A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions and MS Detections
2.2. In-Study Validation
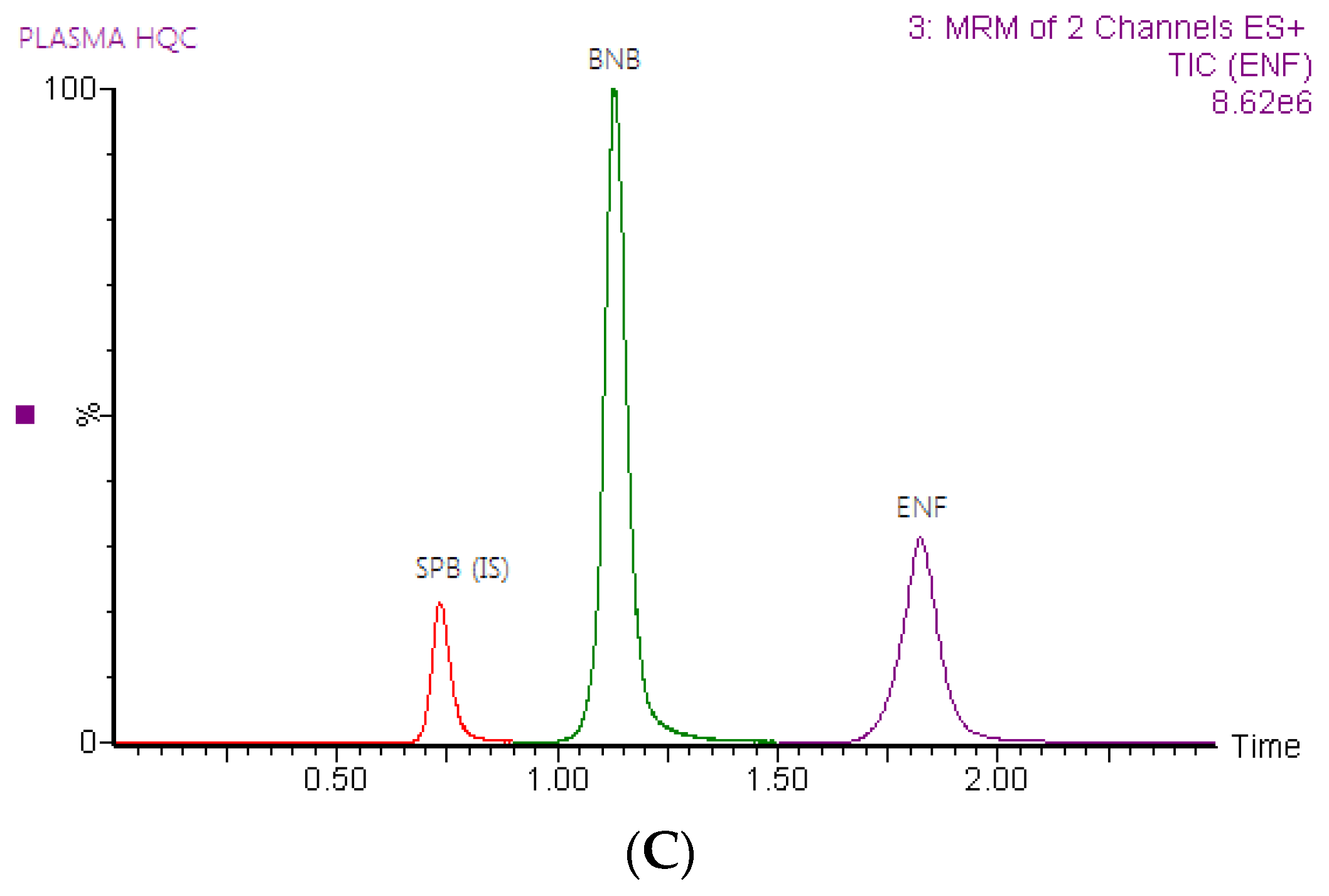
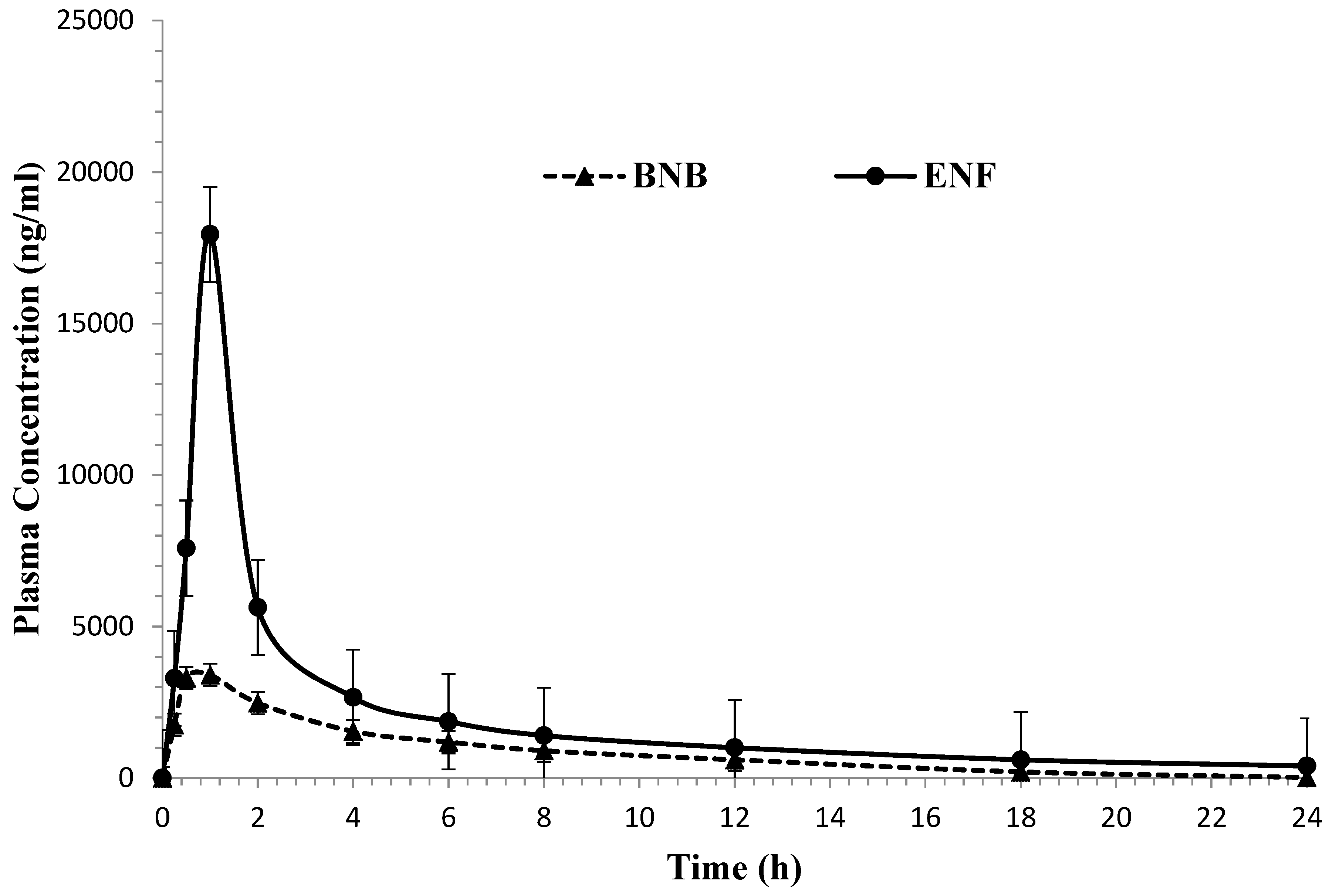
2.3. Application to the Pharmacokinetic Study
3. Experimental
3.1. Chemicals and Reagents
3.2. LC-MS/MS Conditions
3.3. Preparation of Stock, Standard, Calibrators and Quality Control Samples
3.4. Sample Preparation
3.5. Pre-Study Validation
3.6. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.; Fuchs, H.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lopez-Beltran, A.; Massari, F.; MacLennan, G.T.; Montironi, R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod. Pathol. 2018, 31, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.R.M.; Baig, M.; Mohamoud, H.S.A.; Ulhaq, Z.; Hoessli, D.C.; Khogeer, G.S.; Al-Sayed, R.R.; Al-Aama, J.Y. BRAF gene: From human cancers to developmental syndromes. Saudi J. Biol. Sci. 2015, 22, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Eroglu, Z.; Infante, J.; Patel, S.; Daud, A.; Johnson, D.B.; Gonzalez, R.; Kefford, R.; Hamid, O.; Schuchter, L.; et al. Long-Term Outcomes in Patients With BRAF V600-Mutant Metastatic Melanoma Who Received Dabrafenib Combined with Trametinib. J. Clin. Oncol. 2018, 36, 667–673. [Google Scholar] [CrossRef] [PubMed]
