Edible Halophytes with Functional Properties: In Vitro Protein Digestibility and Bioaccessibility and Intestinal Absorption of Minerals and Trace Elements from Australian Indigenous Halophytes
Abstract
1. Introduction
2. Results and Discussion
2.1. Halophytes as Sources of Protein and Amino Acids
2.1.1. In Vitro Protein Digestibility of Halophyte Digesta Samples
2.1.2. Amino Acid Profile of Halophyte Samples
2.2. Halophytes as Sources of Minerals and Trace Elements
2.2.1. In Vitro Bioaccessibility of Minerals and Trace Elements
2.2.2. In Vitro Intestinal Absorption of Minerals and Trace Elements
Effects of Halophyte Digesta Samples on Cell Viability
Intestinal Absorption of Halophyte Minerals and Trace Elements
In Vitro Intestinal Iron Absorption Measured through Cellular Ferritin Production
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation of Samphire and Saltbush Test Foods
3.3. In Vitro Simulated Oral and Gastrointestinal Digestion
3.4. In Vitro Protein Digestibility
3.5. Analysis of Amino Acids
3.6. Cell Culture
3.7. Cell Viability Studies
3.8. Caco-2-HT29-MTX-E12 Co-Culture on Transwells
3.9. Intestinal Absorption Assay
3.10. Ferritin Measurements
3.11. Preparation and Analysis of Digesta Samples
3.11.1. Sample Preparation for Elemental Composition (Minerals and Trace Elements)
3.11.2. Sample Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant. Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Srivarathan, S.; Phan, A.D.T.; Hong, H.T.; Chua, E.T.; Wright, O.; Sultanbawa, Y.; Netzel, M.E. Tecticornia sp. (Samphire)—A Promising Underutilized Australian Indigenous Edible Halophyte. Front. Nutr. 2021, 8, 607799. [Google Scholar] [CrossRef]
- Srivarathan, S.; Phan, A.D.T.; Hong, H.T.; Netzel, G.; Wright, O.R.L.; Sultanbawa, Y.; Netzel, M.E. Nutritional composition and anti-nutrients of underutilized Australian indigenous edible halophytes—Saltbush, Seablite and Seapurslane. J. Food Compost. Anal. 2023, 115, 104876. [Google Scholar] [CrossRef]
- Stein, A. Global impacts of human mineral malnutrition. Plant Soil 2010, 335, 133–154. [Google Scholar] [CrossRef]
- Fleet, J.C.; Replogle, R.; Salt, D.E. Systems Genetics of Mineral Metabolism. J. Nutr. 2011, 141, 520–525. [Google Scholar] [CrossRef]
- Thompson, B.; Amoroso, L. Improving Diets and Nutrition: Food-Based Approaches; Centre for Agriculture and Bioscience International: Wallingford, UK, 2014. [Google Scholar]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Akter, S.; Addepalli, R.; Netzel, M.; Tinggi, U.; Fletcher, M.; Sultanbawa, Y.; Osborne, S. In vitro Bioaccessibility and Intestinal Absorption of Selected Bioactive Compounds in Terminalia ferdinandiana. Front. Nutr. 2021, 8, 818195. [Google Scholar] [CrossRef]
- Filbido, G.S.; Narita, I.M.P.; de Oliveira Pinheiro, A.P.; da Cruz e Silva, D.; Ferreira, B.A.; Nascimento, E.; Villa, R.D.; de Oliveira, A.P. In vitro bioaccessibility of minerals in fortified infant foods and correlation between mineral absorption facilitators and inhibitors. J. Food Meas. Charact. 2021, 15, 5648–5656. [Google Scholar] [CrossRef]
- Bertin, R.L.; Maltez, H.F.; Gois, J.S.d.; Borges, D.L.G.; Borges, G.d.S.C.; Gonzaga, L.V.; Fett, R. Mineral composition and bioaccessibility in Sarcocornia ambigua using ICP-MS. J. Food Compost. Anal. 2016, 47, 45–51. [Google Scholar] [CrossRef]
- Khouzam, R.B.; Pohl, P.; Lobinski, R. Bioaccessibility of essential elements from white cheese, bread, fruit and vegetables. Talanta 2011, 86, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento da Silva, E.; de Farias, L.O.; Cadore, S. The total concentration and bioaccessible fraction of nutrients in purées, instant cereals and infant formulas by ICP OES: A study of Dietary Recommended Intakes and the importance of using a standardized in vitro digestion method. J. Food Compost. Anal. 2018, 68, 65–72. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- De Oliveira Gonçalves, T.; Filbido, G.S.; de Oliveira Pinheiro, A.P.; Pinto Piereti, P.D.; Dalla Villa, R.; de Oliveira, A.P. In vitro bioaccessibility of the Cu, Fe, Mn and Zn in the baru almond and bocaiúva pulp and, macronutrients characterization. J. Food Compost. Anal. 2020, 86, 103356. [Google Scholar] [CrossRef]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef]
