Crystallization-Enhanced Emission and Room-Temperature Phosphorescence of Cyclic Triimidazole-Monohexyl Thiophene Derivatives
Abstract
1. Introduction
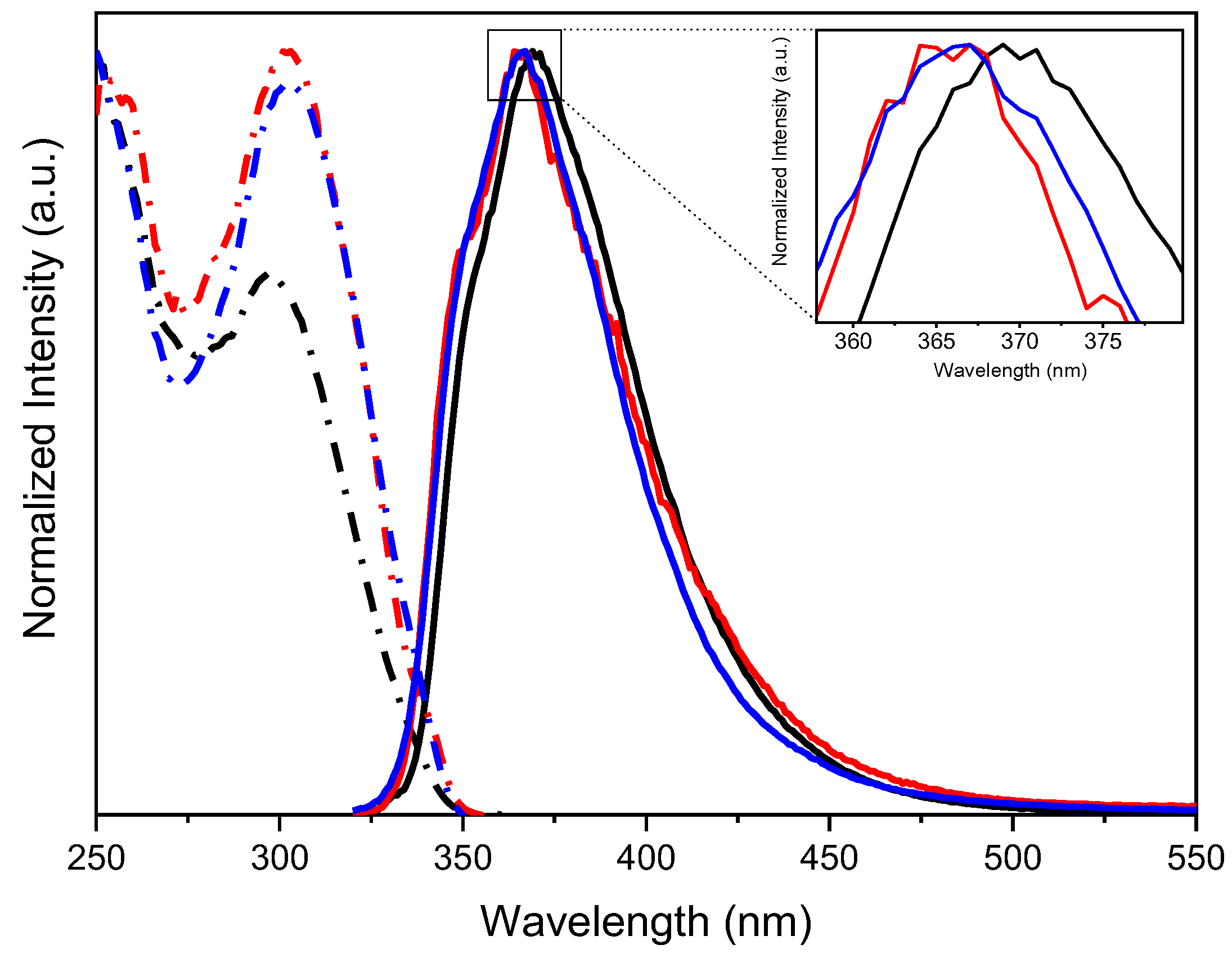
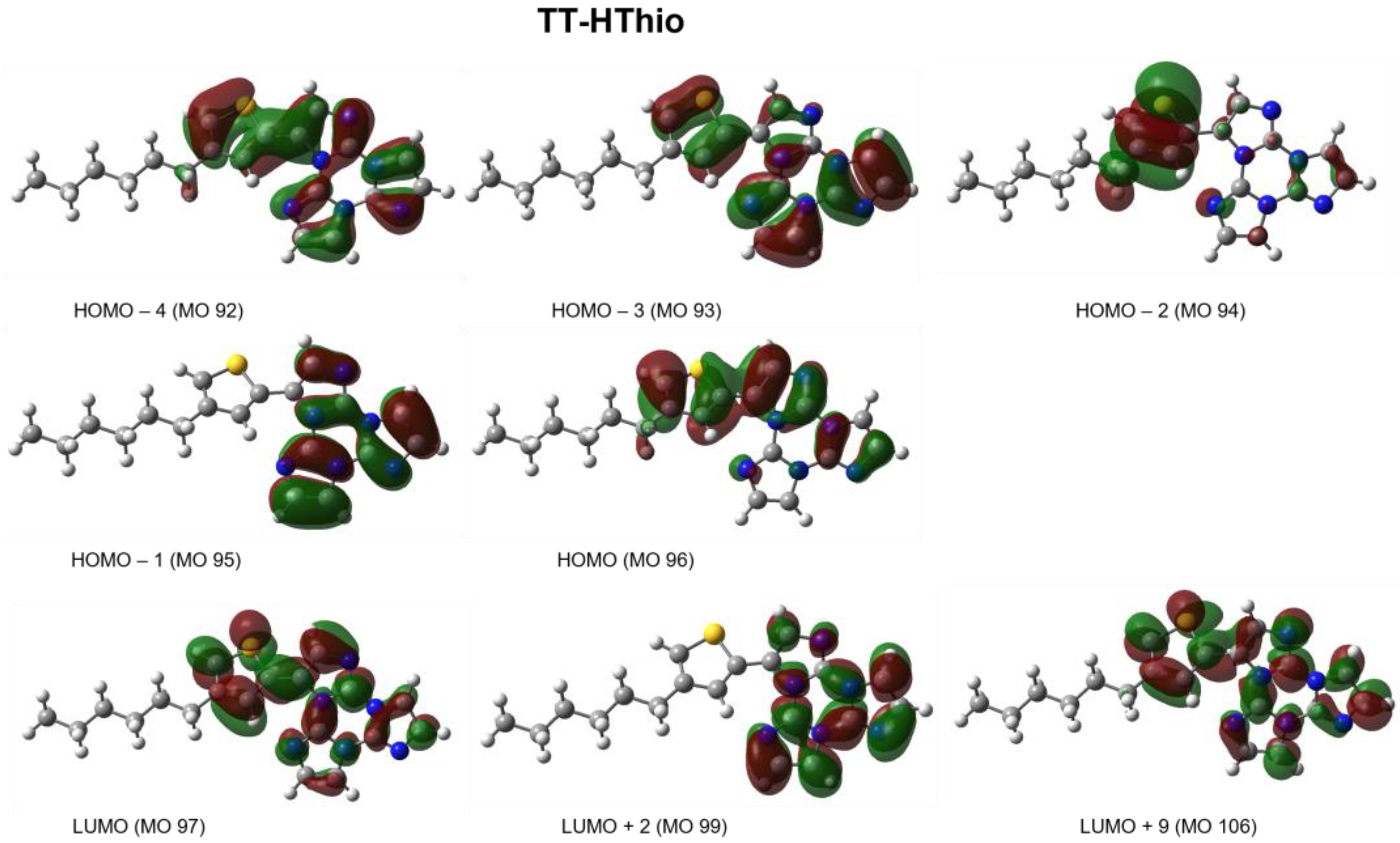
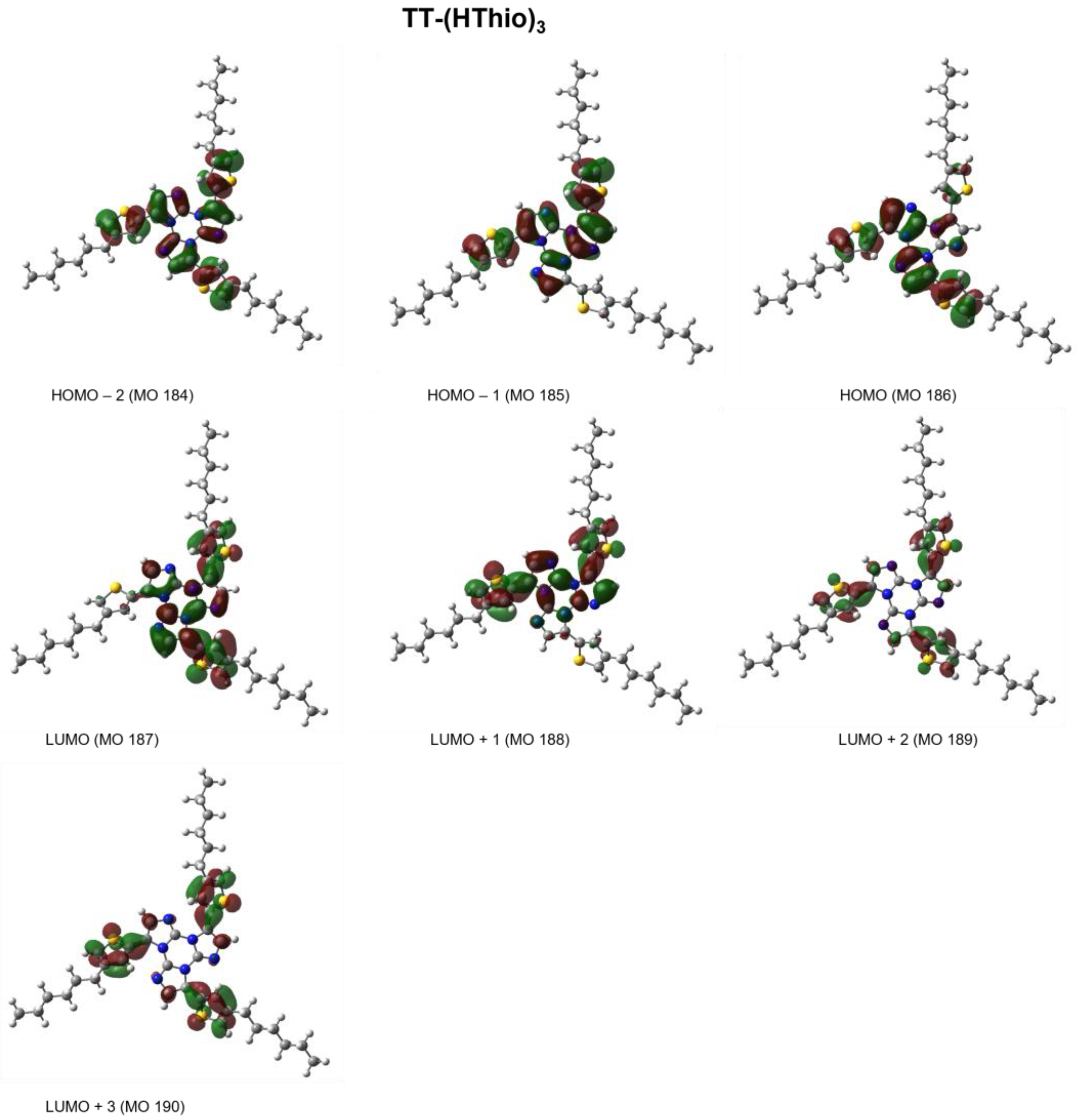
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
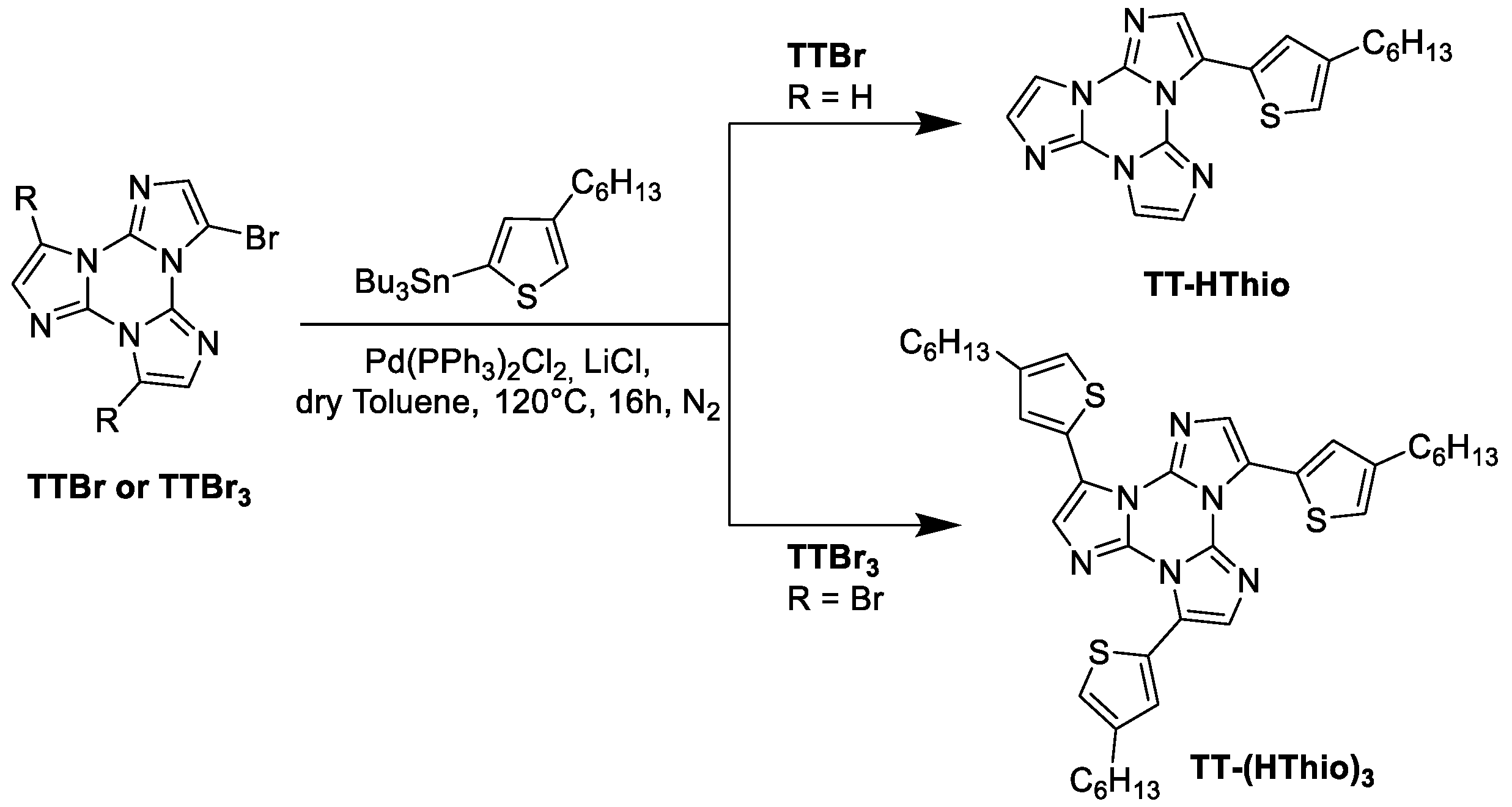
4.2. Synthesis of TT-HThio
4.3. Synthesis of TT-(HThio)3
4.4. Single-Crystal X-ray Studies
4.5. Computational Details
4.6. Photophysical Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qin, W.; Zhang, P.; Li, H.; Lam, J.W.Y.; Cai, Y.; Kwok, R.T.K.; Qian, J.; Zheng, W.; Tang, B.Z. Ultrabright Red AIEgens for Two-Photon Vascular Imaging with High Resolution and Deep Penetration. Chem. Sci. 2018, 9, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Zhou, Q.; Shi, H.; An, Z.; Huang, W. Organic Room Temperature Phosphorescence Materials for Biomedical Applications. Chem. Asian J. 2020, 15, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wu, H.; Ma, H.; Ye, W.; Jia, W.; Wang, H.; Chen, H.; Zhang, N.; Wang, D.; Qian, C.; et al. Color-Tunable Ultralong Organic Room Temperature Phosphorescence from a Multicomponent Copolymer. Nat. Commun. 2020, 11, 944. [Google Scholar] [CrossRef]
