Polymerization-Enhanced Photophysical Performances of AIEgens for Chemo/Bio-Sensing and Therapy
Abstract
1. Introduction
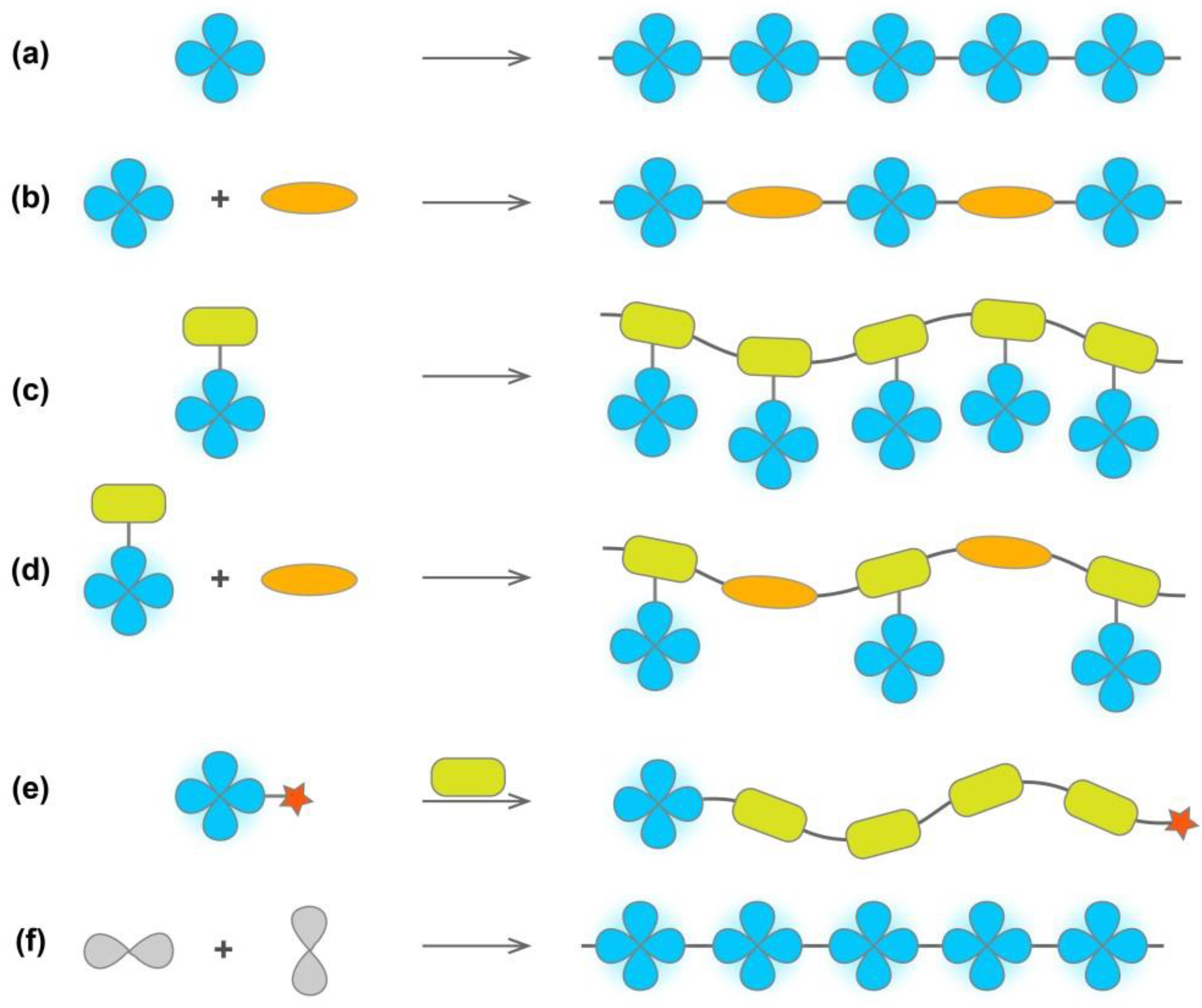
2. Design of AIE-Active Polymers
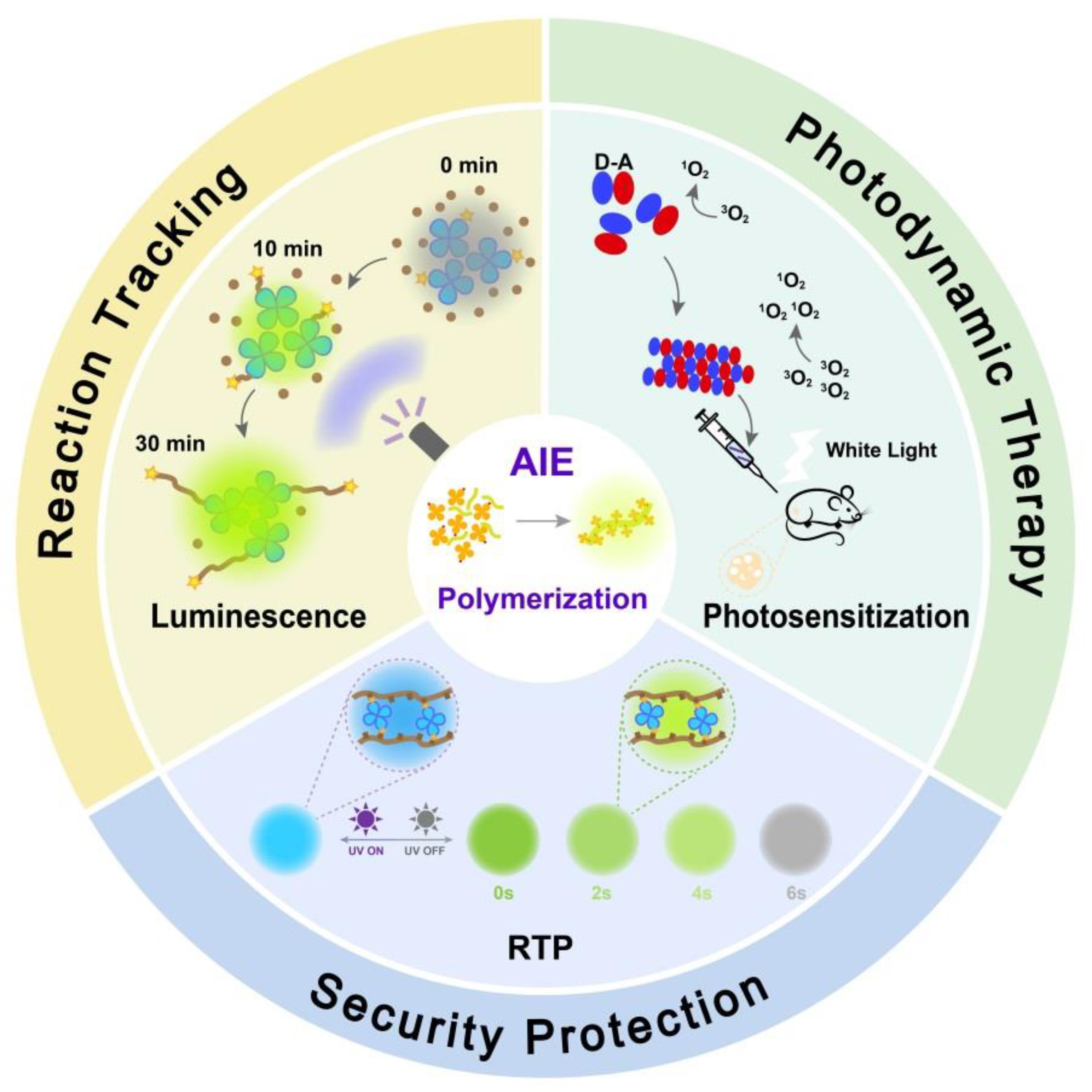
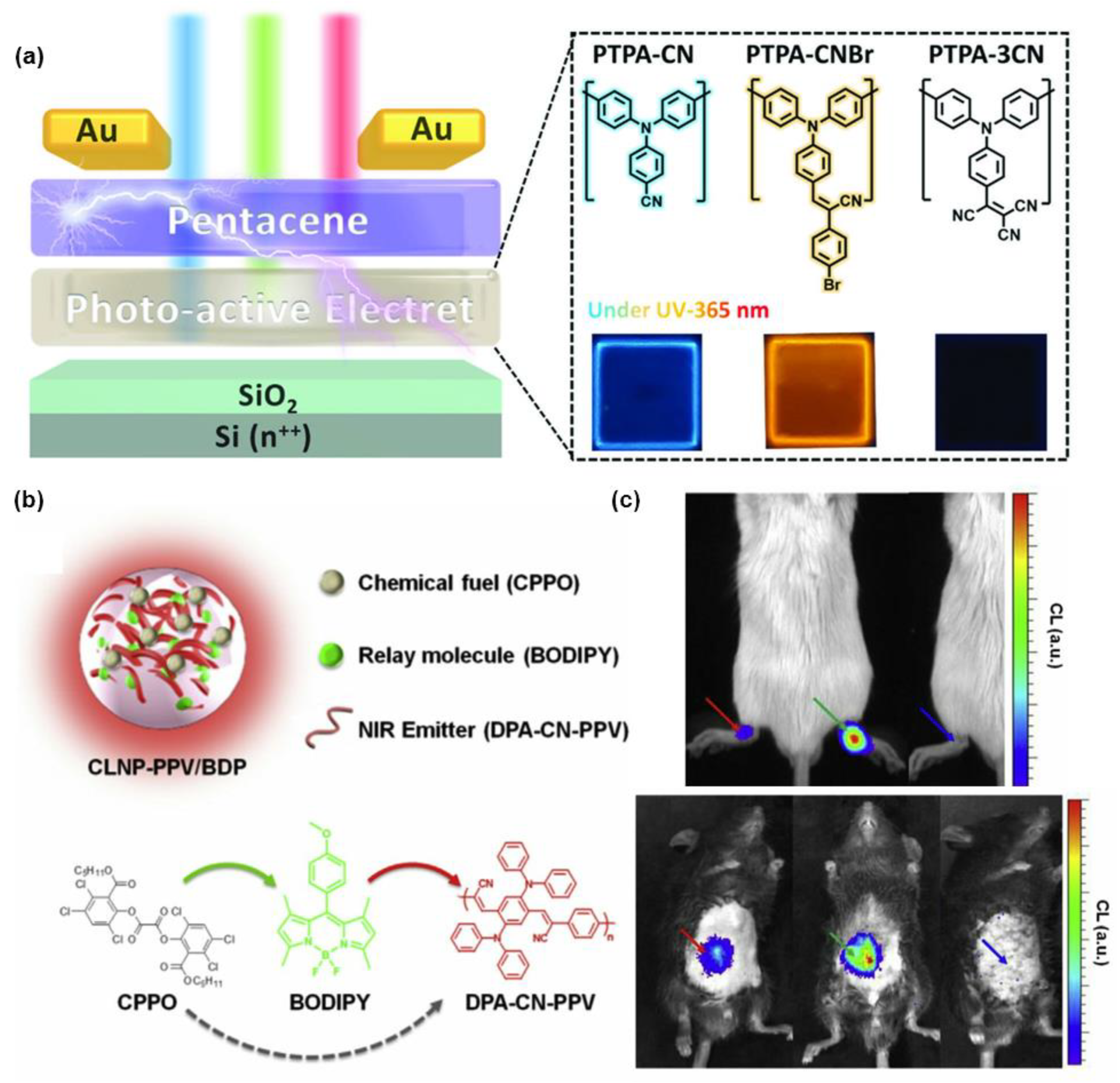
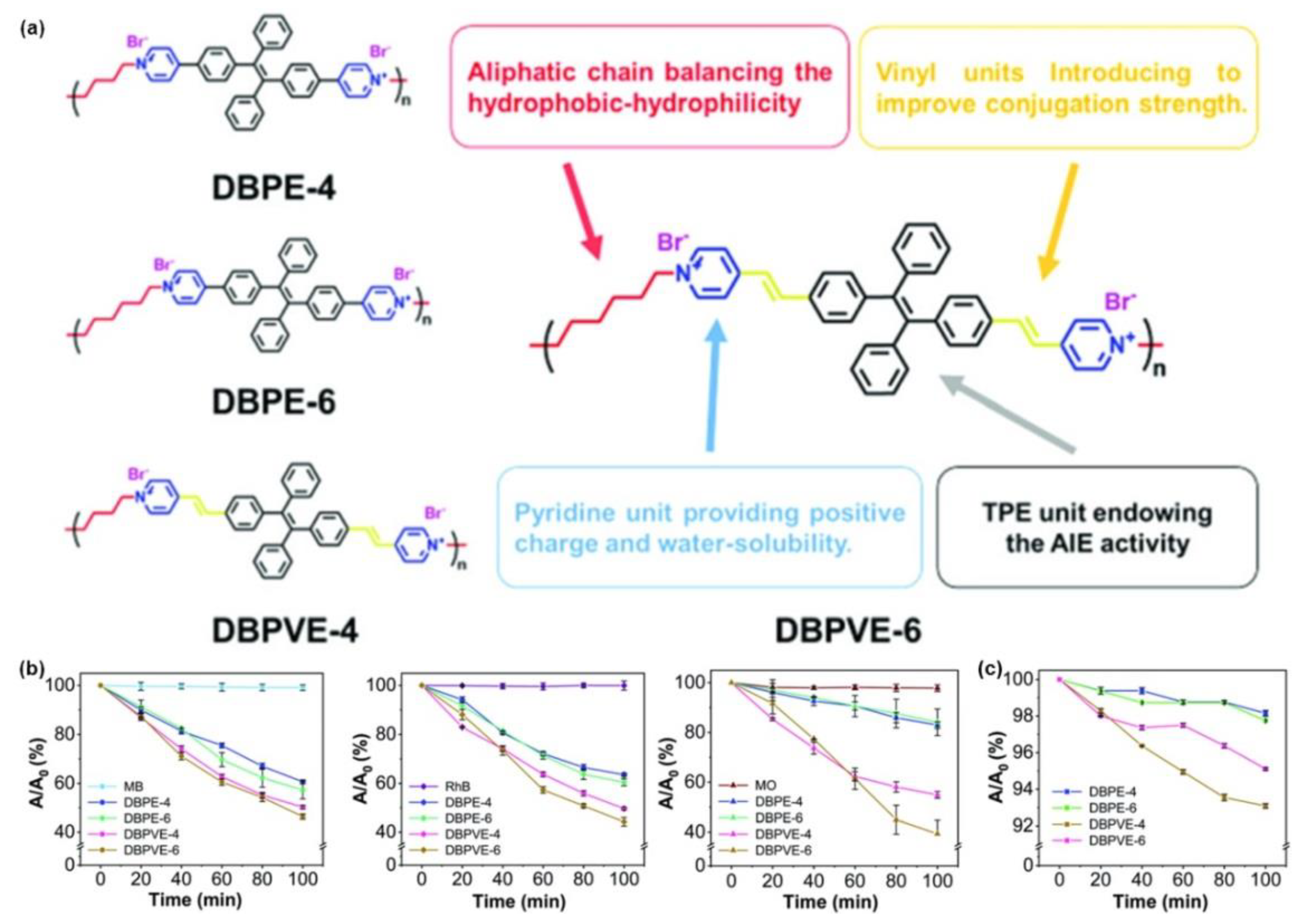
3. Polymerization-Enhanced Luminescence for Reaction Tracking and Responsive Materials
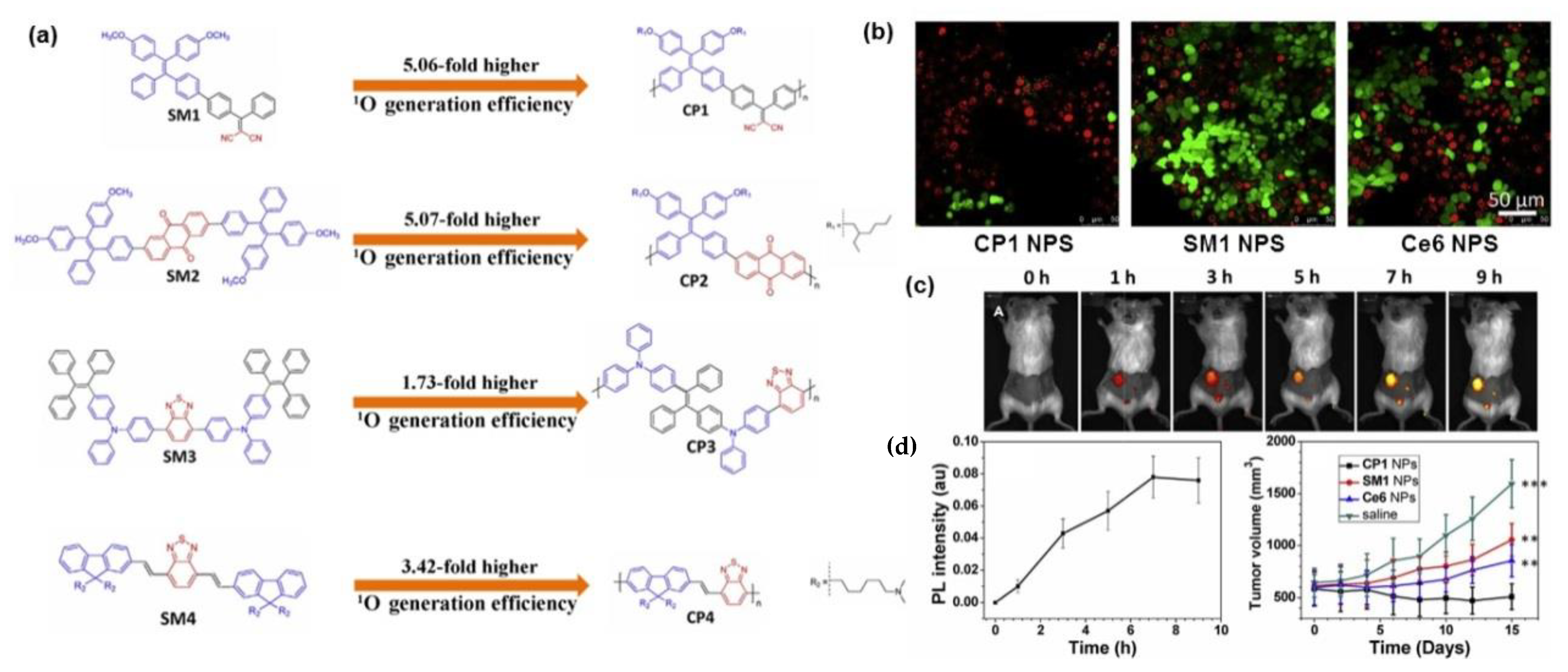
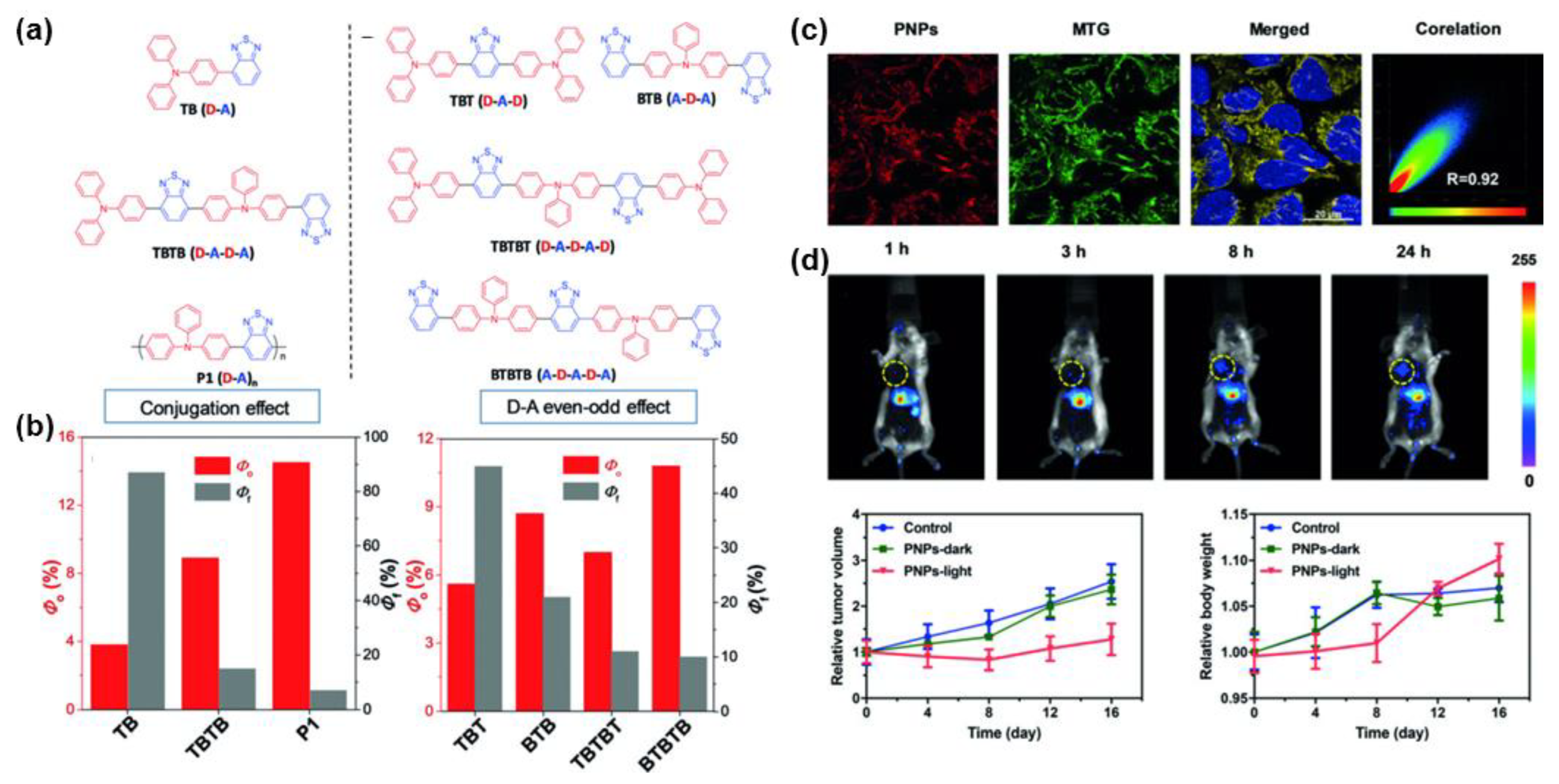
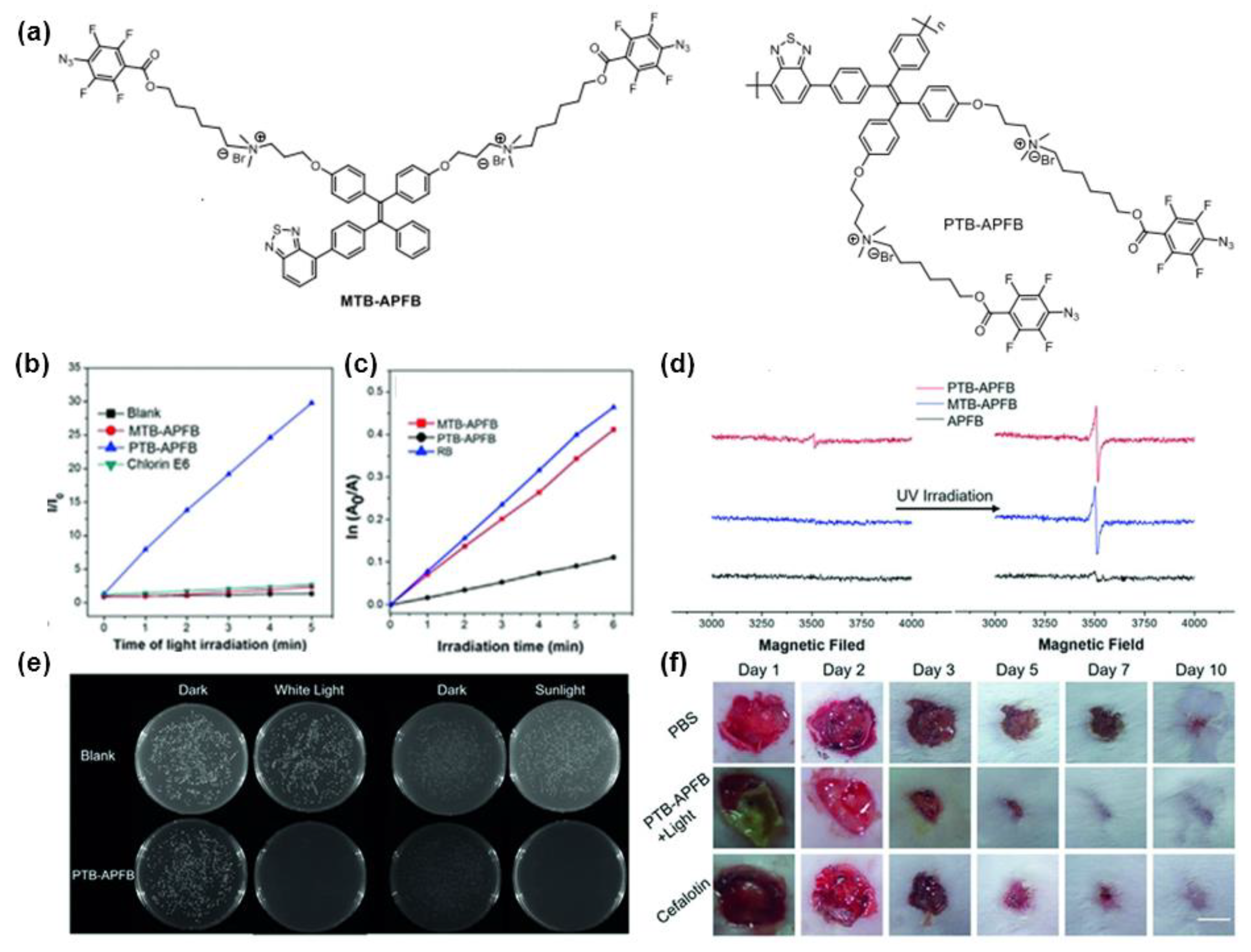
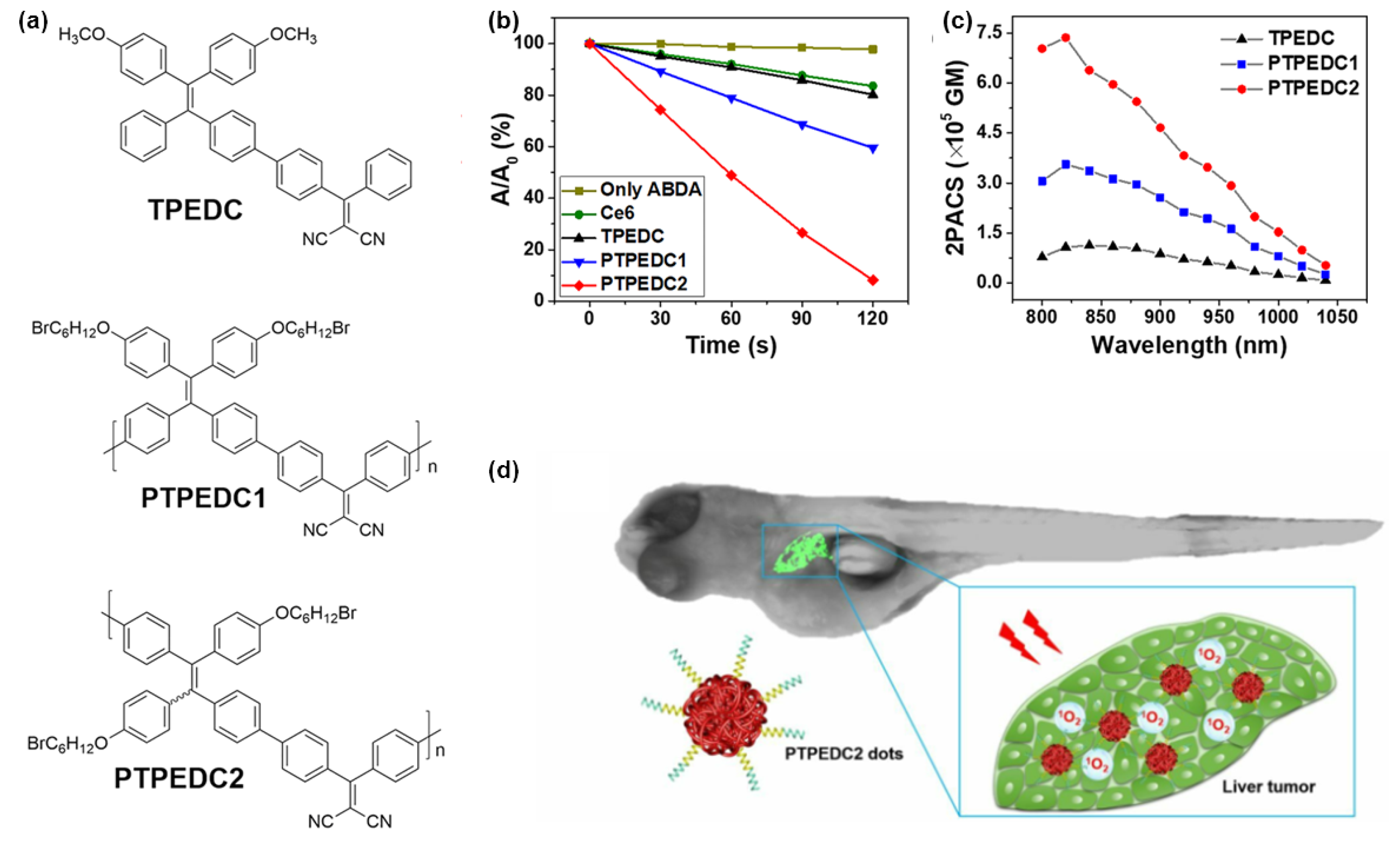
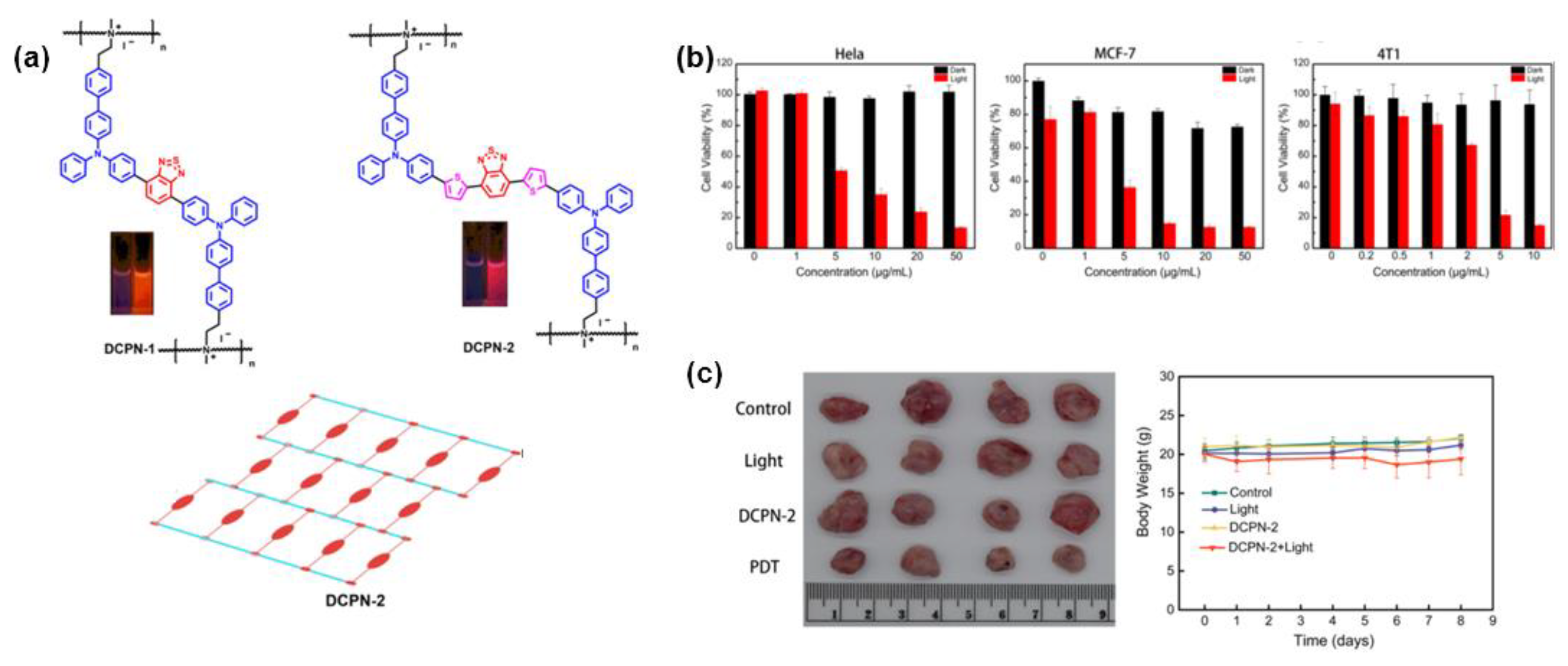
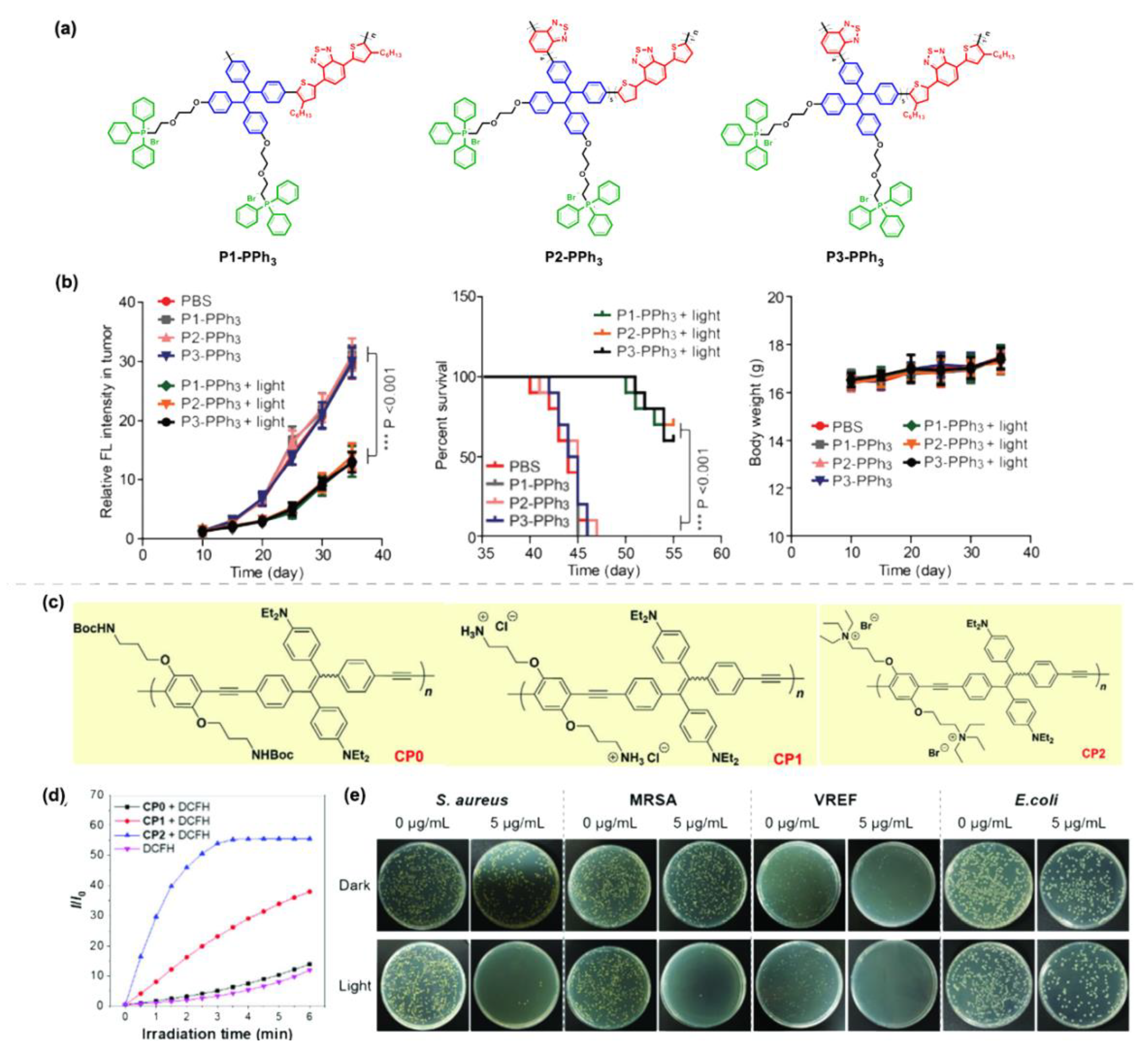
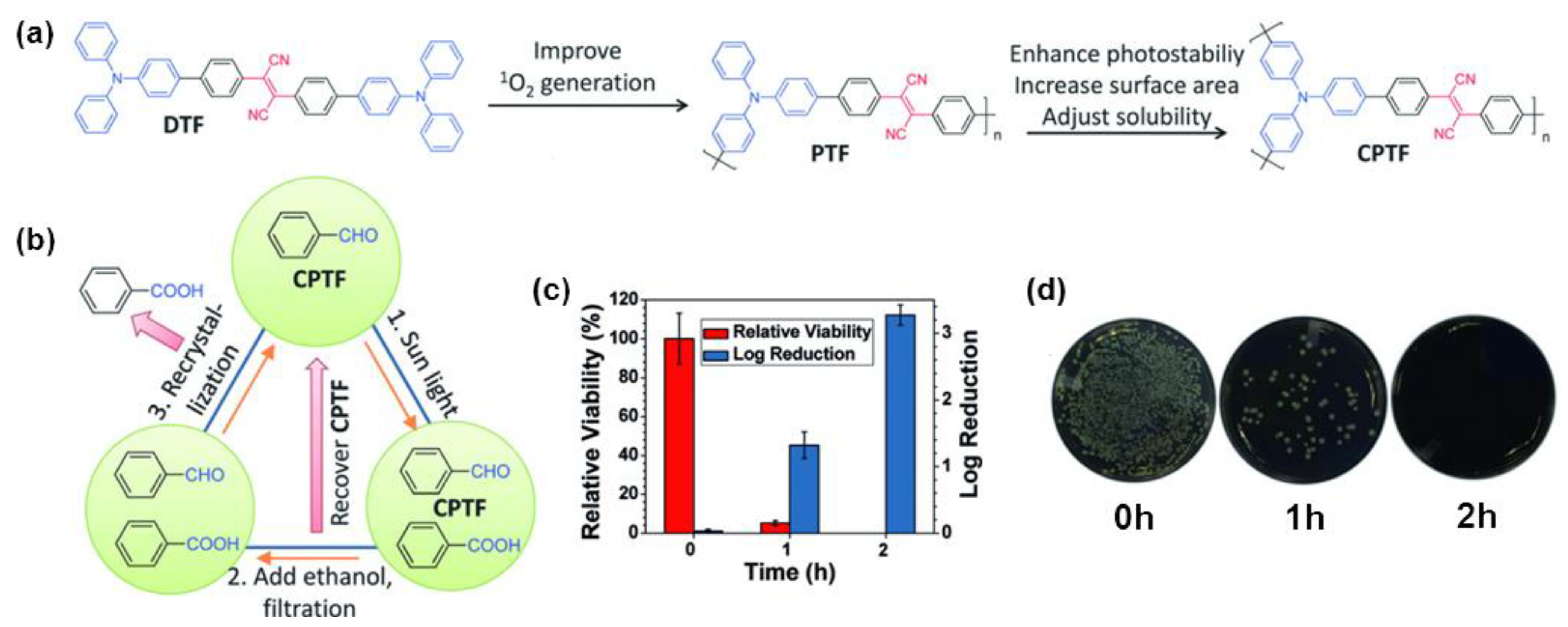
4. Polymerization-Enhanced Photosensitization for Photodynamic Therapy and Photocatalysis
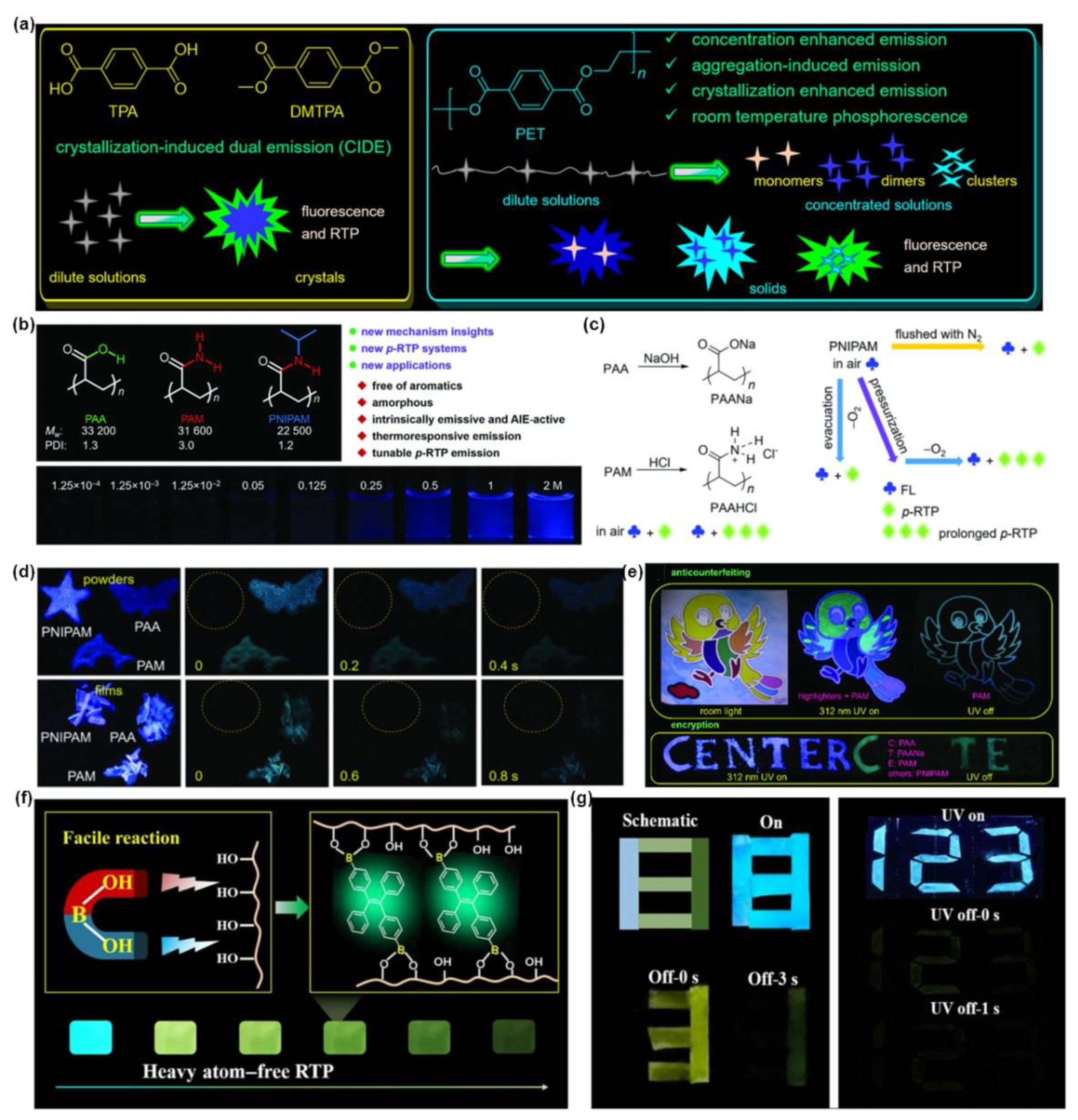
5. Polymerization-Enhanced Room-Temperature Phosphorescence for Security Protection
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 2021, 50, 1030–1069. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, L. Through-space charge transfer polymers for solution-processed organic light-emitting diodes. Aggregate 2020, 1, 45–56. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Qin, A.-J.; Tang, B.Z. AIE-active polymers for explosive detection. Chin. J. Polym. Sci. 2016, 35, 141–154. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Abdelmohsen, L.K.E.A.; Hest, J.C.M. Amphiphilic AIEgen-polymer aggregates: Design, self-assembly and biomedical applications. Aggregate 2021, 3, e128. [Google Scholar] [CrossRef]
- Feng, L.; Li, C.; Liu, L.; Chen, X.; Jiang, G.; Wang, J.; Tang, B.Z. A Facile Structural Isomerization-Induced 3D Spatial D-A Interlocked Network for Achieving NIR-II Phototheranostic Agents. Angew. Chem. Int. Ed. 2022, 61, e202212673. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zehra, N.; Tanwar, A.S.; Khatun, M.N.; Adil, L.R.; Iyer, P.K. AIE active polymers for biological applications. In Progress in Molecular Biology and Translational Science, 6th ed.; Bhosale, R.S., Singh, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 185, pp. 137–177. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2014, 44, 4228–4238. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Wang, S. Water-Soluble Conjugated Organic Molecules as Optical and Electrochemical Materials for Interdisciplinary Biological Applications. Acc. Chem. Res. 2019, 52, 3211–3222. [Google Scholar] [CrossRef]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Hu, R.; Qin, A.; Tang, B.Z. AIE polymers: Synthesis and applications. Prog. Polym. Sci. 2020, 100, 101176. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- He, X.; Kwok, R.; Jacky, W.; Tang, B. AIE Stereoisomers with Huge Differences in Luminescence Behavior and Biomedical Activity. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Alam, P.; Leung, N.L.; Zhang, J.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 2020, 429, 213693. [Google Scholar] [CrossRef]