- Cairns, M.T.; Gupta, A.; Naughton, J.A.; Kane, M.; Clyne, M.; Joshi, L. Glycosylation-related gene expression in HT29-MTX-E12 cells upon infection by Helicobacter pylori. World J. Gastroenterol. 2017, 23, 6817–6832. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Srivarathan, S.; Phan, A.D.T.; Wright, O.; Sultanbawa, Y.; Netzel, M.E.; Cozzolino, D. The Measurement of Antioxidant Capacity and Colour Attributes in Wild Harvest Samphire (Tecticornia sp.) Samples Using Mid-infrared Spectroscopy. Food Anal. Methods 2021, 14, 2328–2334. [Google Scholar] [CrossRef]
- Srivarathan, S.; Phan, A.D.T.; Wright, O.; Cozzolino, D.; Sultanbawa, Y.; Netzel, M.E. Saltbush (Atriplex sp.). In Handbook of Phytonutrients in Indigenous Fruits and Vegetables; Centre for Agriculture and Bioscience International: Wallingford, UK, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Abbeddou, S.; Rihawi, S.; Hess, H.D.; Iñiguez, L.; Mayer, A.C.; Kreuzer, M. Nutritional composition of lentil straw, vetch hay, olive leaves, and saltbush leaves and their digestibility as measured in fat-tailed sheep. Small Rumin. Res. 2011, 96, 126–135. [Google Scholar] [CrossRef]
- Wright, K.H.; Pike, O.A.; Fairbanks, D.J.; Huber, C.S. Composition of Atriplex hortensis, Sweet and Bitter Chenopodium quinoa Seeds. J. Food Sci. 2002, 67, 1383–1385. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Comparative effects of high pressure processing and heat treatment on in vitro digestibility of pea protein and starch. NPJ Sci. Food 2022, 6, 2. [Google Scholar] [CrossRef]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef]
- Habiba, R.A. Changes in anti-nutrients, protein solubility, digestibility, and HCl-extractability of ash and phosphorus in vegetable peas as affected by cooking methods. Food Chem. 2002, 77, 187–192. [Google Scholar] [CrossRef]
- Nestares, T.; López-Frías, M.; Barrionuevo, M.; Urbano, G. Nutritional assessment of raw and processed chickpea (Cicer arietinum L.) protein in growing rats. J. Agric. Food Chem. 1996, 44, 2760–2765. [Google Scholar] [CrossRef]
- Alain Mune Mune, M.; Nyobe, E.C.; Bakwo Bassogog, C.; Minka, S.R. A comparison on the nutritional quality of proteins from Moringa oleifera leaves and seeds. Cogent Food Agric. 2016, 2, 1213618. [Google Scholar] [CrossRef]
- Darragh, A.J.; Moughan, P.J. The Effect of Hydrolysis Time on Amino Acid Analysis. J. AOAC Int. 2019, 88, 888–893. [Google Scholar] [CrossRef]
- Skylas, D.J.; Blanchard, C.L.; Quail, K.J. Variation in nutritional composition of Australian mungbean varieties. J. Agric. Sci. 2017, 9, 45–53. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Kassem, L.T.A.; Shaher, F.M.; Al-Farawati, R.; Ghandourah, M. Phytochemical Compositions of Some Red Sea Halophyte Plants with Antioxidant and Anticancer Potentials. Molecules 2022, 27, 3415. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.d.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef] [PubMed]
- Nasir, F.A.; Batarseh, M.; Abdel-Ghani, A.H.; Jiries, A. Free amino acids content in some halophytes under salinity stress in arid environment, Jordan. CLEAN Soil Air Water 2010, 38, 592–600. [Google Scholar] [CrossRef]
- Min, J.-G.; Lee, D.-S.; Kim, T.-J.; Park, J.-H.; Cho, T.-Y.; Park, D.-I. Chemical Composition of Salicornia herbacea L. Prev. Nutr. Food Sci. 2002, 7, 105–107. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 364976. [Google Scholar] [CrossRef]
- Marolt, G.; Gričar, E.; Pihlar, B.; Kolar, M. Complex Formation of Phytic Acid With Selected Monovalent and Divalent Metals. Front. Chem. 2020, 8, 582746. [Google Scholar] [CrossRef]
- Khoja, K.K.; Aslam, M.F.; Sharp, P.A.; Latunde-Dada, G.O. In vitro bioaccessibility and bioavailability of iron from fenugreek, baobab and moringa. Food Chem. 2021, 335, 127671. [Google Scholar] [CrossRef]
- Aspuru, K.; Villa, C.; Bermejo, F.; Herrero, P.; López, S.G. Optimal management of iron deficiency anemia due to poor dietary intake. Int. J. Gen. Med. 2011, 4, 741–750. [Google Scholar] [CrossRef]