- Lei, Y.; Dai, W.; Guan, J.; Guo, S.; Ren, F.; Zhou, Y.; Shi, J.; Tong, B.; Cai, Z.; Zheng, J.; et al. Wide-Range Color-Tunable Organic Phosphorescence Materials for Printable and Writable Security Inks. Angew. Chem. Int. Ed. 2020, 59, 16054–16060. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Ordered Assembly of Hybrid Room-Temperature Phosphorescence Thin Films Showing Polarized Emission and the Sensing of VOCs. Chem. Commun. 2017, 53, 5408–5411. [Google Scholar] [CrossRef]
- Hirata, S.; Totani, K.; Kaji, H.; Vacha, M.; Watanabe, T.; Adachi, C. Reversible Thermal Recording Media Using Time-Dependent Persistent Room Temperature Phosphorescence. Adv. Opt. Mater. 2013, 1, 438–442. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing Triplet Excited States for Ultralong Organic Phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-Tunable Ultra-Long Organic Phosphorescence of a Single-Component Molecular Crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. H-Aggregates Granting Crystallization-Induced Emissive Behavior and Ultralong Phosphorescence from a Pure Organic Molecule. J. Phys. Chem. Lett. 2017, 8, 1894–1898. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Pavanello, A.; Previtali, A.; Righetto, S.; Cariati, E. Cyclic Triimidazole Derivatives: Intriguing Examples of Multiple Emissions and Ultralong Phosphorescence at Room Temperature. Angew. Chem. Int. Ed. 2017, 56, 16302–16307. [Google Scholar] [CrossRef]