- Mei, J.; Huang, Y.; Tian, H. Progress and Trends in AIE-Based Bioprobes: A Brief Overview. ACS Appl. Mater. Interfaces 2017, 10, 12217–12261. [Google Scholar] [CrossRef]
- Boreham, A.; Brodwolf, R.; Walker, K.; Haag, R.; Alexiev, U. Time-Resolved Fluorescence Spectroscopy and Fluorescence Lifetime Imaging Microscopy for Characterization of Dendritic Polymer Nanoparticles and Applications in Nanomedicine. Molecules 2017, 22, 17. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous organic polymers as a platform for sensing applications. Chem. Soc. Rev. 2022, 51, 2031–2080. [Google Scholar] [CrossRef]
- Honeybone, D.; Peace, H.; Green, M. Infrared emitting and absorbing conjugated polymer nanoparticles as biological imaging probes. J. Mater. Chem. C 2022. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Z.; Lam, J.W.Y.; Law, A.C.C.W.; Tang, B.Z. Silole-Containing Polyacetylenes. Synthesis, Thermal Stability, Light Emission, Nanodimensional Aggregation, and Restricted Intramolecular Rotation. Macromolecules 2003, 36, 1108–1117. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, G.; Lustig, W.P.; Wang, F.; Wang, H.; Teat, S.J.; Banerjee, D.; Zhang, D.; Li, J. Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal–organic framework. Chem. Commun. 2015, 51, 3045–3048. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.; Pieler, T. Programming Pluripotent Precursor Cells Derived from Xenopus Embryos to Generate Specific Tissues and Organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef]
- Wu, W.; Liu, B. Modulating the optical properties and functions of organic molecules through polymerization. Mater. Horiz. 2022, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-Y.; Wan, H.-B.; Zhou, F.; Gu, P.-Y.; Xu, Q.-F.; Lu, J.-M. AIEgens-lightened Functional Polymers: Synthesis, Properties and Applications. Chin. J. Polym. Sci. 2019, 37, 302–326. [Google Scholar] [CrossRef]
- Hu, Y.B.; Lam, J.W.Y.; Tang, B.Z. Recent Progress in AIE-active Polymers. Chin. J. Polym. Sci. 2019, 37, 289–301. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-Enhanced Two-Photon Photosensitization for Precise Photodynamic Therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, D.; Seo, S.; Jung, J.; Kim, J. Tailoring Intermolecular Interactions for Efficient Room-Temperature Phosphorescence from Purely Organic Materials in Amorphous Polymer Matrices. Angew. Chem. Int. Ed. 2014, 53, 11177–11181. [Google Scholar] [CrossRef]