- D’Imperio, M.; Montesano, F.F.; Serio, F.; Santovito, E.; Parente, A. Mineral Composition and Bioaccessibility in Rocket and Purslane after Zn Biofortification Process. Foods 2022, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Sachdev, H.S.; Boy, E. Effect of iron-fortified foods on hematologic and biological outcomes: Systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 309–324. [Google Scholar] [CrossRef]
- Joshi, V.; Thatte, P.; Prakash, J.; Jyothi Lakshmi, A. Effect of oilseed protein concentrates and exogenous amino acids on the dialysability of iron and zinc. LWT Food Sci. Technol. 2014, 59, 540–546. [Google Scholar] [CrossRef]
- Bodiga, S.; Krishnapillai, M.N. Concurrent repletion of iron and zinc reduces intestinal oxidative damage in iron- and zinc-deficient rats. World J. Gastroenterol. 2007, 13, 5707–5717. [Google Scholar] [CrossRef]
- Hemalatha, S.; Platel, K.; Srinivasan, K. Zinc and iron contents and their bioaccessibility in cereals and pulses consumed in India. Food Chem. 2007, 102, 1328–1336. [Google Scholar] [CrossRef]
- Cámara, F.; Amaro, M.A.; Barberá, R.; Clemente, G. Bioaccessibility of minerals in school meals: Comparison between dialysis and solubility methods. Food Chem. 2005, 92, 481–489. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Mattera, M.; Aguzzi, A.; Pusztai, A. Investigation of the mechanisms affecting Cu and Fe bioavailability from legumes. Biol. Trace Elem. Res. 2001, 84, 181–196. [Google Scholar] [CrossRef]
- Anderson, J.J.B. Minerals. In Krause’s Food Nutrition and Diet Therapy; Saunders: Philadelphia, PA, USA, 2000; pp. 111–152. [Google Scholar]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- WHO. Report of the Formal Meeting of Member States to Conclude the Work on the Comprehensive Global Monitoring Framework, Including Indicators, and a Set of Voluntary Global Targets for the Prevention and Control of Noncommunicable Diseases; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- NHRMC. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2017.
- Zimmermann, M.B.; Chaouki, N.; Hurrell, R.F. Iron deficiency due to consumption of a habitual diet low in bioavailable iron: A longitudinal cohort study in Moroccan children. Am. J. Clin. Nutr. 2005, 81, 115–121. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461s–1467s. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Lombardi-Boccia, G.; Carbonaro, M.; Lullo, G.D.; Carnovale, E. Influence of protein components (G1, G2 and albumin) on Fe and Zn dialysability from bean (Phaseolus vulgaris L.). Int. J. Food Sci. Nutr. 1994, 45, 183–190. [Google Scholar] [CrossRef]
- Yun, S.; Habicht, J.-P.; Miller, D.D.; Glahn, R.P. An In Vitro Digestion/Caco-2 Cell Culture System Accurately Predicts the Effects of Ascorbic Acid and Polyphenolic Compounds on Iron Bioavailability in Humans. J. Nutr. 2004, 134, 2717–2721. [Google Scholar] [CrossRef]
- Miller, D.D.; Berner, L.A. Is solubility in vitro a reliable predictor of iron bioavailability? Biol. Trace Elem. Res. 1989, 19, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Chadare, F.J.; Linnemann, A.R.; Hounhouigan, J.D.; Nout, M.J.; Van Boekel, M.A. Baobab food products: A review on their composition and nutritional value. Crit. Rev. Food Sci. Nutr. 2009, 49, 254–274. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (ascorbic acid). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 Cell Ferritin Formation Predicts Nonradiolabeled Food Iron Availability in an In Vitro Digestion/Caco-2 Cell Culture Model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef]
- Beard, J.L.; Dawson, H.; Piñero, D.J. Iron metabolism: A comprehensive review. Nutr. Rev. 1996, 54, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Hernandez, X.; Nichols, G.M.; Glass, J. Caco-2 cell line: A system for studying intestinal iron transport across epithelial cell monolayers. Biochim. Biophys. Acta BBA Biomembr. 1991, 1070, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Gangloff, M.B.; Lai, C.; Van Campen, D.R.; Miller, D.D.; Norvell, W.A.; Glahn, R.P. Ferrous iron uptake but not transfer is down-regulated in Caco-2 cells grown in high iron serum-free medium. J. Nutr. 1996, 126, 3118–3127. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H. Defining the Unique Flavours of Australian Native Foods; Rural Industries Research and Development Corporation: Canberra, Australia, 2010.