- US FDA. FDA Approves Encorafenib and Binimetinib in Combination for Unresectable or Metastatic Melanoma with BRAF Mutations. 2018. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-and-binimetinib-combination-unresectable-or-metastatic-melanoma-braf (accessed on 5 November 2022).
- Trojaniello, C.; Festino, L.; Vanella, V.; Ascierto, P.A. Encorafenib in combination with binimetinib for unresectable or metastatic melanoma with BRAF mutations. Expert Rev. Clin. Pharmacol. 2019, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jayachandiran, R.; Pandey, S. Current advancements and novel strategies in the treatment of metastatic melanoma. Integr. Cancer Ther. 2021, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Wayman, M. Encorafenib and Binimetinib Combination Therapy in Metastatic Melanoma. J. Adv. Pract. Oncol. 2022, 13, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.C.; Sagar, G.V.; Rao, K.R.S. Validation of LC-MS/MS Electrospray Ionisation method for the Estimation of Binimetinib in Human Plasma. Int. J. Sci. Res. 2019, 8, 2841–2854. [Google Scholar]
- Rousset, M.; Titier, K.; Bouchet, S.; Dutriaux, C.; Pham-Ledard, A.; Prey, S.; Canal-Raffin, M.; Molimard, M. An UPLC-MS/MS method for the quantification of BRAF inhibitors (vemurafenib, dabrafenib) and MEK inhibitors (cobimetinib, trametinib, binimetinib) in human plasma. Application to treated melanoma patients. Clin. Chim. Acta 2017, 470, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Jadav, T.; Sahu, A.K.; Sengupta, P. LC–MS/MS bioanalytical