An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities
Abstract
1. Introduction
2. Results and Discussion
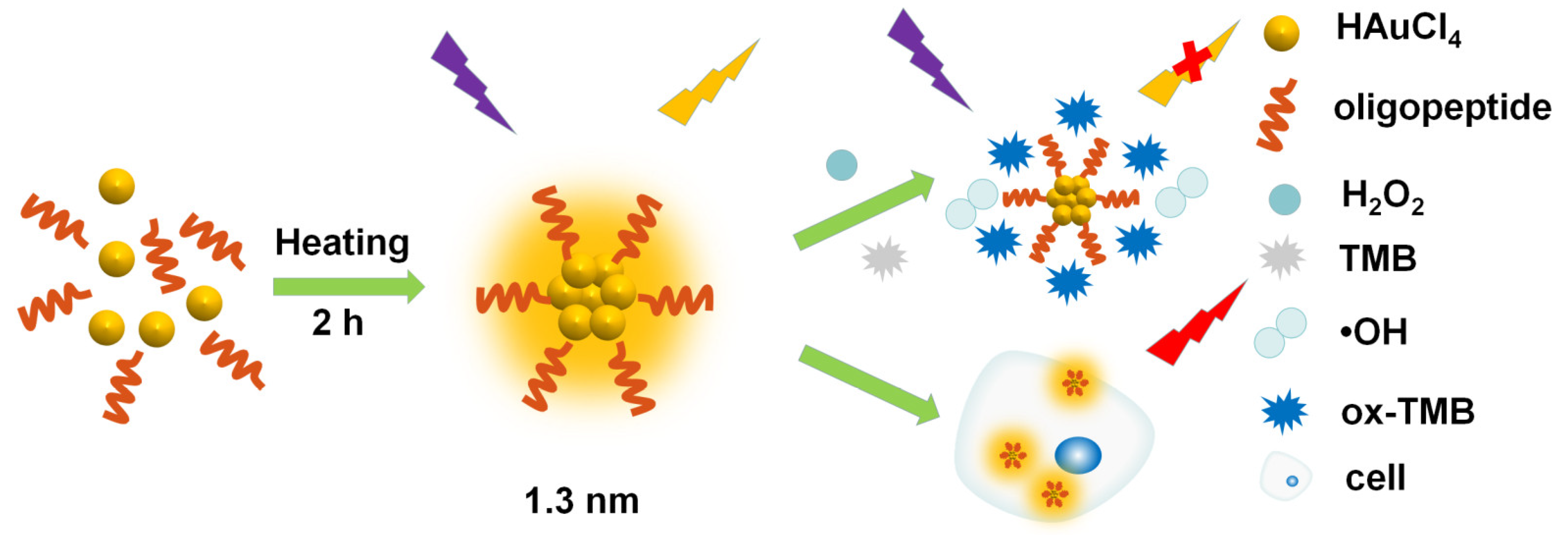
2.1. Characterization of Materials
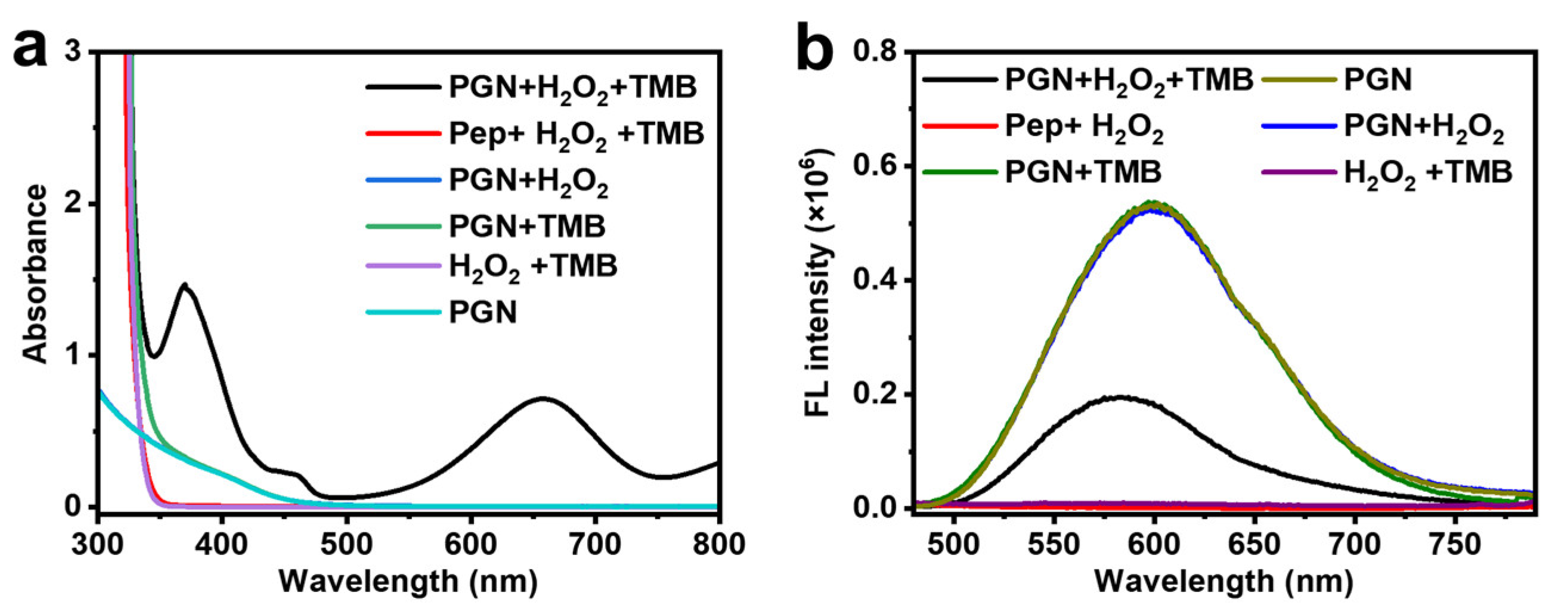
2.2. Peroxidase-like Activity of PGN
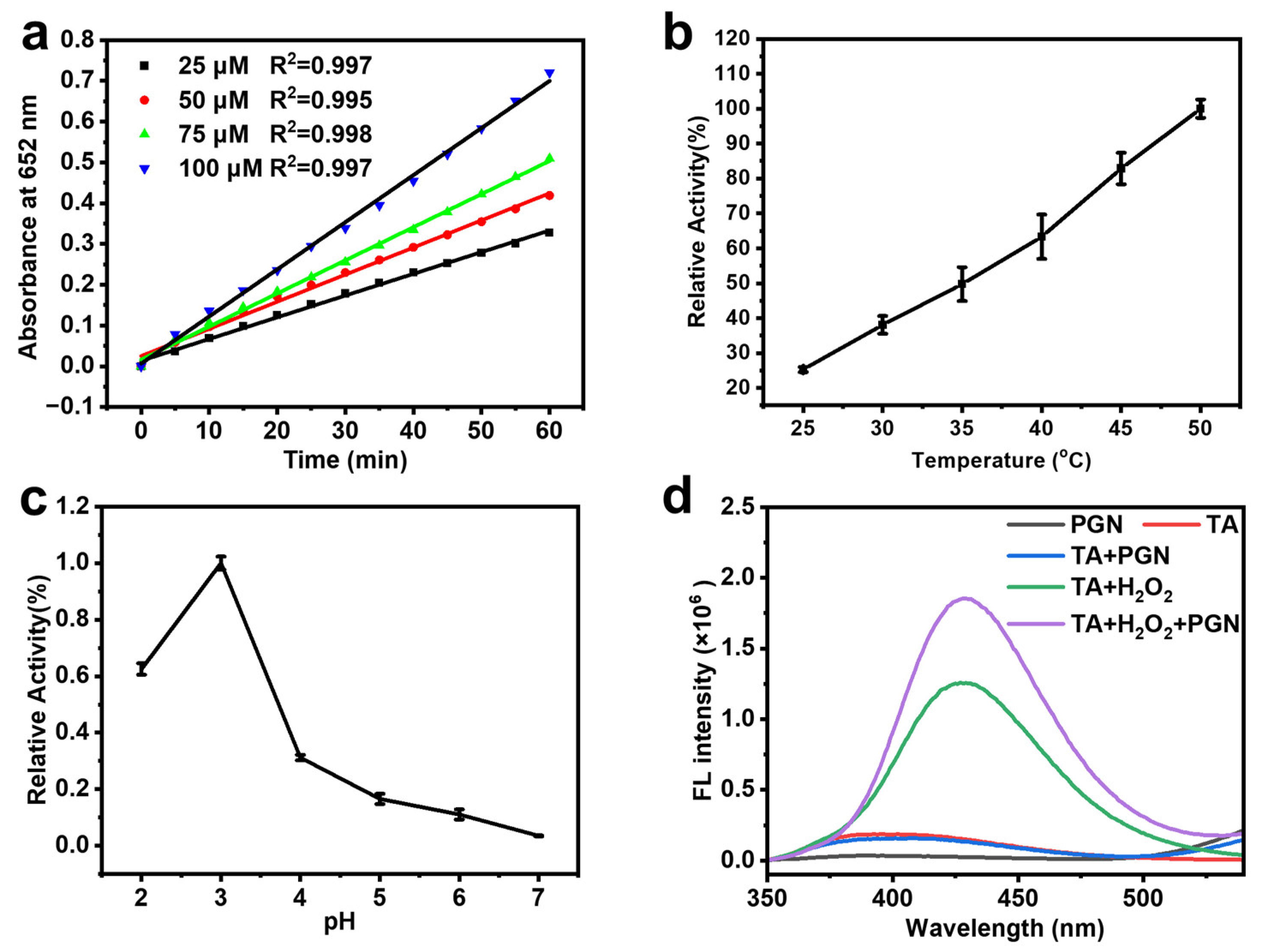
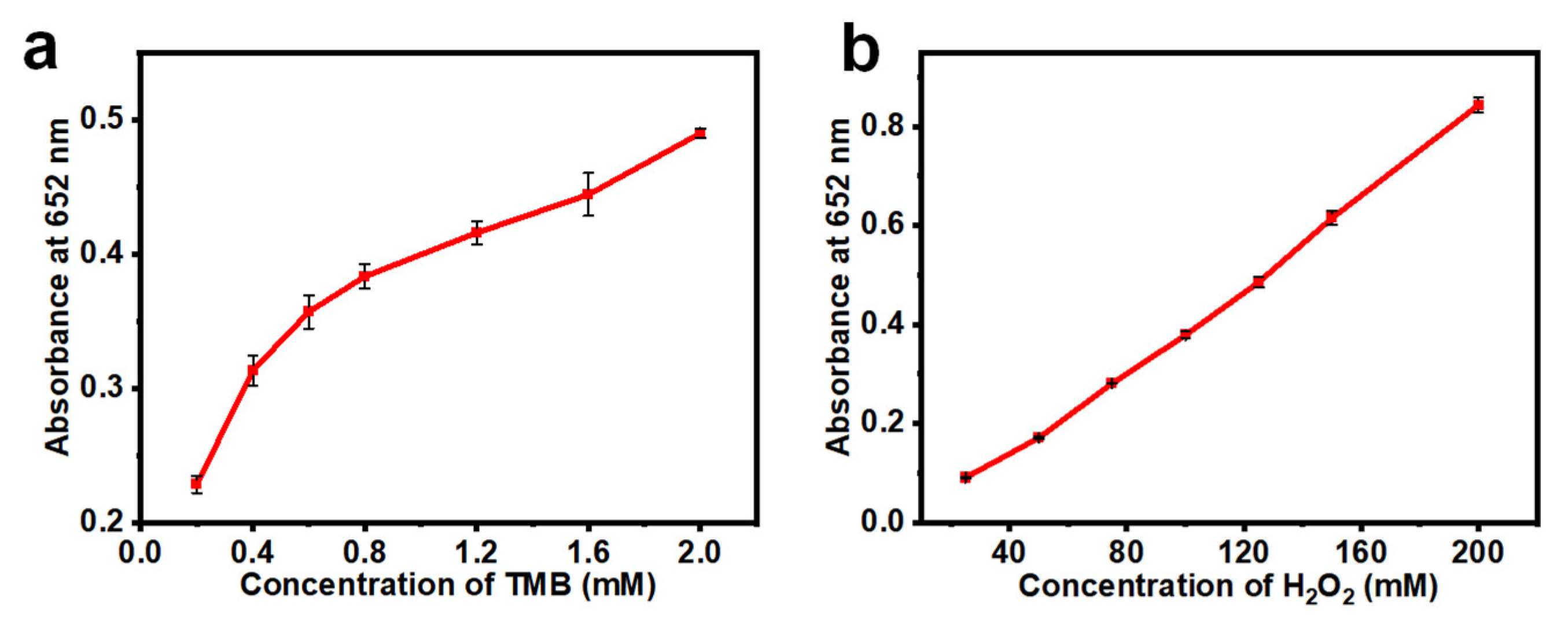
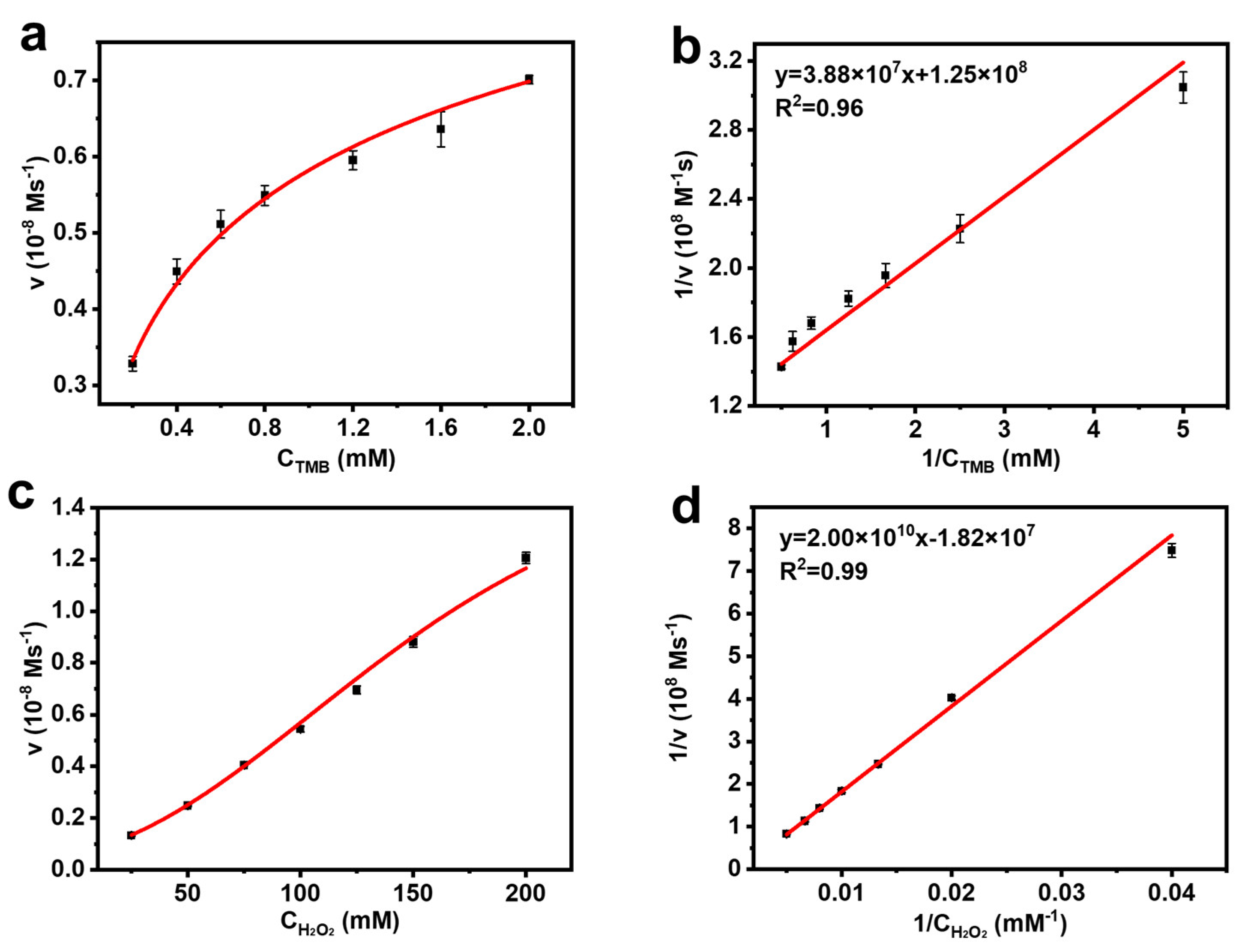
2.3. Kinetic Assay of the Peroxidase-like Activity
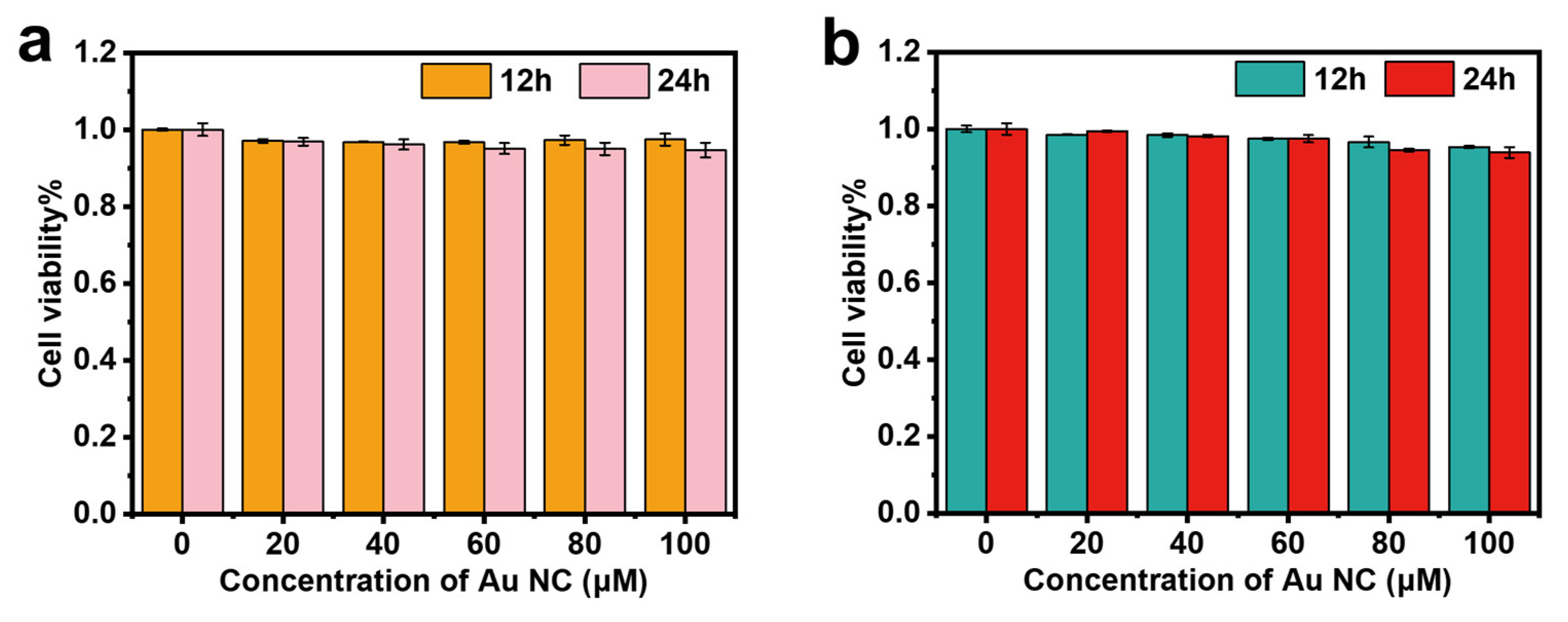
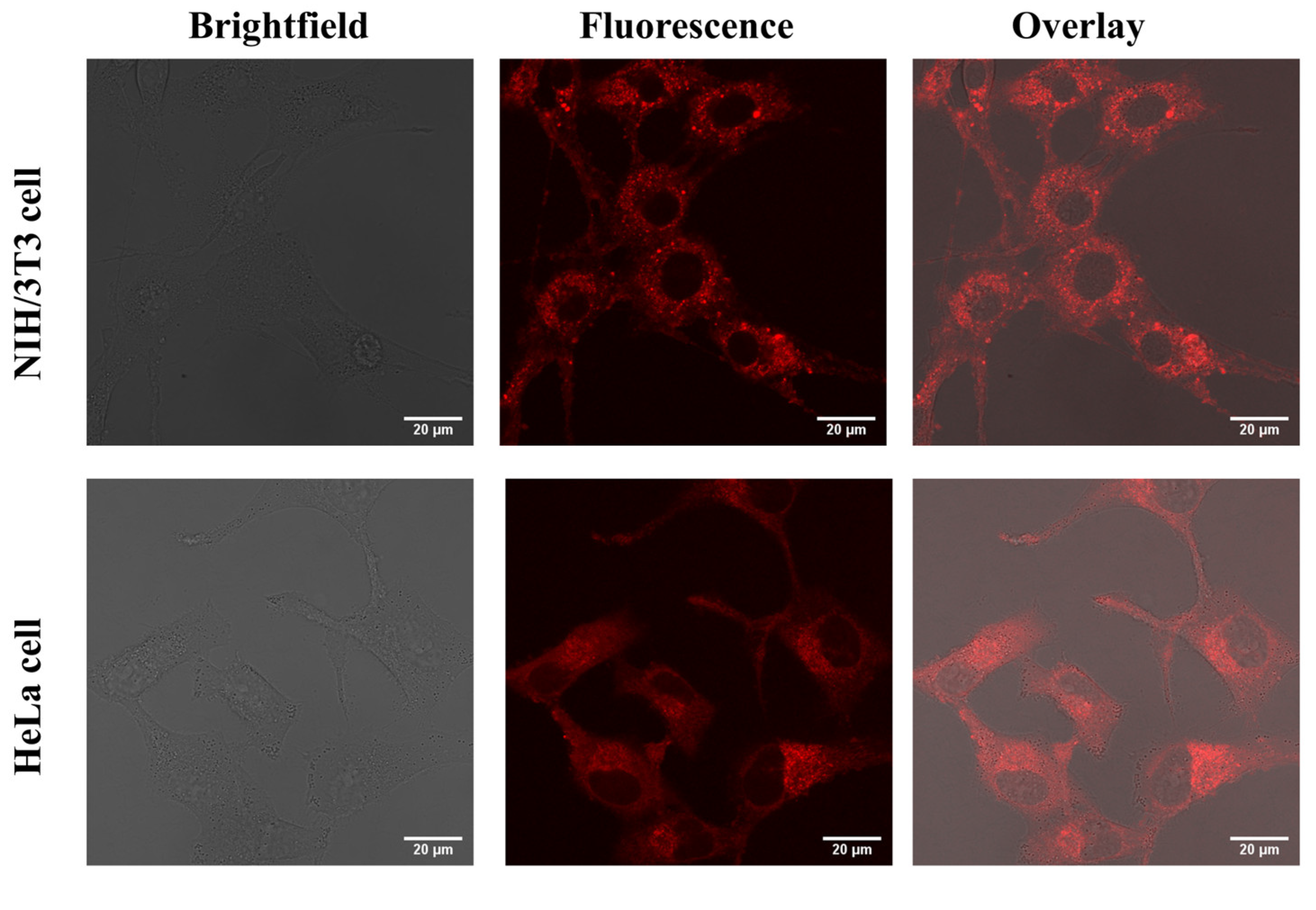
2.4. Cytotoxicity Study and In Vitro Imaging
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Equipment
3.3. Synthesis of the PGN
3.4. Peroxidase-like Activity
3.5. Fluorescence Quenching Experiments with Adding H2O2 into the Cluster-TMB System
3.6. Concentration-Dependent Peroxidase-like Activity of the Prepared Clusters
3.7. Determination of the Hydroxyl (·OH) Radical
3.8. Steady-State Kinetic Assay of the Peroxidase-like Activity of the Clusters
3.9. Cytotoxicity Experiment
3.10. Cell Culture and Confocal Fluorescence Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, J.; Yang, G.; Yang, J.; Ding, D.; Zhang, M. Copper(II) ions enhance the peroxidase-like activity and stability of keratin-capped gold nanoclusters for the colorimetric detection of glucose. Mikrochim Acta 2019, 186, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Liu, H.; Long, W.; Zhang, X.D. Enzyme-Like Properties of Gold Clusters for Biomedical Application. Front. Chem. 2020, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Krishna Kumar, A.S.; Tseng, W.