- Mieno, H.; Kabe, R.; Notsuka, N.; Allendorf, M.D.; Adachi, C. Long-Lived Room-Temperature Phosphorescence of Coronene in Zeolitic Imidazolate Framework ZIF-8. Adv. Opt. Mater. 2016, 4, 1015–1021. [Google Scholar] [CrossRef]
- Kabe, R.; Adachi, C. Organic Long Persistent Luminescence. Nature 2017, 550, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, F.; Wang, J.; Hu, W.; Cao, X.M.; Ma, X.; Tian, H. Amorphous Metal-Free Room-Temperature Phosphorescent Small Molecules with Multicolor Photoluminescence via a Host-Guest and Dual-Emission Strategy. J. Am. Chem. Soc. 2018, 140, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xu, W.W.; Xu, W.S.; Niu, J.; Sun, X.H.; Liu, Y. A Synergistic Enhancement Strategy for Realizing Ultralong and Efficient Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 18748–18754. [Google Scholar] [CrossRef] [PubMed]
- Hayduk, M.; Riebe, S.; Voskuhl, J. Phosphorescence through Hindered Motion of Pure Organic Emitters. Chem. Eur. J. 2018, 24, 12221–12230. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, M.; Bergamini, G.; Ceroni, P. Rigidification or Interaction-Induced Phosphorescence of Organic Molecules. Chem. Commun. 2017, 53, 2081–2093. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, W.; Yang, F.; Li, B.; Ren, X.; Zhang, X.; Hu, W. Molecular Cocrystals: Design, Charge-Transfer and Optoelectronic Functionality. Phys. Chem. Chem. Phys. 2018, 20, 6009–6023. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Lee, K.; Kim, H.-J.; Lin, K.Y.; Kim, J. Activating Efficient Phosphorescence from Purely Organic Materials by Crystal Design. Nat. Chem. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- Shi, H.; An, Z.; Li, P.-Z.; Yin, J.; Xing, G.; He, T.; Chen, H.; Wang, J.; Sun, H.; Huang, W.; et al. Enhancing Organic Phosphorescence by Manipulating Heavy-Atom Interaction. Cryst. Growth Des. 2016, 16, 808–813. [Google Scholar] [CrossRef]
- Lin, Z.; Kabe, R.; Nishimura, N.; Jinnai, K.; Adachi, C. Organic Long-Persistent Luminescence from a Flexible and Transparent Doped Polymer. Adv. Mater. 2018, 30, 1803713. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Colombo, A.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. The Effect of Bromo Substituents on the Multifaceted Emissive and Crystal-Packing Features of Cyclic Triimidazole Derivatives. ChemPhotoChem 2018, 2, 801–805. [Google Scholar] [CrossRef]
- Lucenti, E.; Forni, A.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. Intrinsic and Extrinsic Heavy-Atom Effects on the Multifaceted Emissive Behavior of Cyclic Triimidazole. Chem. Eur. J. 2019, 25, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Previtali, A.; Carlucci, L.; Cariati, E. Room Temperature Phosphorescence from Organic Materials: Unravelling the Emissive Behaviour of Chloro-Substituted Derivatives of Cyclic Triimidazole. Eur. J. Org. Chem. 2021, 2021, 2041–2049. [Google Scholar] [CrossRef]