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Osborne, S.; Chen, W.; Addepalli, R.; Colgrave, M.; Singh, T.; Tran, C.; Day, L. In vitro transport and satiety of a beta-lactoglobulin dipeptide and beta-casomorphin-7 and its metabolites. Food Funct. 2014, 5, 2706–2718. [Google Scholar] [CrossRef]
- Yu, K.-F.; Kamber, B.S.; Lawrence, M.G.; Greig, A.; Zhao, J.-X. High-precision analysis on annual variations of heavy metals, lead isotopes and rare earth elements in mangrove tree rings by inductively coupled plasma mass spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 255, 399–408. [Google Scholar] [CrossRef]
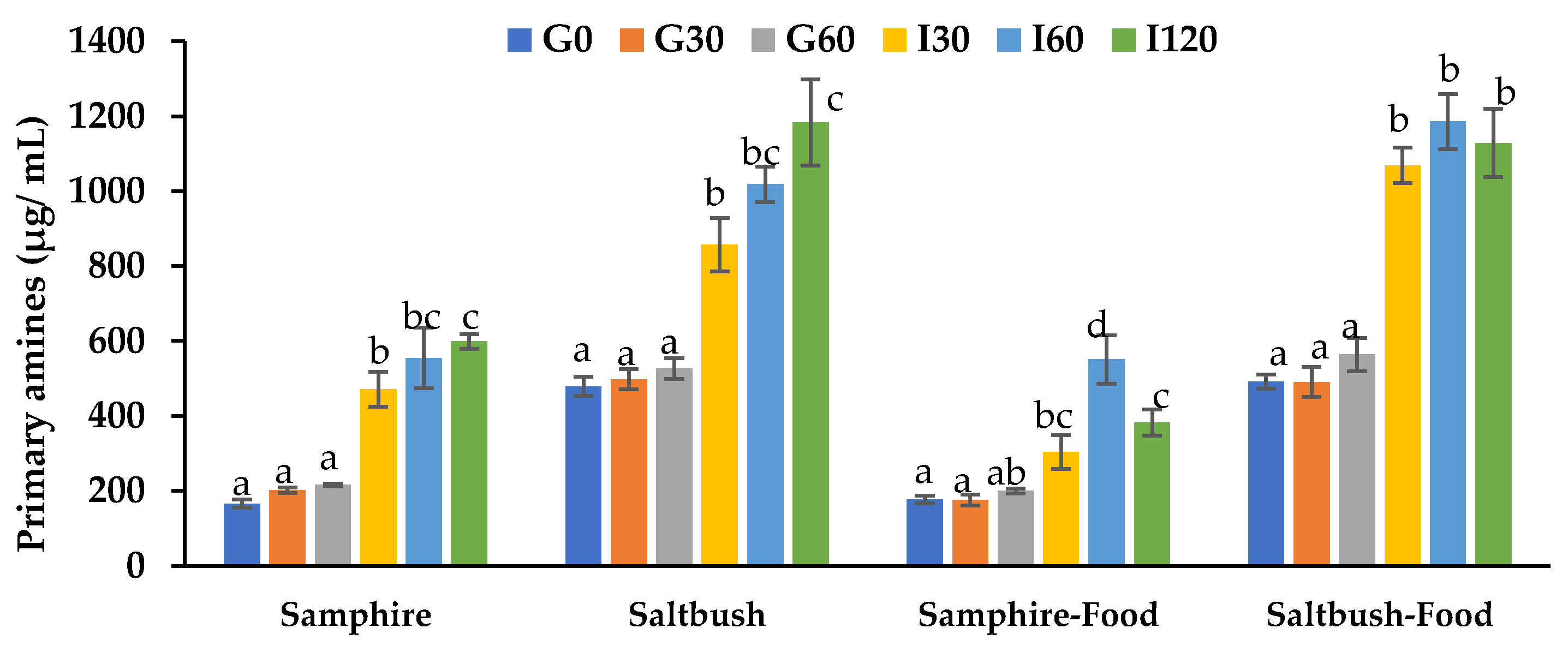



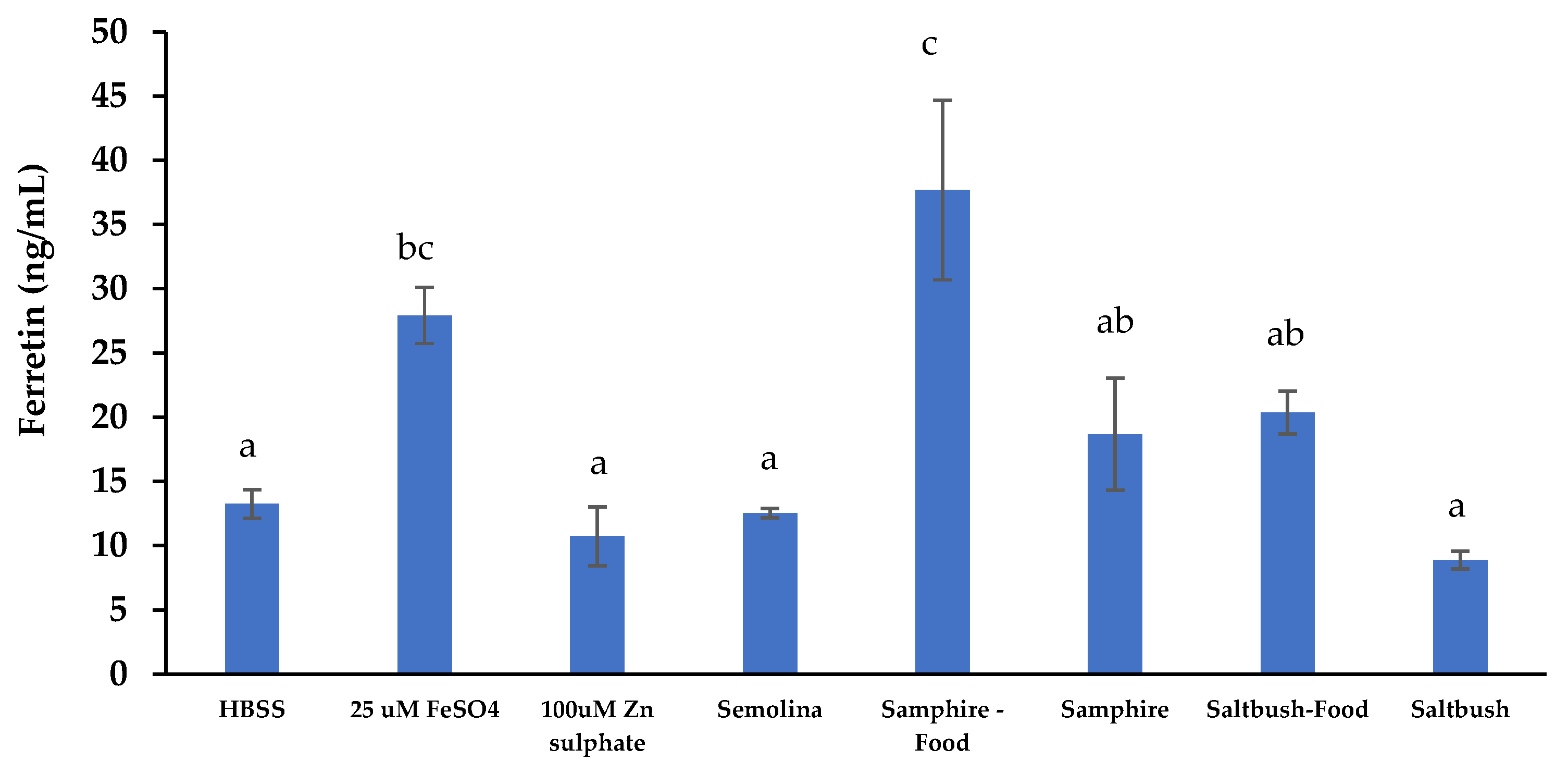
| Samphire | Saltbush | |
|---|---|---|
| Essential amino acids (mg/g DW) | ||
| Histidine * | 1.2 | 2.4 |
| Threonine * | 2.2 | 4.5 |
| Lysine * | 2.6 | 6.3 |
| Valine * | 2.8 | 5.7 |
| Isoleucine * | 2.2 | 4.7 |
| Leucine * | 3.7 | 7.8 |
| Phenylalanine * | 2.3 | 5.2 |
| Methionine * | ND | 0.8 |
| Non-essential amino acids (mg/g DW) | ||
| Alanine | 2.8 | 5.3 |
| Proline | 2.3 | 5.6 |
| Tyrosine | 1.4 | 3.0 |
| Serine | 2.6 | 4.6 |
| Arginine | 2.5 | 5.1 |
| Glycine | 3.4 | 5.7 |
| Aspartic acid | 4.8 | 9.5 |
| Glutamic acid | 5.6 | 11.1 |