- Wei, Q.; Kleine, P.; Karpov, Y.; Qiu, X.; Komber, H.; Sahre, K.; Kiriy, A.; Lygaitis, R.; Lenk, S.; Reineke, S.; et al. Conjugation-Induced Thermally Activated Delayed Fluorescence (TADF): From Conventional Non-TADF Units to TADF-Active Polymers. Adv. Funct. Mater. 2017, 27, 1605051. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tang, B.Z.; Xu, J. Aggregation-induced emission (AIE)-active polymers for explosive detection. Polym. Chem. 2019, 10, 3822–3840. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Pan, Y.; Manghnani, P.N.; Liu, B. AIE Polymers: Synthesis, Properties, and Biological Applications. Macromol. Biosci. 2017, 17, 1600433. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, X.; Lam, J.W.Y.; Tang, B.Z. The Marriage of Aggregation-Induced Emission with Polymer Science. Macromol. Rapid Commun. 2019, 40, e1800568. [Google Scholar] [CrossRef]
- Ge, S.; Wang, E.; Li, J.; Tang, B.Z. Aggregation-Induced Emission Boosting the Study of Polymer Science. Macromol. Rapid Commun. 2022, 43, e2200080. [Google Scholar] [CrossRef]
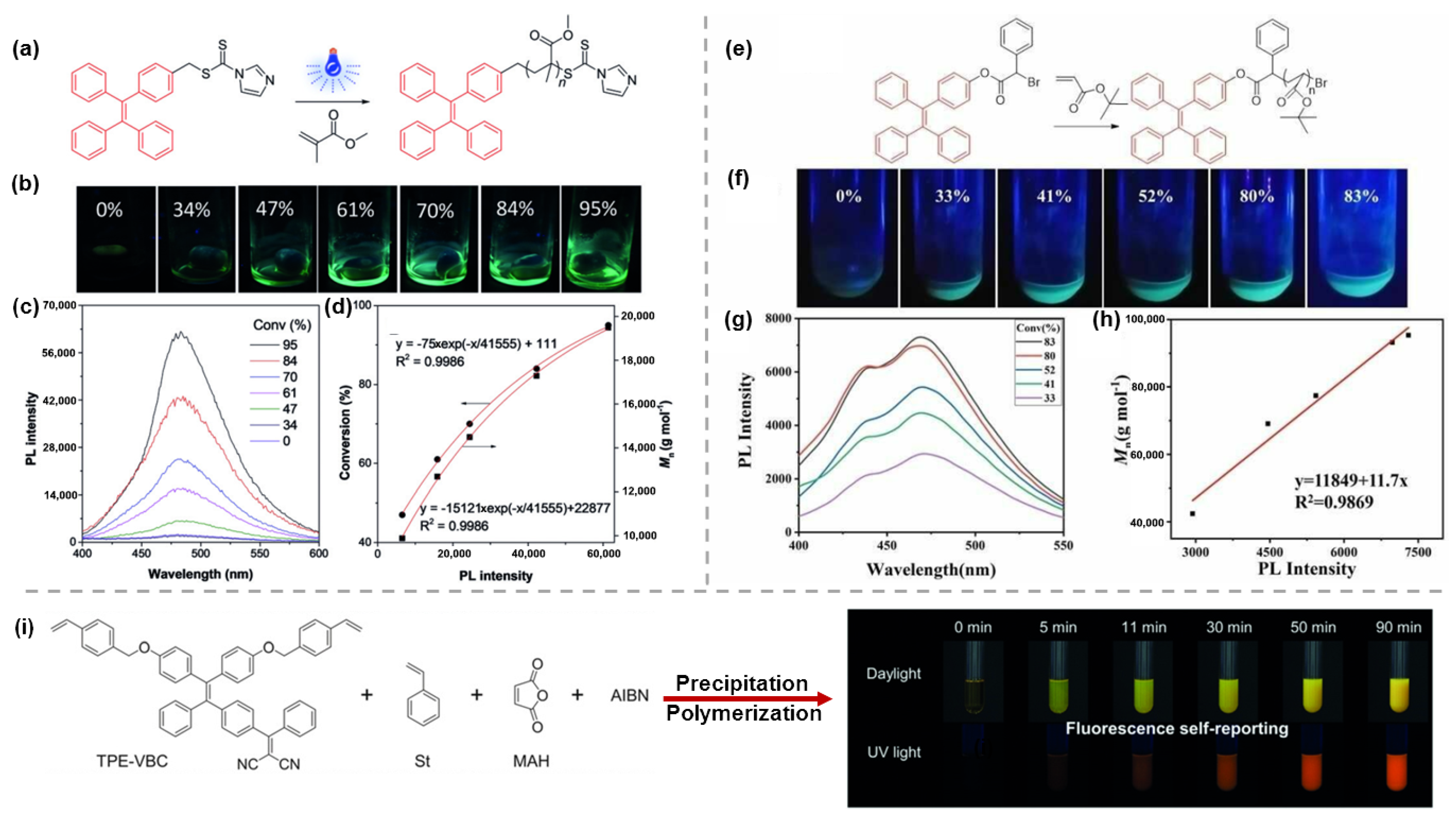
- Liu, S.; Cheng, Y.; Zhang, H.; Qiu, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. In Situ Monitoring of RAFT Polymerization by Tetraphenylethylene-Containing Agents with Aggregation-Induced Emission Characteristics. Angew. Chem. Int. Ed. 2018, 57, 6274–6278. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Guo, C.; Dong, A.; Zhang, J. Facile and Efficient Synthesis of Fluorescence-Labeled RAFT Agents and Their Application in the Preparation of α-,ω- and α,ω-End-Fluorescence-Labeled Polymers. Macromol. Chem. Phys. 2012, 213, 1851–1862. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, X.; Yin, X.; Cui, Z.; Fu, P.; Liu, M.; Wang, G.; Pan, X.; Pang, X. Visualization of Atom Transfer Radical Polymerization by Aggregation-Induced Emission Technology. Chem. Asian J. 2020, 15, 1014–1017. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, L.; Zhang, P.; Zhao, E.; Zhou, L.; Chen, D.; Sun, J.; Gu, X.; Yang, W.; Tang, B.Z. Fluorescence Self-Reporting Precipitation Polymerization Based on Aggregation-Induced Emission for Constructing Optical Nanoagents. Angew. Chem. Int. Ed. 2019, 59, 10122–10128. [Google Scholar] [CrossRef]
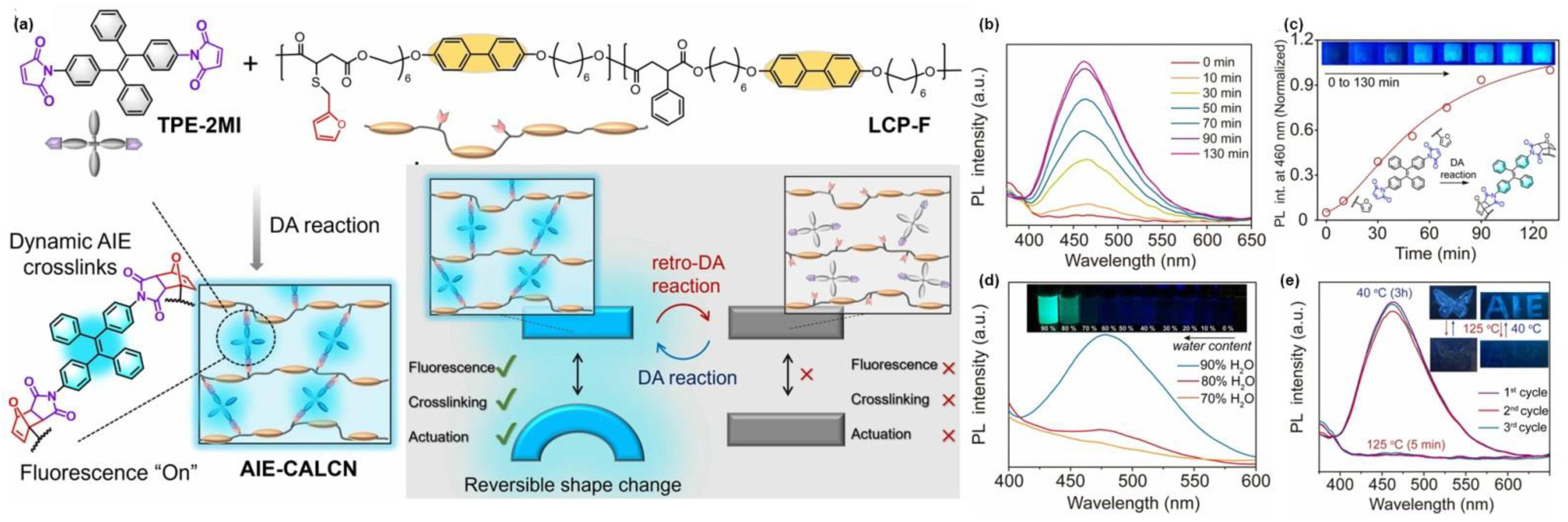
- Jiang, Z.-C.; Xiao, Y.-Y.; Hou, J.-B.; Chen, X.-S.; Yang, N.; Zeng, H.; Zhao, Y. Dynamic AIE crosslinks in liquid crystal networks: Visualizing for actuation-guiding, re-bonding for actuation-altering. Angew. Chem. Int. Ed. 2022, 61, e202211959. [Google Scholar] [CrossRef] [PubMed]