- Previtali, A.; Lucenti, E.; Forni, A.; Mauri, L.; Botta, C.; Giannini, C.; Malpicci, D.; Marinotto, D.; Righetto, S.; Cariati, E. Solid State Room Temperature Dual Phosphorescence from 3-(2-Fluoropyridin-4-Yl)Triimidazo [1,2-a:1′,2′-c:1″,2″-e][1,3,5]Triazine. Molecules 2019, 24, 2552. [Google Scholar] [CrossRef] [PubMed]
- Lucenti, E.; Forni, A.; Previtali, A.; Marinotto, D.; Malpicci, D.; Righetto, S.; Giannini, C.; Virgili, T.; Kabacinski, P.; Ganzer, L.; et al. Unravelling the Intricate Photophysical Behavior of 3-(Pyridin-2-Yl)Triimidazotriazine AIE and RTP Polymorphs. Chem. Sci. 2020, 11, 7599–7608. [Google Scholar] [CrossRef] [PubMed]
- Previtali, A.; He, W.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Carlucci, L.; Mercandelli, P.; Ortenzi, M.A.; Terraneo, G.; et al. Tunable Linear and Nonlinear Optical Properties from Room Temperature Phosphorescent Cyclic Triimidazole-Pyrene Bio-Probe. Chem. Eur. J. 2021, 27, 16690–16700. [Google Scholar] [CrossRef]
- Malpicci, D.; Giannini, C.; Lucenti, E.; Forni, A.; Marinotto, D.; Cariati, E. Mono-, Di-, Tri-Pyrene Substituted Cyclic Triimidazole: A Family of Highly Emissive and RTP Chromophores. Photochem 2021, 1, 477–487. [Google Scholar] [CrossRef]