method for quantification of binimetinib and venetoclax, and their pharmacokinetic interaction. Bioanalysis 2021, 14, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Rosing, H.; Rood, J.J.M.; Schellens, J.H.M.; Beijnen, J.H. Liquid chromatography-tandem mass spectrometric assay for therapeutic drug monitoring of the B-Raf inhibitor encorafenib, the EGFR inhibitors afatinib, erlotinib and gefitinib and the O-desmethyl metabolites of erlotinib and gefitinib in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Attwa, M.W.; Darwish, H.H.; Al-Shakliah, N.S.; Kadi, A.A. A Validated LC–MS/MS Assay for the Simultaneous Quantification of the FDA-Approved Anticancer Mixture (Encorafenib and Binimetinib): Metabolic Stability Estimation. Molecules 2021, 26, 2717. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm070107.pdf (accessed on 5 November 2022).
- US FDA. MEKTOVI ® (Binimetinib) Tablets: US Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210498Orig1s000MultidisciplineR.pdf (accessed on 5 November 2022).
- European Medicines Agency: EMA/CHMP/554701/2018-Annual Report of the European Medicines Agency. 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/braftovi-epar-public-assessment-report_en.pdf (accessed on 5 November 2022).
- US FDA. BRAFTOVI® (Encorafenib) Tablets: US Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210496Orig1s000MultidisciplineR.pdf (accessed on 5 November 2022).