-L. Perspective on recent developments of near infrared-emitting gold nanoclusters: Applications in sensing and bio-imaging. Anal. Methods 2020, 12, 1809–1826. [Google Scholar] [CrossRef]
- You, J.G.; Lu, C.Y.; Krishna Kumar, A.S.; Tseng, W.L. Cerium(iii)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale 2018, 10, 17691–17698. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Dai, X.; Ding, L.; Chen, J.; Yao, G.; Liu, X.; Luo, S.; Shi, J.; Wang, L.; et al. Single-Stranded DNA-Encoded Gold Nanoparticle Clusters as Programmable Enzyme Equivalents. J. Am. Chem. Soc. 2022, 144, 6311–6320. [Google Scholar] [CrossRef]
- Xu, J.; Sun, F.; Li, Q.; Yuan, H.; Ma, F.; Wen, D.; Shang, L. Ultrasmall Gold Nanoclusters-Enabled Fabrication of Ultrafine Gold Aerogels as Novel Self-Supported Nanozymes. Small 2022, 18, e2200525. [Google Scholar] [CrossRef]
- Qiu, N.; Liu, Y.; Guo, R. Electrodeposition-Assisted Rapid Preparation of Pt Nanocluster/3D Graphene Hybrid Nanozymes with Outstanding Multiple Oxidase-Like Activity for Distinguishing Colorimetric Determination of Dihydroxybenzene Isomers. ACS Appl. Mater. Interfaces 2020, 12, 15553–15561. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Bao, M.; Zhang, L.; Carraro, C.; Maboudian, R.; Liu, A.; Wei, W.; Zhang, Y.; Liu, S. Pd Nanoclusters Confined in ZIF-8 Matrixes for Fluorescent Detection of Glucose and Cholesterol. ACS Appl. Nano Mater. 