHz, DMSO-d6) δ 13.25 (d, J = 59.9 Hz, 1H), 8.56 (d, J = 52.0 Hz, 1H), 7.67 (br s, 1H), 7.61 (d, J = 10.5 Hz, 1H), 7.56–7.39 (m, 2H), 7.38 (s, 1H), 7.22–7.05 (m, 2H), 6.71 (d, J = 8.2 Hz, 1H), 6.65 (s, 3H), 4.68 (dt, J = 48.0, 3.8 Hz, 2H), 4.13 (dt, J = 30.3, 3.8 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 162.6 (d, J = 242.5 Hz), 153.6, 150.1, 143.1, 140.4, 135.2, 134.6, 131.7, 130.7 (d, J = 18.8 Hz), 124.7, 122.8, 121.1, 117.3, 117.1, 115.2, 114.3, 114.2 (d, J = 7.0 Hz), 114.1, 112.5, 111.5 (d, J = 11.9 Hz), 99.0, 82.2 (d, J = 166.4 Hz), 67.5 (d, J = 19.1 Hz). HPLC-MS (ESI): m/z calcd for C23H17F2N3OS 421.11; [M + MeCN + 2H]+ found 464.10.
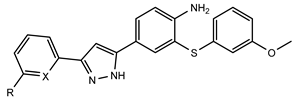
3.1.38. 2-((3-Methoxyphenyl)thio)-4-(3-phenyl-1H-pyrazol-5-yl)aniline (DAP3a)
The synthesis was carried out according to general procedure E (23.0 mg, 81%). Rf: 0.42 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 13.02 (s, 1H), 7.84 (d, J = 2.1 Hz, 1H), 7.81 (d, J = 7.7 Hz, 2H), 7.66 (d, J = 8.4 Hz, 1H), 7.42 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 7.20 (t, J = 8.0 Hz, 1H), 6.99 (s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.73 (ddd, J = 8.0, 2.1, 0.9 Hz, 1H), 6.68 (dt, J = 8.0, 2.1, 0.9 Hz, 1H), 6.66 (t, J = 2.1 Hz, 1H), 5.57 (s, 2H), 3.69 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.6, 150.1, 150.0, 143.2, 137.9, 137.8, 133.7, 129.9, 128.6, 128.5, 128.3, 127.4, 125.0, 118.5, 115.0, 112.1, 112.0, 110.8, 98.1, 55.0. HPLC-MS (ESI): m/z calcd for C22H19N3OS 373.12; [M + H]+ found 374.10.
3.1.39. 2-((3-Methoxyphenyl)thio)-4-(3-(pyridin-2-yl)-1H-pyrazol-5-yl)aniline (DAP3b)
The synthesis was carried out according to general procedure E (21.0 mg, 47%). Rf: 0.42 (PE/EtOAc 1:4). 1H NMR (600 MHz, DMSO-d6) δ 13.27 (d, J = 84.4 Hz, 1H), 8.59 (d, J = 4.9 Hz, 1H), 7.92 (s, 1H), 7.85 (d, J = 2.0 Hz, 2H), 7.69 (d, J = 6.1 Hz, 1H), 7.31 (br s, 1H), 7.20 (t, J = 8.0 Hz, 1H), 7.10 (d, J = 8.1 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.73 (dd, J = 8.4, 2.3 Hz, 1H), 6.70–6.62 (m, 2H), 5.65 (br s, 2H), 3.68 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 159.7, 152.1, 150.3, 149.2, 148.9, 141.9, 138.0, 136.8, 133.7, 130.0, 128.6, 128.5, 122.6, 119.2, 118.6, 115.1, 112.2, 111.9, 110.8, 99.2, 55.1. HPLC-MS (ESI): m/z calcd for C21H18N4OS 374.12; [M + H]+ found 375.15.
3.1.40. 4-(3-(3-Fluorophenyl)-1H-pyrazol-5-yl)-2-((3-methoxyphenyl)thio)aniline (DAP3c)
The synthesis was carried out according to general procedure E (16.0 mg, 68%). Rf: 0.45 (PE/EtOAc 1:1). 1H NMR (600 MHz, DMSO-d6) δ 13.10 (s, 1H), 7.84 (d, J = 2.0 Hz, 1H), 7.66 (t, J = 8.8 Hz, 2H), 7.62 (dt, J = 10.6, 2.0 Hz, 1H), 7.46 (q, J = 7.0, 6.6 Hz, 1H), 7.20 (t, J = 8.2 Hz, 1H), 7.12 (t, J = 9.7 Hz, 1H), 7.07 (s, 1H), 6.91 (d, J = 8.4 Hz, 1H), 6.73 (dd, J = 8.2, 2.4 Hz, 1H), 6.68 (dt, J = 8.2, 2.1, 1.0 Hz, 1H), 6.66 (t, J = 2.1 Hz, 1H), 5.61 (br s, 2H), 3.69 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 162.5 (d, J = 242.7 Hz), 159.7, 152.6, 150.2 (d, J = 14.8 Hz), 143.7, 137.8, 135.1 (d, J = 2.1 Hz), 133.8, 130.6 (d, J = 5.3 Hz), 130.1, 129.9, 128.3, 121.0 (d, J = 1.8 Hz), 118.5, 115.1, 112.1, 112.0, 111.5 (d, J = 23.1 Hz), 111.4, 110.8, 97.2, 55.0. HPLC-MS (ESI): m/z calcd for C22H18FN3OS 391.12; [M + H]+ found 392.10.