- Cavell, A.C.; Krasecki, V.K.; Li, G.; Sharma, A.; Sun, H.; Thompson, M.P.; Forman, C.J.; Guo, S.Y.; Hickman, R.J.; Parrish, K.; et al. Optical monitoring of polymerizations in droplets with high temporal dynamic range. Chem. Sci. 2020, 11, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Chen, M.; Chen, M.; Li, Y.; Chiu, Y.; Liou, G. Novel Authentic and Ultrafast Organic Photorecorders Enhanced by AIE-Active Polymer Electrets via Interlayer Charge Recombination. Adv. Funct. Mater. 2021, 31, 2101288. [Google Scholar] [CrossRef]
- Seo, Y.H.; Singh, A.; Cho, H.-J.; Kim, Y.; Heo, J.; Lim, C.-K.; Park, S.Y.; Jang, W.-D.; Kim, S. Rational design for enhancing inflammation-responsive in vivo chemiluminescence via nanophotonic energy relay to near-infrared AIE-active conjugated polymer. Biomaterials 2016, 84, 111–118. [Google Scholar] [CrossRef]
- Lee, E.; Li, X.; Oh, J.; Kwon, N.; Kim, G.; Kim, D.; Yoon, J. A boronic acid-functionalized phthalocyanine with an aggregation-enhanced photodynamic effect for combating antibiotic-resistant bacteria. Chem. Sci. 2020, 11, 5735–5739. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, D.; Huang, J.-D.; Yoon, J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 9885–9890. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Chien, C.-H.; Chang, C.-C.; Chang, T.-C. Aggregation induced photodynamic therapy enhancement based on linear and nonlinear excited FRET of fluorescent organic nanoparticles. J. Mater. Chem. B 2013, 1, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yin, Z.; Wang, P.; Chen, D.; Shao, J.; Zhang, Q.; Sun, L.; Huang, W.; Dong, X.; Zou, J.; et al. Photosensitizer synergistic effects: D–A–D structured organic molecule with enhanced fluorescence and singlet oxygen quantum yield for photodynamic therapy. Chem. Sci. 2018, 9, 2188–2194. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; He, X.; He, Z.; Yang, X.; Tian, S.; Meng, F.; Ding, D.; Luo, L.; Tang, B.Z. A Dual-Functional Photosensitizer for Ultraefficient Photodynamic Therapy and Synchronous Anticancer Efficacy Monitoring. Adv. Funct. Mater. 2019, 29, 1902673. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Zha, M.; Yang, G.; Li, Y.; Zhang, C.; Li, B.; Li, K. Recent Advances in AIEgen-Based Photodynamic Therapy and Immunotherapy. Adv. Health Mater. 2021, 10, e2101066. [Google Scholar] [CrossRef] [PubMed]
- Wu, W. High-Performance Conjugated Polymer Photosensitizers. Chem 2018, 4, 1762–1764. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, Y.; Liu, J.; Du, L.; Chen, M.; Kwok, R.T.K.; Lam, J.W.Y.; Phillips, D.L.; Tang, B.Z. Strategies to Enhance the Photosensitization: Polymerization and the Donor–Acceptor Even–Odd Effect. Angew. Chem. Int. Ed. 2018, 57, 15189–15193. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Wu, P.-J. Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-Polymer-Amplified Sensing, Imaging, and Therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, B.; Lv, F.; Liu, L.; Wang, S. Conjugated-Polymer-Based Energy-Transfer Systems for Antimicrobial and Anticancer Applications. Adv. Mater. 