2021, 4, 9132–9142. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, J.; Zhou, Z.; Xu, H.; Cui, L.; Lv, Z.; Qin, Y. TiO2 Nanoflowers Decorated with FeOx Nanocluster and Single Atoms by Atomic Layer Deposition for Peroxidase-Mimicking Nanozymes. ACS Appl. Nano Mater. 2022, 5, 13090–13099. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Han, X.; Fang, Y.; Liu, X.; Wang, X.; Waterhouse, G.I.N.; Xu, C.; Yin, H.; Gao, X. Polypeptide-Templated Au Nanoclusters with Red and Blue Fluorescence Emissions for Multimodal Imaging of Cell Nuclei. ACS Appl. Bio. Mater. 2020, 3, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bain, D.; Chakraborty, S.; Kolay, S.; Patra, A. Copper Nanocluster (Cu23 NC)-Based Biomimetic System with Peroxidase Activity. ACS Sustain. Chem. Eng. 2020, 8, 18335–18344. [Google Scholar] [CrossRef]
- Wang, G.L.; Jin, L.Y.; Dong, Y.M.; Wu, X.M.; Li, Z.J. Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens. Bioelectron. 2015, 64, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J.; Ganjali, M.R. New Colorimetric DNA Sensor for Detection of Campylobacter jejuni in Milk Sample Based on Peroxidase-Like Activity of Gold/Platinium Nanocluster. ChemistrySelect 2019, 4, 11687–11692. [Google Scholar] [CrossRef]