- Ramaswamy, M.; Wallace, T.L.; Cossum, P.A.; Wasan, K.M. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob. Agents Chemother. 1999, 43, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, M.; Hefnawy, M.; Abuelizz, H.; Alzamil, A.; Mohammed, M.; Alsaif, N.; Almehizia, A.; Alkahtani, H.; Abounassif, M. Development and validation of an UHPLC-MS/MS method for simultaneous determination of palbociclib, letrozole and its metabolite carbinol in rat plasma and pharmacokinetic study application. Arab. J. Chem. 2020, 13, 4024–4034. [Google Scholar] [CrossRef]
- Hefnawy, M.; Hammad, S.; Kadi, A.; Alsaif, N.; Mohammed, M.; Al-Hossaini, A.; Bin Jardan, Y.; Attwa, M.; Abou-El-Alamin, M. Effective quantification of ravidasvir (an NS5A inhibitor) and sofosbuvir in rat plasma by validated LC-MS/MS method and its application to pharmacokinetic study. Arab. J. Chem. 2020, 13, 8160–8171. [Google Scholar] [CrossRef]
- Hefnawy, M.; Al-Majed, A.; Alrabiah, H.; Algrain, N.; Mohammed, M.; Bin Jardand, Y. Rapid and sensitive LC-MS/MS method for the enantioanalysis ofverapamil in rat plasma using superficially porous silicaisopropyl-cyclofructan 6 chiral stationary phase after SPE: Applicationto a stereoselective pharmacokinetic study. J. Pharm. Biomed. Anal. 2021, 201, 114108. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhoua, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]

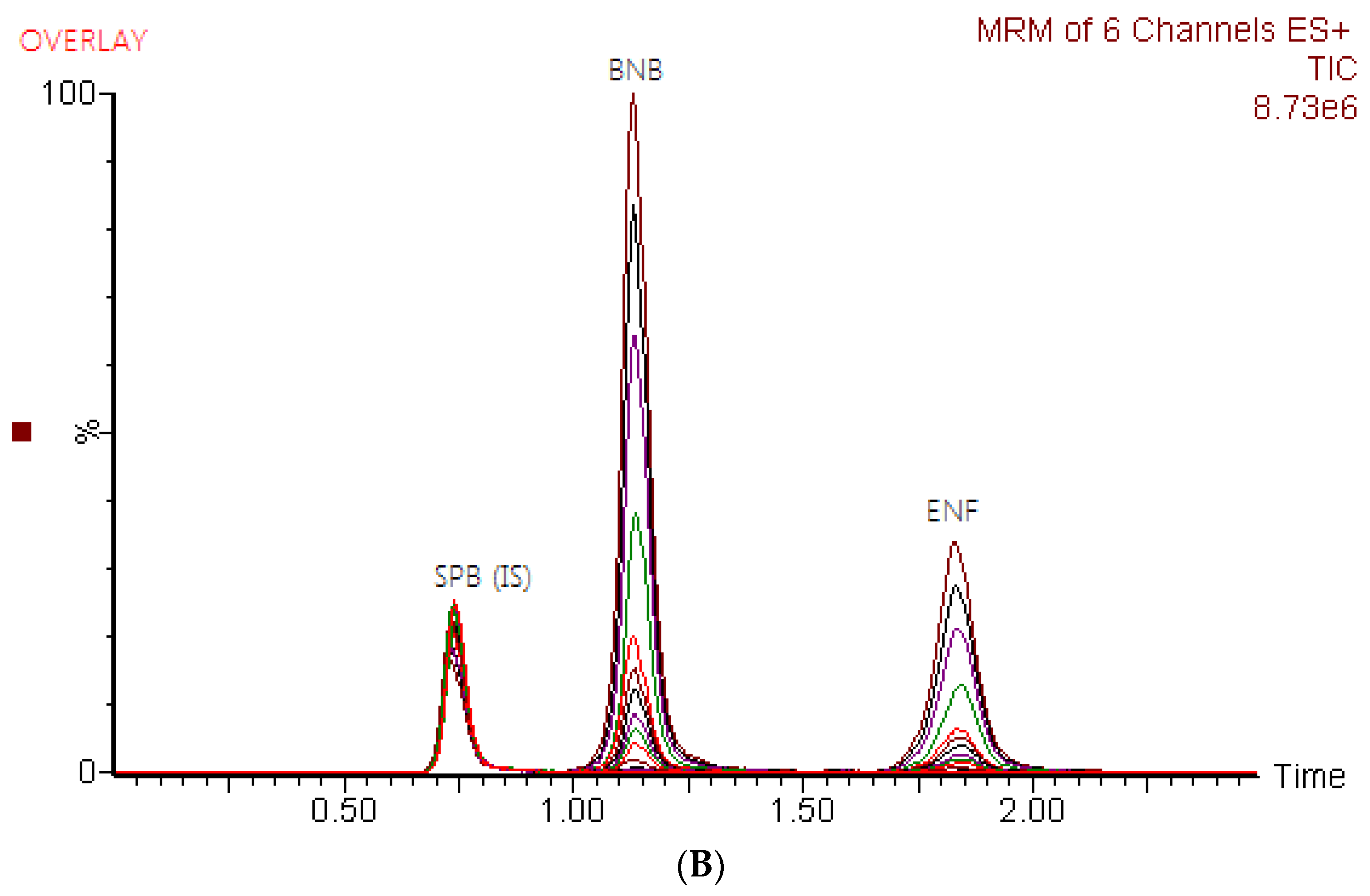
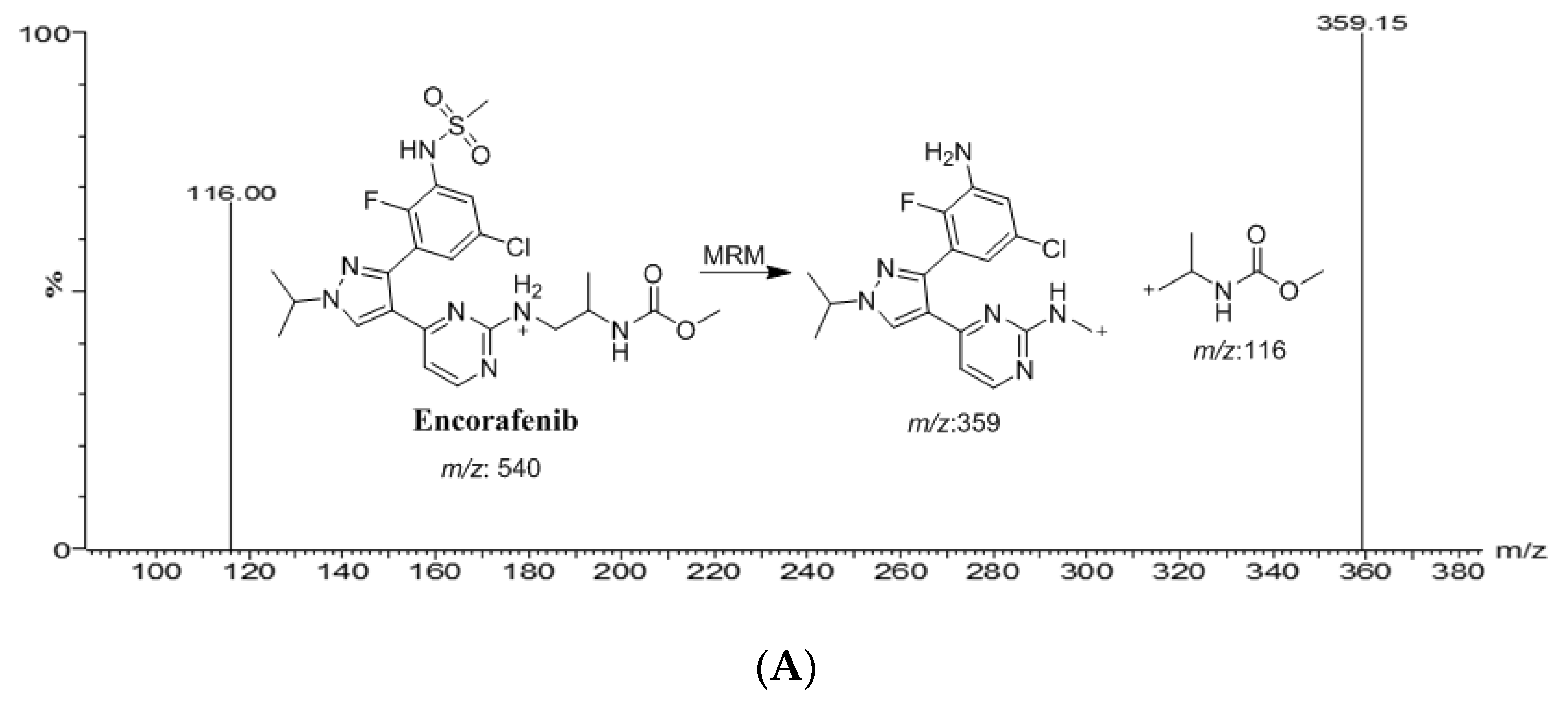
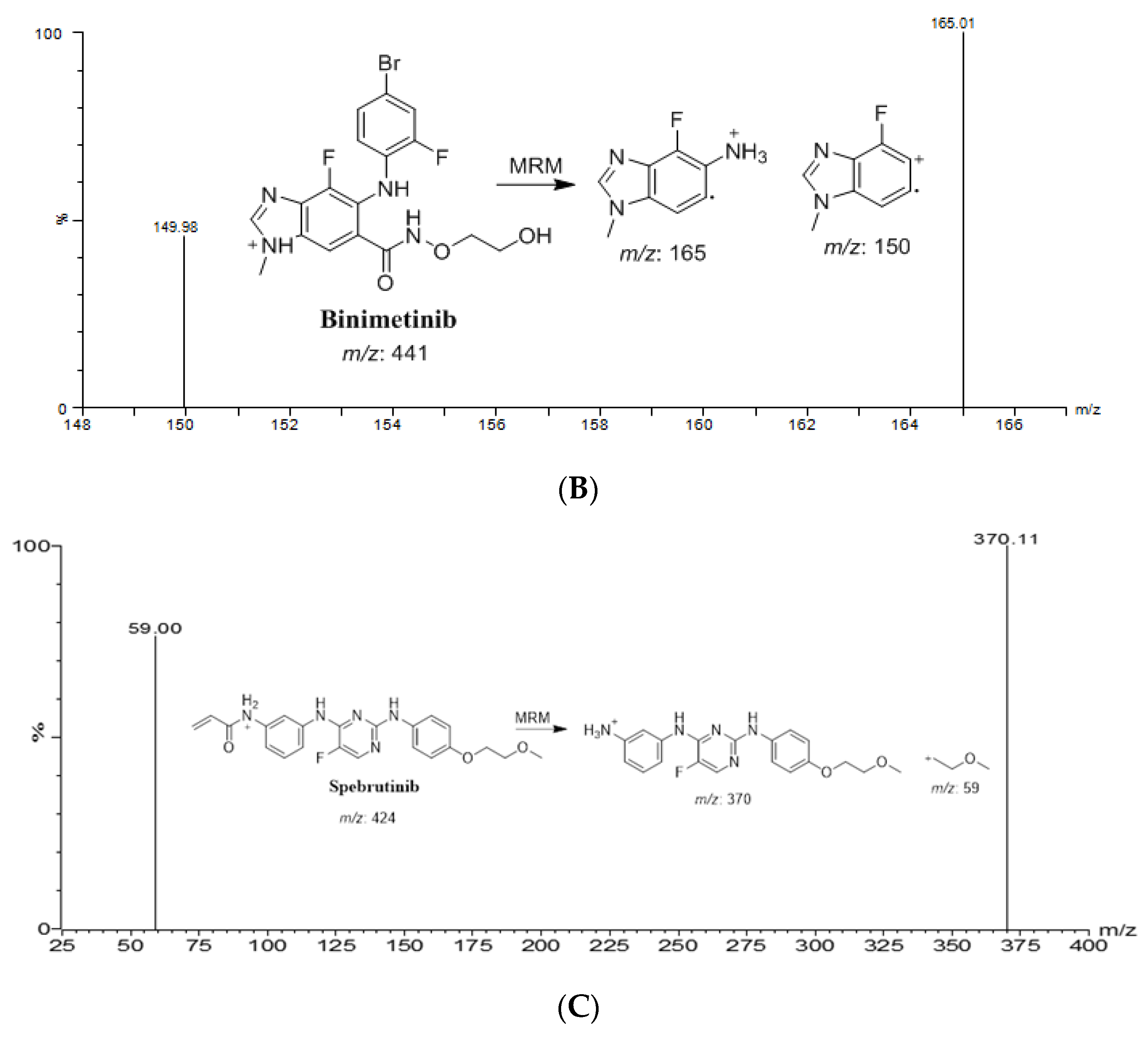

| Drug | Ion Mode | Precursor (m/z) | Quantification traces (m/z) | Qualification traces (m/z) | Cone Voltage (V) | Collision energy (CE, eV) |
|---|---|---|---|---|---|---|
| BNB | +ve | 441.0 | 165.0 | 149.9 | 44 | 54/32 |
| ENF | +ve | 540.1 | 359.1 | 116.0 | 54 | 46/44 |
| IS | +ve | 424.1 | 370.1 | 58.9 | 58 | 32/26 |
| Parameters | BNB | ENF |
|---|---|---|
| Concentration range (ng/mL) | 0.5–3000 | 0.5–3000 |
| Intercept (a) | 4.31 × 10−2 | 6.45 × 10−2 |
| Slope (b) | 2.36 × 10−3 | 1.17 × 10−3 |
| Coefficient of determination (r2) | 0.998 | 0.999 |
| SY/N a | 7.70 × 10−3 | 6.48 × 10−3 |
| Sa b | 2.42 × 10−3 | 2.04 × 10−3 |
| Sb c | 2.08 × 10−4 | 1.75 × 10−4 |
| LLOQ (ng/mL) | 0.5 | 0.5 |
| LLOD (ng/mL | 0.2 | 0.2 |
| Analyte | Concentration ng/mL | Inter-Day | Intra-Day | |||