2014, 26, 6978–6982. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Hong, Y.; Lin, R.; Liu, Z.; Chen, M.; Lam, J.W.Y.; Ning, G.; Zheng, X.; Qin, A.; et al. Click Synthesis Enabled Sulfur Atom Strategy for Polymerization-Enhanced and Two-Photon Photosensitization. Angew. Chem. Int. Ed. 2022, 61, e202202005. [Google Scholar] [CrossRef]
- Wu, W.; Feng, G.; Xu, S.; Liu, B. A Photostable Far-Red/Near-Infrared Conjugated Polymer Photosensitizer with Aggregation-Induced Emission for Image-Guided Cancer Cell Ablation. Macromolecules 2016, 49, 5017–5025. [Google Scholar] [CrossRef]
- Xie, H.; Hu, W.; Zhang, F.; Zhao, C.; Peng, T.; Zhu, C.; Xu, J. AIE-active polyelectrolyte based photosensitizers: The effects of structure on antibiotic-resistant bacterial sensing and killing and pollutant decomposition. J. Mater. Chem. B 2021, 9, 5309–5317. [Google Scholar] [CrossRef]
- Yu, H.; Chen, B.; Huang, H.; He, Z.; Sun, J.; Wang, G.; Gu, X.; Tang, B.Z. AIE-Active Photosensitizers: Manipulation of Reactive Oxygen Species Generation and Applications in Photodynamic Therapy. Biosensors 2022, 12, 348. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956. [Google Scholar] [CrossRef]
- Yao, H.; Dai, J.; Zhuang, Z.; Yao, J.; Wu, Z.; Wang, S.; Xia, F.; Zhou, J.; Lou, X.; Zhao, Z. Red AIE conjugated polyelectrolytes for long-term tracing and image-guided photodynamic therapy of tumors. Sci. China Chem. 2020, 63, 1815–1824. [Google Scholar] [CrossRef]
- Xu, Q.; Lv, F.; Liu, L.; Wang, S. Development of A Thermo-Responsive Conjugated Polymer with Photobleaching-Resistance Property and Tunable Photosensitizing Performance. Macromol. Rapid Commun. 2020, 41, e2000249. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, Z.; Liu, Y.; Tian, B.; Guo, T.; Liu, R.; Wang, C.; Ying, L. In Vivo Bioimaging and Photodynamic Therapy Based on Two-Photon Fluorescent Conjugated Polymers Containing Dibenzothiophene-S,S-dioxide Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 57281–57289. [Google Scholar] [CrossRef] [PubMed]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, L.-P.; Wu, F.; Zhao, Y. Photosensitizers for Two-Photon Excited Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1704079. [Google Scholar] [CrossRef]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Wu, W.; Liu, B. Two-photon Excitable Photosensitizers with Aggregation-induced Emission and Their Biomedical Applications. Chem. J. Chin. Univ. 2020, 41, 191–203. [Google Scholar]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Cong, Z.; Xie, S.; Jiang, Z.; Zheng, S.; Wang, W.; Wang, W.; Song, H. In vivo photodynamic therapy based on Near-Infrared AIE cationic polymers. Chem. Eng. J. 2022, 431, 133748. [Google Scholar] [CrossRef]