- Li, M.; Lao, Y.H.; Mintz, R.L.; Chen, Z.; Shao, D.; Hu, H.; Wang, H.X.; Tao, Y.; Leong, K.W. A multifunctional mesoporous silica-gold nanocluster hybrid platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay. Nanoscale 2019, 11, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, J.; Lei, J.; Fan, Q.; Tang, X.; Zhu, G.; Yan, Q.; Feng, X.; Shi, B. An efficient treatment of biofilm-induced periodontitis using Pt nanocluster catalysis. Nanoscale 2021, 13, 17912–17919. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Gao, Y.-C.; Li, H.-W.; Wu, Y. Gold–Platinum Bimetallic Nanoclusters for Oxidase-like Catalysis. ACS Appl. Nano Mater. 2020, 3, 9318–9328. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Fan, A. Improvement of mimetic peroxidase activity of gold nanoclusters on the luminol chemiluminescence reaction by surface modification with ethanediamine. Luminescence 2018, 33, 751–758. [Google Scholar] [CrossRef]
- Dehghani, Z.; Mohammadnejad, J.; Hosseini, M. A new colorimetric assay for amylase based on starch-supported Cu/Au nanocluster peroxidase-like activity. Anal. Bioanal. Chem. 2019, 411, 3621–3629. [Google Scholar] [CrossRef]
- Garcia-Lopez, V.; Chen, F.; Nilewski, L.G.; Duret, G.; Aliyan, A.; Kolomeisky, A.B.; Robinson, J.T.; Wang, G.; Pal, R.; Tour, J.M. Molecular machines open cell membranes. Nature 2017, 548, 567–572. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Shukla, S.; Gu, Y.; Yu, X.; Steinmetz, N.F. Dysprosium-Modified Tobacco Mosaic Virus Nanoparticles for Ultra-High-Field Magnetic Resonance and Near-Infrared Fluorescence Imaging of Prostate Cancer. ACS Nano 2017, 11, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.