|---|---|---|---|---|---|---|
| RSD (%) | Accuracy (%) | RSD (%) | Accuracy (%) | |||
| Binimetinib | LLOQ | 0.5 | 94.00 | 7.52 | 95.71 | 5.17 |
| LQC | 1.5 | 95.61 | 2.39 | 96.45 | 3.24 | |
| MQC | 1800 | 97.53 | 1.53 | 99.18 | 0.38 | |
| HQC | 2400 | 101.31 | 0.62 | 100.31 | 0.58 | |
| Encorafenib | LLOQ | 0.5 | 95.15 | 6.23 | 92.88 | 5.67 |
| LQC | 1.5 | 97.51 | 2.62 | 96.65 | 2.61 | |
| MQC | 1800 | 96.65 | 1.57 | 99.57 | 0.33 | |
| HQC | 2400 | 99.84 | 0.97 | 102.28 | 1.26 | |
| n | 6 | 18 | ||||
| Nominal Concentration (ng/mL) | Binimetinib | Encorafenib | IS | ||||
|---|---|---|---|---|---|---|---|
| 1.5 | 1800 | 2400 | 1.5 | 1800 | 2400 | 100 | |
| Mean a | 1.39 | 1706.77 | 2220.74 | 1.40 | 1735.89 | 2216.03 | 95.28 |
| RSD | 1.09 | 0.28 | 2.07 | 1.08 | 0.95 | 1.10 | 1.74 |
| Recovery (%) | 92.33 | 94.82 | 92.53 | 93.33 | 96.43 | 92.33 | 95.28 |
| Mean recovery (%) | 93.41 | 94.18 | 95.28 | ||||
| Analyte | Spiked Conc. (ng/ mL) | Dilution Fold | Mean Recovery (%) ± RSD a |
|---|---|---|---|
| Binimetinib | 4500 | 1:2 | 94.66 ± 1.13 |
| 1:4 | 96.86 ± 1.08 | ||
| Encorafenib | 4500 | 1:2 | 99.26 ± 1.82 |
| 1:4 | 98.71 ± 1.10 |