- Su, X.; Liu, R.; Li, Y.; Han, T.; Zhang, Z.; Niu, N.; Kang, M.; Fu, S.; Wang, D.; Wang, D.; et al. Aggregation-Induced Emission-Active Poly(phenyleneethynylene)s for Fluorescence and Raman Dual-Modal Imaging and Drug-Resistant Bacteria Killing. Adv. Health Mater. 2021, 10, e2101167. [Google Scholar] [CrossRef]
- Kabe, R.; Notsuka, N.; Yoshida, K.; Adachi, C. Afterglow Organic Light-Emitting Diode. Adv. Mater. 2016, 28, 655–660. [Google Scholar] [CrossRef]
- Huang, L.; Chen, B.; Zhang, X.; Trindle, C.O.; Liao, F.; Wang, Y.; Miao, H.; Luo, Y.; Zhang, G. Proton-Activated “Off–On” Room-Temperature Phosphorescence from Purely Organic Thioethers. Angew. Chem. Int. Ed. 2018, 57, 16046–16050. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Gao, X.; Cai, C.; Lin, H. Facile, Quick, and Gram-Scale Synthesis of Ultralong-Lifetime Room-Temperature-Phosphorescent Carbon Dots by Microwave Irradiation. Angew. Chem. Int. Ed. 2018, 57, 6216–6220. [Google Scholar] [CrossRef]
- Jinnai, K.; Kabe, R.; Adachi, C. Wide-Range Tuning and Enhancement of Organic Long-Persistent Luminescence Using Emitter Dopants. Adv. Mater. 2018, 30, e1800365. [Google Scholar] [CrossRef]
- Louis, M.; Thomas, H.; Gmelch, M.; Haft, A.; Fries, F.; Reineke, S. Blue-Light-Absorbing Thin Films Showing Ultralong Room-Temperature Phosphorescence. Adv. Mater. 2019, 31, e1807887. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, K.; Ma, H.; Lv, A.; Pei, D.; Li, G.; Zhang, Y.; An, Z.; Li, A.; He, G. Ultralong Organic Phosphorescent Nanocrystals with Long-Lived Triplet Excited States for Afterglow Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 18385–18394. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, S.; Yang, X.; Wang, Y.; Zhou, X.; Xie, X. A Porphyrinic Donor–Acceptor Conjugated Porous Polymer as Highly Efficient Photocatalyst for PET–RAFT Polymerization. Macromol. Rapid Commun. 2022, 43, e22001743. [Google Scholar] [CrossRef]
- Zhu, S.-S.; Liu, Y.; Chen, X.-L.; Qu, L.-B.; Yu, B. Polymerization-Enhanced Photocatalysis for the Functionalization of C(sp3)–H Bonds. ACS Catal. 2022, 12, 126–134. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, G.; Guo, S.; Kong, L.; Lu, T.; Zhang, Z. Charge Transfer from Donor to Acceptor in Conjugated Microporous Polymer for Enhanced Photosensitization. Angew. Chem. Int. Ed. 2021, 60, 22062–22069. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, E.; Guo, H.; Du, J.; Wu, Y.; Liu, C.; Che, G. Visible Light-Driven D–A Conjugated Linear Polymer and Its Coating for Dual Highly Efficient Photocatalytic Degradation and Disinfection. ACS Appl. Mater. Interfaces 2021, 13, 51447–51458. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xu, S.; Qi, G.; Zhu, H.; Hu, F.; Liu, Z.; Zhang, D.; Liu, B. A Cross-linked Conjugated Polymer Photosensitizer Enables Efficient Sunlight-Induced Photooxidation. Angew. Chem. Int. Ed. 2019, 58, 3062–3066. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Ji, S.; Hu, F.; Ding, D.; Kong, D.; Liu, B. High performance photosensitizers with aggregation-induced emission for image-guided photodynamic anticancer therapy. Mater. Horiz. 2017, 4, 1110–1114. [Google Scholar] [CrossRef]
- Xu, S.; Yuan, Y.; Cai, X.; Zhang, C.-J.; Hu, F.; Liang, J.; Zhang, G.; Zhang, D.; Liu, B. Tuning the singlet-triplet energy gap: A unique approach to efficient photosensitizers with aggregation-induced emission (AIE) characteristics. Chem. Sci. 2015, 6, 5824–5830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Palmer, G.; Dewhirst, M.W.; Fraser, C.L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 2009, 8, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Bessière, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Zheng, C.; Huang, W. Excited State Modulation for Organic Afterglow: Materials and Applications. Adv. Mater. 2016, 28, 9920–9940. [Google Scholar] [CrossRef]
- Bian, L.; Shi, H.; Wang, X.; Ling, K.; Ma, H.; Li, M.; Cheng, Z.; Ma, C.; Cai, S.; Wu, Q.; et al. Simultaneously Enhancing Efficiency and Lifetime of Ultralong Organic Phosphorescence Materials by Molecular Self-Assembly. J. Am. Chem. Soc. 2018, 140, 10734–10739. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Gu, M.; Ling, K.; Ma, H.; Cai, S.; Song, L.; Ma, C.; Li, H.; Xing, G.; et al. Dynamic Ultralong Organic Phosphorescence by Photoactivation. Angew. Chem. Int. Ed. 2018, 57, 8425–8431. [Google Scholar] [CrossRef]
- Dang, Q.; Jiang, Y.; Wang, J.; Wang, J.; Zhang, Q.; Zhang, M.; Luo, S.; Xie, Y.; Pu, K.; Li, Q.; et al. Room-Temperature Phosphorescence Resonance Energy Transfer for Construction of Near-Infrared Afterglow Imaging Agents. Adv. Mater. 2020, 32, e2006752. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, S.; Wu, M.; Xiao, L.; Fan, Y.; Han, M.; Chang, K.; Zhang, Y.; Zhen, X.; Li, Q.; et al. Mobile Phone Flashlight-Excited Red Afterglow Bioimaging. Adv. Mater. 2022, 34, 2201280. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gao, H.; Ou, H.; Kwok, R.T.K.; Tang, Y.; Zheng, D.; Ding, D. Amplification of Activated Near-Infrared Afterglow Luminescence by Introducing Twisted Molecular Geometry for Understanding Neutrophil-Involved Diseases. J. Am. Chem. Soc. 2022, 144, 3429–3441. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Q.; Lv, A.; Liu, L.; Gong, J.-Y.; Ma, H.; Wang, J.; Tang, B.Z. Modulation of the intramolecular hydrogen bonding and push–pull electron effects toward realizing highly efficient organic room temperature phosphorescence. J. Mater. Chem. C 2022, 10, 13797–13804. [Google Scholar] [CrossRef]