-K.; Liu, J.-C.; Cheng, B.; Liu, Y.-C.; Lai, T.-S.; Lin, H.-C.; Yeh, M.-Y. Tumor targeting with DGEA peptide ligands: A new aromatic peptide amphiphile for imaging cancers. Chem. Comm. 2019, 55, 1060–1063. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, M.Q.; Ma, G.F.; Wang, Y.L.; Zhao, L.N.; Yuan, Q.; Gao, F.P.; Liu, R.; Zhai, J.; Chai, Z.F.; et al. Peptide-Conjugated Gold Nanoprobe: Intrinsic Nanozyme-Linked Immunsorbant Assay of Integrin Expression Level on Cell Membrane. ACS Nano 2015, 9, 10979–10990. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 11. [Google Scholar] [CrossRef]
- Sugiuchi, M.; Maeba, J.; Okubo, N.; Iwamura, M.; Nozaki, K.; Konishi, K. Aggregation-Induced Fluorescence-to-Phosphorescence Switching of Molecular Gold Clusters. J. Am. Chem. Soc. 2017, 139, 17731–17734. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef]
- Yu, H.; Rao, B.; Jiang, W.; Yang, S.; Zhu, M. The photoluminescent metal nanoclusters with atomic precision. Coord. Chem. Rev. 2019, 378, 595–617. [Google Scholar] [CrossRef]
- Zheng, Y.; Lai, L.; Liu, W.; Jiang, H.; Wang, X. Recent advances in biomedical applications of fluorescent gold nanoclusters. Adv. Colloid Interface Sci. 2017, 242, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, T.; Chen, J.; Shi, J.; Wang, L.; Shen, J.; Liu, X. Water-Dispersible Gold Nanoclusters: Synthesis Strategies, Optical Properties, and Biological Applications. Chem. Eur. J. 2022, 28, e202103736. [Google Scholar] [CrossRef]