| Analyte | Concentration ng/mL | Short Term Stability at Room Temperature (24 h) | Autosampler Stabilityat 10 °C (24 h) | Freeze and Thaw Stability at −80 °C (3 Cycles) | Long Term Stability at −80 °C (30 Days) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |||
| Binimetinib | LQC | 1.5 | 96.23 | 2.57 | 97.35 | 4.79 | 104.33 | 2.45 | 95.54 | 2.92 |
| MQC | 1800 | 103.14 | 1.75 | 104.26 | 2.29 | 103.67 | 1.93 | 103.92 | 2.26 | |
| HQC | 2400 | 93.39 | 2.92 | 95.41 | 2.24 | 95.21 | 2.97 | 94.76 | 3.21 | |
| Encorafenib | LQC | 1.5 | 95.25 | 2.89 | 96.57 | 2.46 | 99.47 | 0.83 | 97.47 | 1.71 |
| MQC | 1800 | 102.64 | 1.64 | 99.83 | 2.55 | 96.78 | 2.61 | 102.24 | 2.24 | |
| HQC | 2400 | 94.48 | 3.53 | 92.44 | 5.15 | 96.53 | 2.12 | 94.62 | 3.65 | |
| n | 3 | 3 | 3 | 3 | ||||||
| Parameters | Unit | Binimetinib * | Encorafenib * |
|---|---|---|---|
| AUC0-t a | µg/mL·h | 12.35 ± 1.86 | 30.30 ± 3.01 |
| AUC0-∞ b | µg/mL·h | 18.16 ± 1.31 | 36.52 ± 3.92 |
| Cmax c | µg/mL | 3.43 ± 0.46 | 16.42 ± 1.47 |
| Tmax d | h | 1 | 1 |
| Cl/F e | L/h | 0.21 ± 0.03 | 0.55 ± 0.06 |
| t1/2kel f | h | 3.39 ± 0.43 | 2.48 ± 0.24 |