- Formenti, M.; Blasi, D.; Cariati, E.; Carlucci, L.; Forni, A.; Giannini, C.; Guidotti, M.; Econdi, S.; Malpicci, D.; Marinotto, D.; et al. Pyrene-Substituted Cyclic Triimidazole: An Appealing and Versatile Luminescent Scaffold for Explosive Detection. Dye Pigment 2022, 206, 110637. [Google Scholar] [CrossRef]
- Ikeya, M.; Katada, G.; Ito, S. Tunable Mechanochromic Luminescence of 2-Alkyl-4-(Pyren-1-Yl)Thiophenes: Controlling the Self-Recovering Properties and the Range of Chromism. Chem. Commun. 2019, 55, 12296–12299. [Google Scholar] [CrossRef]
- Yoshida, R.; Tachikawa, T.; Ito, S. Mechano- and Thermo-Responsive Luminescence of Crystalline Thienylbenzothiadiazole Derivatives: Stepwise Hypsochromic Switching of Near-Infrared Emission. Cryst. Growth Des. 2022, 22, 547–558. [Google Scholar] [CrossRef]
- Magni, M.; Lucenti, E.; Previtali, A.; Mussini, P.R.; Cariati, E. Electrochemistry of Cyclic Triimidazoles and Their Halo Derivatives: A Casebook for Multiple Equivalent Centers and Electrocatalysis. Electrochim. Acta 2019, 317, 272–280. [Google Scholar] [CrossRef]
- Malpicci, D.; Lucenti, E.; Giannini, C.; Forni, A.; Botta, C.; Cariati, E. Prompt and Long-Lived Anti-Kasha Emission from Organic Dyes. Molecules 2021, 26, 6999. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Huang, Y.C.; Hsiow, C.Y.; Yang, Y.W.; Huang, C.I.; Rwei, S.P.; Wang, H.L.; Wang, L. Effect of Side-Chain Architecture on the Optical and Crystalline Properties of Two-Dimensional Polythiophenes. Macromolecules 2013, 46, 5985–5997. [Google Scholar] [CrossRef]
- Bruker. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Chai, J.-D.; Head-Gordon, M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
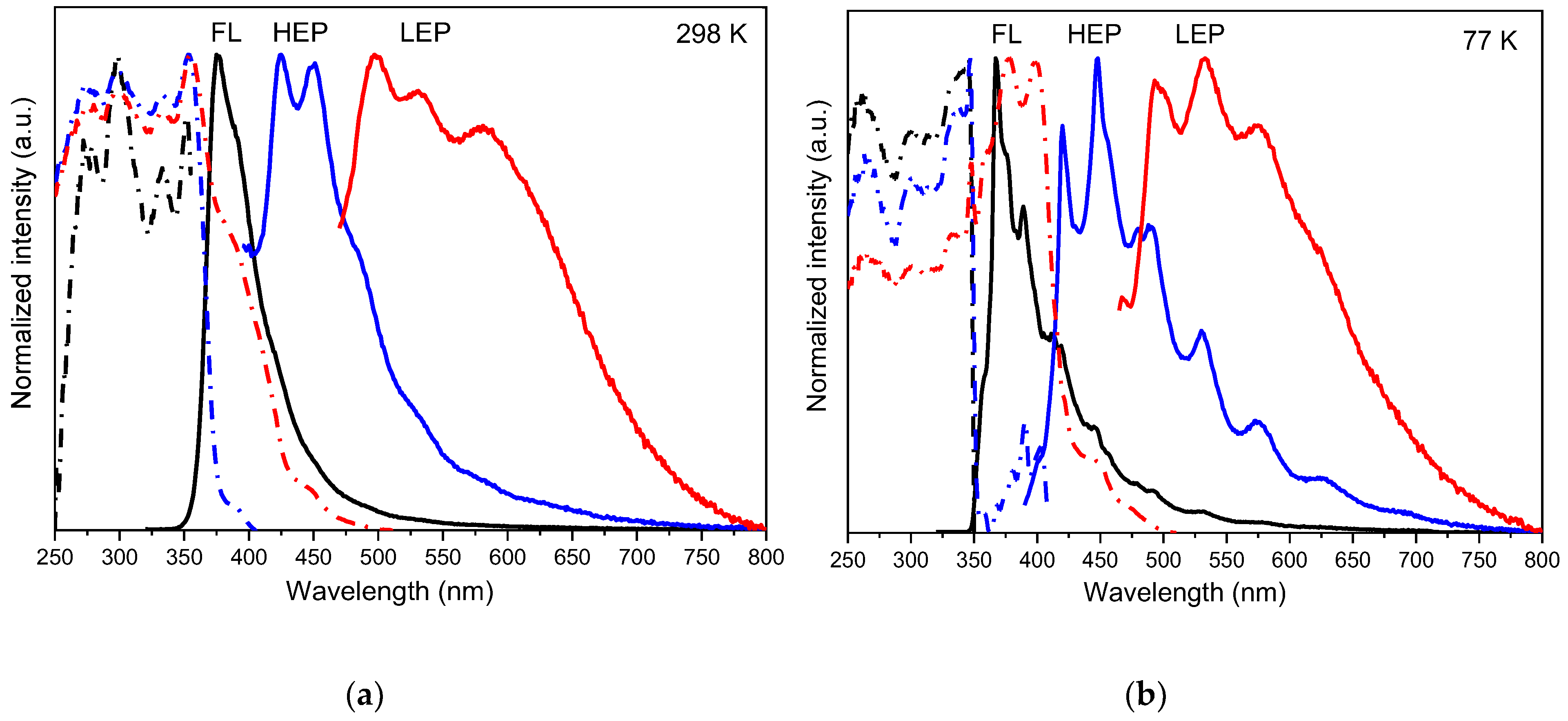

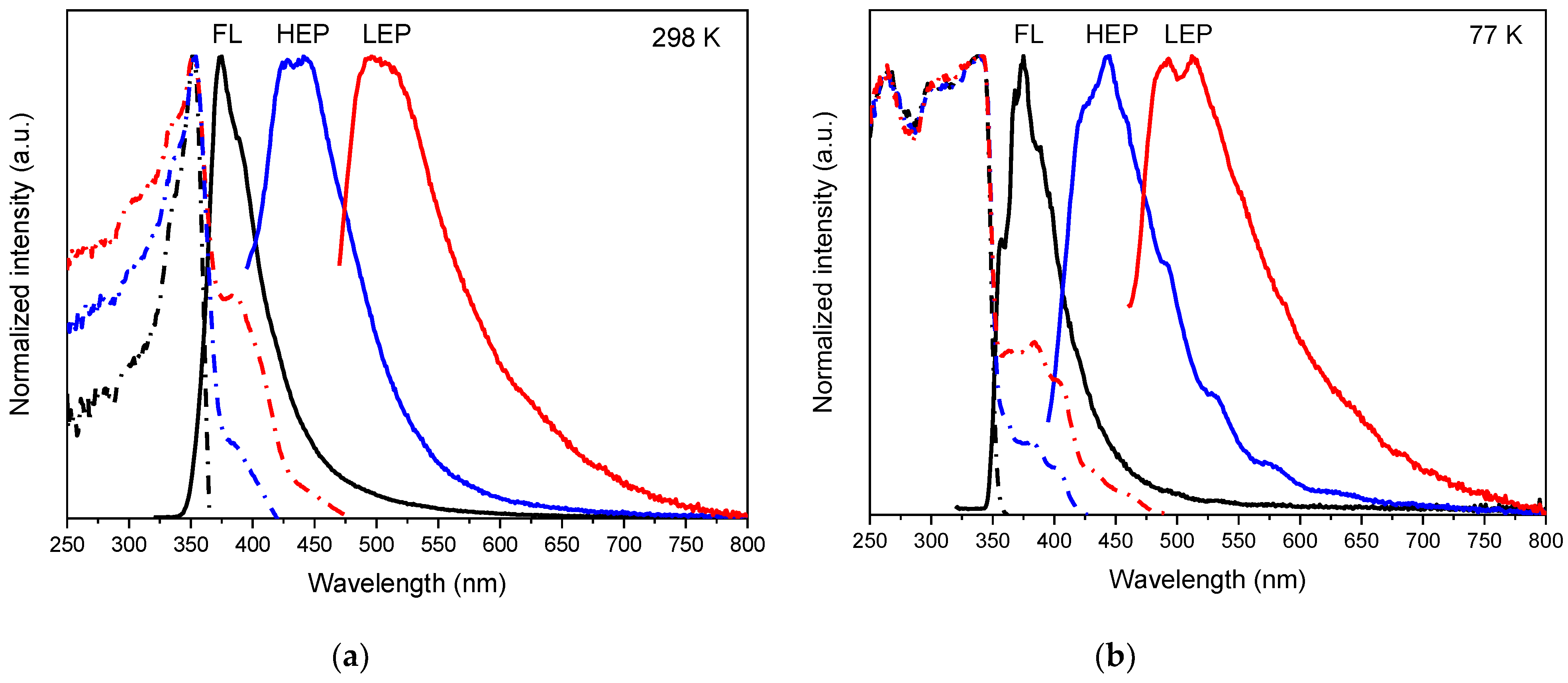
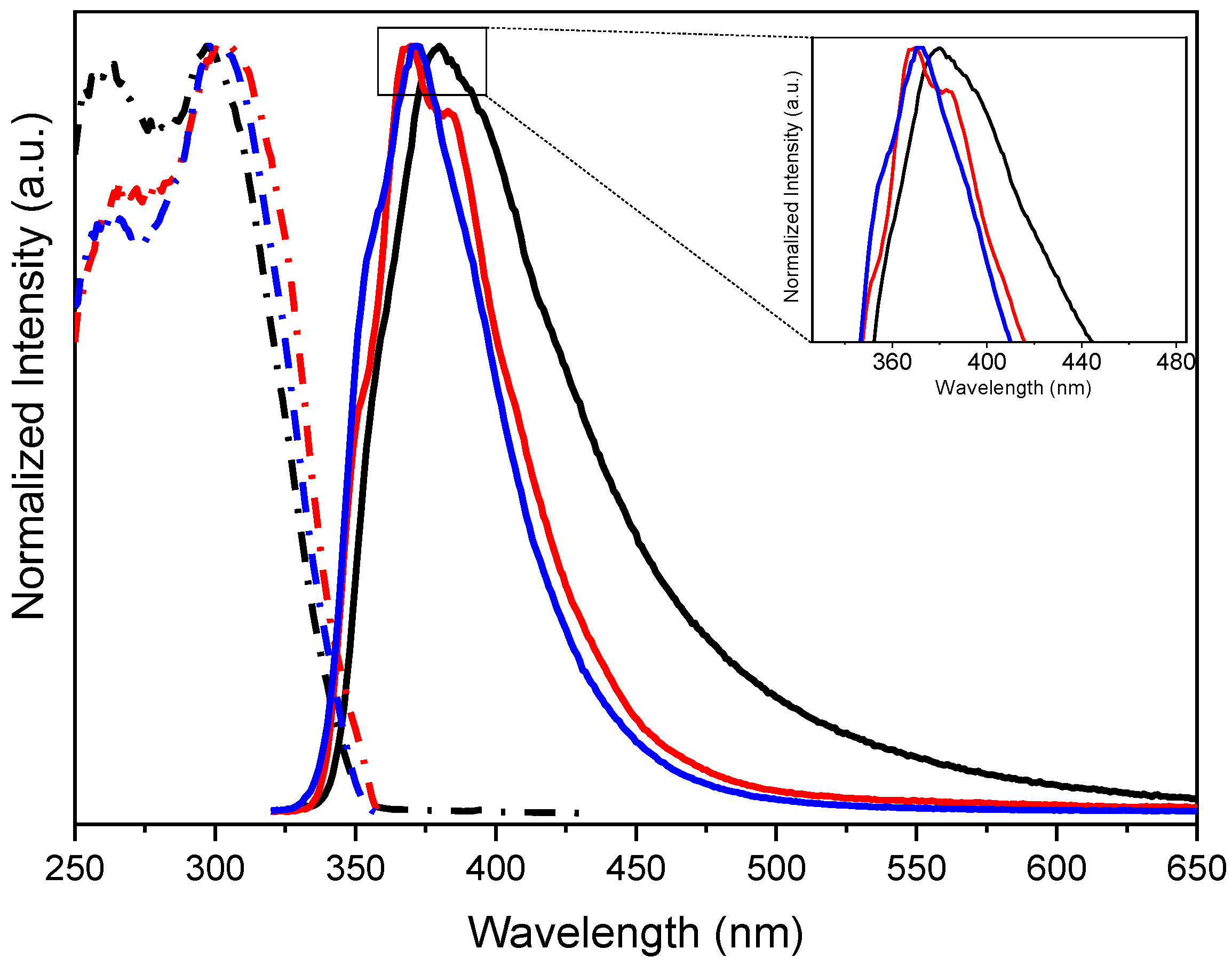

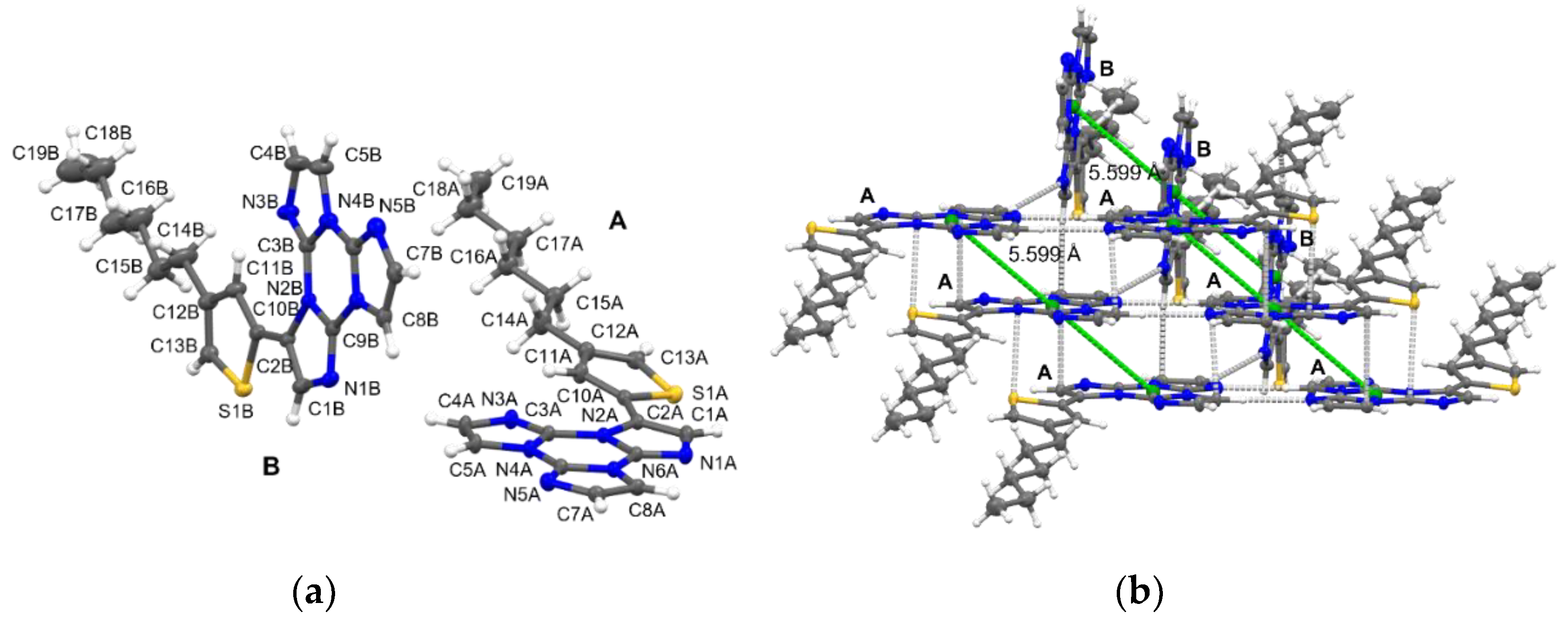
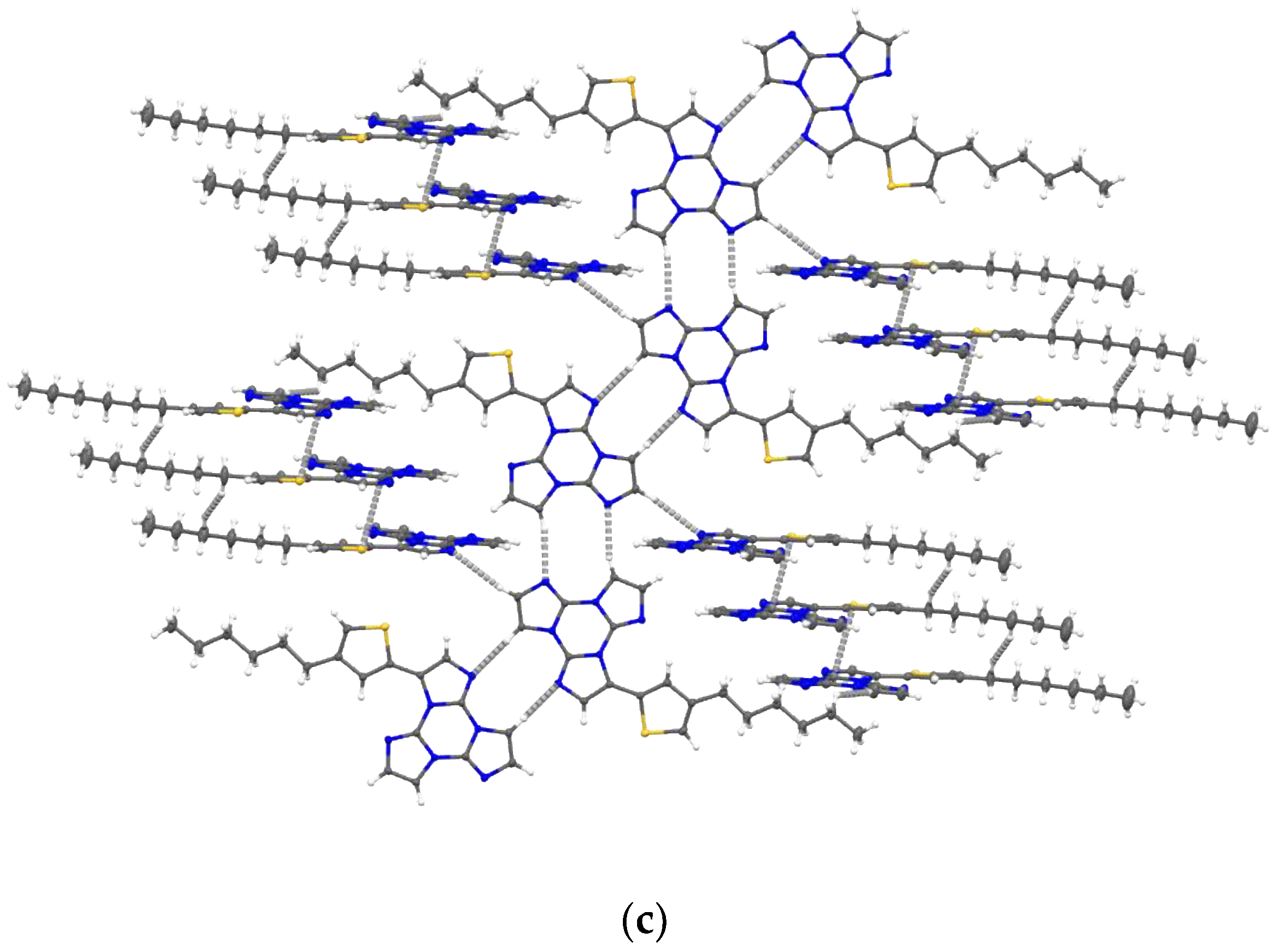

| Compound | 298 K | 77 K | |||||
|---|---|---|---|---|---|---|---|
| Φ (%) | λem | τav | λem | τav | |||
| TT-HThio | DCM | 11 | 370 | 0.81 ns | 365 | 1.71 ns | |
| PMMA | 15 | 365 | 0.72 ns | ||||
| Solid state | Crystals | 26 | 376 | 1.15 ns | 368 | 1.48 ns | |
| 425, 451 | 5.91 ms | 420, 448 | 10.61 ms | ||||
| 497, 530, 578 | 50.22 ms | 494, 533, 577 | 132.01 ms | ||||
| Ground Crystals | 18 | 376 | 1.11 ns | 375 | 1.91 ns | ||
| 428, 441 | 5.20 ms | 423, 443 | 9.56 ms | ||||
| 500 | 29.81 ms | 492, 513 | 60.84 ms | ||||
| TT-(HThio)3 | DCM | 5 | 380 | 1.17 ns | 370 | 1.42 ns | |
| PMMA | 17 | 372 | 0.90 ns | ||||
| Crystalline powders | 22 | 382, 400 | 0.42 ns | 364, 381 | 1.23 ns | ||
| 428, 453 | 15.10 ms | 423, 447 | 153.42 ms | ||||