- Yin, Z.; Gu, M.; Ma, H.; Jiang, X.; Zhi, J.; Wang, Y.; Yang, H.; Zhu, W.; An, Z. Molecular Engineering through Control of Structural Deformation for Highly Efficient Ultralong Organic Phosphorescence. Angew. Chem. Int. Ed. 2021, 60, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Yang, Z.; Fan, Z.; Ubba, E.; Li, W.; Li, Y.; Zhao, J.; Yang, Z.; Aldred, M.P.; Chi, Z. The methylation effect in prolonging the pure organic room temperature phosphorescence lifetime. Chem. Sci. 2018, 10, 179–184. [Google Scholar] [CrossRef]
- Tian, S.; Ma, H.; Wang, X.; Lv, A.; Shi, H.; Geng, Y.; Li, J.; Liang, F.; Su, Z.; An, Z.; et al. Utilizing d–pπ Bonds for Ultralong Organic Phosphorescence. Angew. Chem. Int. Ed. 2019, 58, 6645–6649. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Yan, Y. From aggregation-induced emission to organic room temperature phosphorescence through suppression of molecular vibration. Cell Rep. Phys. Sci. 2022, 3, 100771. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; Kausar, F.; Chen, G.; Zhang, Y.; Yuan, W.Z. Aggregation-Induced Dual Emission and Unusual Luminescence beyond Excimer Emission of Poly(ethylene terephthalate). Macromolecules 2018, 51, 9035–9042. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Z.; Dou, X.; Wang, Y.; Liu, S.; Zhang, Y.; Yuan, W.Z. Emission mechanism understanding and tunable persistent room temperature phosphorescence of amorphous nonaromatic polymers. Mater. Chem. Front. 2019, 3, 257–264. [Google Scholar] [CrossRef]
- Tian, R.; Xu, S.-M.; Xu, Q.; Lu, C. Large-scale preparation for efficient polymer-based room-temperature phosphorescence via click chemistry. Sci. Adv. 2020, 6, eaaz6107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Shan, G.; Qin, C.; Liu, S. Polymerization-Enhanced Photophysical Performances of AIEgens for Chemo/Bio-Sensing and Therapy. Molecules 2023, 28, 78. https://doi.org/10.3390/molecules28010078
Huang S, Shan G, Qin C, Liu S. Polymerization-Enhanced Photophysical Performances of AIEgens for Chemo/Bio-Sensing and Therapy. Molecules. 2023; 28(1):78. https://doi.org/10.3390/molecules28010078
Chicago/Turabian StyleHuang, Shanshan, Guogang Shan, Chao Qin, and Shunjie Liu. 2023. "Polymerization-Enhanced Photophysical Performances of AIEgens for Chemo/Bio-Sensing and Therapy" Molecules 28, no. 1: 78. https://doi.org/10.3390/molecules28010078
APA StyleHuang, S., Shan, G., Qin, C., & Liu, S. (2023). Polymerization-Enhanced Photophysical Performances of AIEgens for Chemo/Bio-Sensing and Therapy. Molecules, 28(1), 78. https://doi.org/10.3390/molecules28010078