| MRT0-∞ g | h | 4.50 ± 0.28 | 3.23 ± 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefnawy, M.M.; Alanazi, M.M.; Al-Hossaini, A.M.; Alnasser, A.I.; El-Azab, A.S.; Jardan, Y.A.B.; Attwa, M.W.; El-Gendy, M.A. A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study. Molecules 2023, 28, 79. https://doi.org/10.3390/molecules28010079
Hefnawy MM, Alanazi MM, Al-Hossaini AM, Alnasser AI, El-Azab AS, Jardan YAB, Attwa MW, El-Gendy MA. A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study. Molecules. 2023; 28(1):79. https://doi.org/10.3390/molecules28010079
Chicago/Turabian StyleHefnawy, Mohamed M., Mohammed M. Alanazi, Abdullah M. Al-Hossaini, Abdulaziz I. Alnasser, Adel S. El-Azab, Yousef A. Bin Jardan, Mohamed W. Attwa, and Manal A. El-Gendy. 2023. "A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study" Molecules 28, no. 1: 79. https://doi.org/10.3390/molecules28010079
APA StyleHefnawy, M. M., Alanazi, M. M., Al-Hossaini, A. M., Alnasser, A. I., El-Azab, A. S., Jardan, Y. A. B., Attwa, M. W., & El-Gendy, M. A. (2023). A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study. Molecules, 28(1), 79. https://doi.org/10.3390/molecules28010079