- Shang, L.; Azadfar, N.; Stockmar, F.; Send, W.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. One-pot synthesis of near-infrared fluorescent gold clusters for cellular fluorescence lifetime imaging. Small 2011, 7, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, Z.; Chevrier, D.M.; Leong, D.T.; Zhang, P.; Jiang, D.E.; Xie, J. Identification of a highly luminescent Au22(SG)18 nanocluster. J. Am. Chem. Soc. 2014, 136, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.S.; Rasaeinezhad, S.; Es’haghi, Z. Evaluation of flutamide loading capacity of biosynthesis of plant-mediated glutathione-modified gold nanoparticles by Dracocephalum Kotschyi Boiss extract. Chem. Zvesti 2020, 74, 2041–2048. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Mechanism of the oxidation of 3,5,3’,5’-tetramethylbenzidine by myeloperoxidase determined by transient- and steady-state kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef]
- Du, C.; Qi, L.; Wang, Y.; Pei, K.; Zhang, R.; Wu, D.; Qi, W. Fluorescence dual “turn-on” detection of acid phosphatase via fluorescence resonance energy transfer and dual quenching strategy to improve sensitivity. Dyes Pigm. 2022, 205, 110554. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Huang, H.; Zhang, L.; Wang, L.; Liu, F.; Chen, J.; Zeng, Y.; Chu, P.K. Colorimetric and ultra-sensitive fluorescence resonance energy transfer determination of H2O2 and glucose by multi-functional Au nanoclusters. Analyst 2014, 139, 1498–1503. [Google Scholar] [CrossRef]