| Total Amino acids | 42.5 | 87.3 |
| Samphire | Saltbush | Samphire-Food | Saltbush-Food | |
|---|---|---|---|---|
| G30 | −0.2 | 0.1 | 0.3 | 0.0 |
| G60 | 6.9 | 5.8 | 14.4 | 14.8 |
| I30 | 133.3 | 72.2 | 73.5 | 117.6 |
| I60 | 174.7 | 104.5 | 214.7 | 141.6 |
| I120 | 196.6 | 137.7 | 118.4 | 129.8 |
| Samples | Na Papp (× 10−6 cm/s) | K Papp (× 10−6 cm/s) | Mg Papp (× 10−6 cm/s) | Zn Papp (× 10−6 cm/s) | Fe Papp (× 10−6 cm/s) |
|---|---|---|---|---|---|
| Samphire | 10.4 ± 1.7 a | 11.4 ± 1.4 b | 2.1 ± 0.2 a | 2.4 ± 0.3 b | 1.0 ± 0.3 a |
| Samphire-Food | 10.8 ± 2.4 a | 26.0 ± 1.6 c | 4.5 ± 0.4 b | 0.9 ± 0.2 a | 1.0 ± 0.2 a |
| Saltbush | 5.0 ± 1.1 a | 4.4 ± 0.0 a | 1.5 ± 0.1 a | 1.5 ± 0.1 a | 2.8 ± 0.3 b |
| Saltbush-Food | 3.7 ± 0.5 a | 35.4 ± 0.6 d | 3.9 ± 0.8 ab | 1.0 ± 0.1 a | 2.3 ± 0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivarathan, S.; Addepalli, R.; Adiamo, O.Q.; Kodagoda, G.K.; Phan, A.D.T.; Wright, O.R.L.; Sultanbawa, Y.; Osborne, S.; Netzel, M.E. Edible Halophytes with Functional Properties: In Vitro Protein Digestibility and Bioaccessibility and Intestinal Absorption of Minerals and Trace Elements from Australian Indigenous Halophytes. Molecules 2023, 28, 4004. https://doi.org/10.3390/molecules28104004