| 514, 550 | 41.51 ms | 478, 512, 544 | 327.16 ms | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpicci, D.; Forni, A.; Cariati, E.; Inoguchi, R.; Marinotto, D.; Maver, D.; Turco, F.; Lucenti, E. Crystallization-Enhanced Emission and Room-Temperature Phosphorescence of Cyclic Triimidazole-Monohexyl Thiophene Derivatives. Molecules 2023, 28, 140. https://doi.org/10.3390/molecules28010140
Malpicci D, Forni A, Cariati E, Inoguchi R, Marinotto D, Maver D, Turco F, Lucenti E. Crystallization-Enhanced Emission and Room-Temperature Phosphorescence of Cyclic Triimidazole-Monohexyl Thiophene Derivatives. Molecules. 2023; 28(1):140. https://doi.org/10.3390/molecules28010140
Chicago/Turabian StyleMalpicci, Daniele, Alessandra Forni, Elena Cariati, Riku Inoguchi, Daniele Marinotto, Daniele Maver, Federico Turco, and Elena Lucenti. 2023. "Crystallization-Enhanced Emission and Room-Temperature Phosphorescence of Cyclic Triimidazole-Monohexyl Thiophene Derivatives" Molecules 28, no. 1: 140. https://doi.org/10.3390/molecules28010140
APA StyleMalpicci, D., Forni, A., Cariati, E., Inoguchi, R., Marinotto, D., Maver, D., Turco, F., & Lucenti, E. (2023). Crystallization-Enhanced Emission and Room-Temperature Phosphorescence of Cyclic Triimidazole-Monohexyl Thiophene Derivatives. Molecules, 28(1), 140. https://doi.org/10.3390/molecules28010140