- Li, J.J.; Qiao, D.; Yang, S.Z.; Weng, G.J.; Zhu, J.; Zhao, J.W. Colorimetric determination of cysteine based on inhibition of GSH-Au/Pt NCs as peroxidase mimic. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119257. [Google Scholar] [CrossRef]
- Hu, D.; Sheng, Z.; Fang, S.; Wang, Y.; Gao, D.; Zhang, P.; Gong, P.; Ma, Y.; Cai, L. Folate receptor-targeting gold nanoclusters as fluorescence enzyme mimetic nanoprobes for tumor molecular colocalization diagnosis. Theranostics 2014, 4, 142–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, S.; Liao, L.; Wang, C.; Xia, N.; Gan, Z.; Gu, W.; Li, J.; Deng, H.; Wu, Z. A Dual Purpose Strategy to Endow Gold Nanoclusters with Both Catalysis Activity and Water Solubility. J. Am. Chem. Soc. 2020, 142, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, Y.; Du, J. Bifunctional gold nanoclusters enable ratiometric fluorescence nanosensing of hydrogen peroxide and glucose. Talanta 2019, 197, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Yadav, P.K.; Chandra, S.; Bano, D.; Talat, M.; Hasan, S.H. Peroxidase mimetic activity of fluorescent NS-carbon quantum dots and their application in colorimetric detection of H2O2 and glutathione in human blood serum. J. Mater. Chem. B 2018, 6, 5256–5268. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, J.; Ou, Y.; Shi, Y.; Liu, L.; Sun, C.; Zheng, H.; Long, Y. Peroxidase-like activity of 2’,7’-difluorofluorescein and its application for galactose detection. Talanta 2018, 182, 422–427. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Gao, P.; Wu, S.; Chang, X.; Liu, F.; Zhang, T.; Wang, B.; Zhang, K.Q. Aprotinin Encapsulated Gold Nanoclusters: A Fluorescent Bioprobe with Dynamic Nuclear Targeting and Selective Detection of Trypsin and Heavy Metal. Bioconjug. Chem. 2018, 29, 4140–4148. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Ou, J.; Chen, L.; Zhang, L.; Zheng, Z.; Yu, H.; Meng, X.; Zhu, M. An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities. Molecules 2023, 28, 70. https://doi.org/10.3390/molecules28010070
Fan D, Ou J, Chen L, Zhang L, Zheng Z, Yu H, Meng X, Zhu M. An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities. Molecules. 2023; 28(1):70. https://doi.org/10.3390/molecules28010070
Chicago/Turabian StyleFan, Daoqing, Jiale Ou, Ling Chen, Lichao Zhang, Zhiren Zheng, Haizhu Yu, Xiangming Meng, and Manzhou Zhu. 2023. "An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities" Molecules 28, no. 1: 70. https://doi.org/10.3390/molecules28010070
APA StyleFan, D., Ou, J., Chen, L., Zhang, L., Zheng, Z., Yu, H., Meng, X., & Zhu, M. (2023). An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities. Molecules, 28(1), 70. https://doi.org/10.3390/molecules28010070