Srivarathan S, Addepalli R, Adiamo OQ, Kodagoda GK, Phan ADT, Wright ORL, Sultanbawa Y, Osborne S, Netzel ME. Edible Halophytes with Functional Properties: In Vitro Protein Digestibility and Bioaccessibility and Intestinal Absorption of Minerals and Trace Elements from Australian Indigenous Halophytes. Molecules. 2023; 28(10):4004. https://doi.org/10.3390/molecules28104004
Chicago/Turabian StyleSrivarathan, Sukirtha, Rama Addepalli, Oladipupo Qudus Adiamo, Gethmini Kavindya Kodagoda, Anh Dao Thi Phan, Olivia Renee Louise Wright, Yasmina Sultanbawa, Simone Osborne, and Michael Erich Netzel. 2023. "Edible Halophytes with Functional Properties: In Vitro Protein Digestibility and Bioaccessibility and Intestinal Absorption of Minerals and Trace Elements from Australian Indigenous Halophytes" Molecules 28, no. 10: 4004. https://doi.org/10.3390/molecules28104004
APA StyleSrivarathan, S., Addepalli, R., Adiamo, O. Q., Kodagoda, G. K., Phan, A. D. T., Wright, O. R. L., Sultanbawa, Y., Osborne, S., & Netzel, M. E. (2023). Edible Halophytes with Functional Properties: In Vitro Protein Digestibility and Bioaccessibility and Intestinal Absorption of Minerals and Trace Elements from Australian Indigenous Halophytes. Molecules, 28(10), 4004. https://doi.org/10.3390/molecules28104004










