Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation
Abstract
1. Introduction
2. Results and Discussion
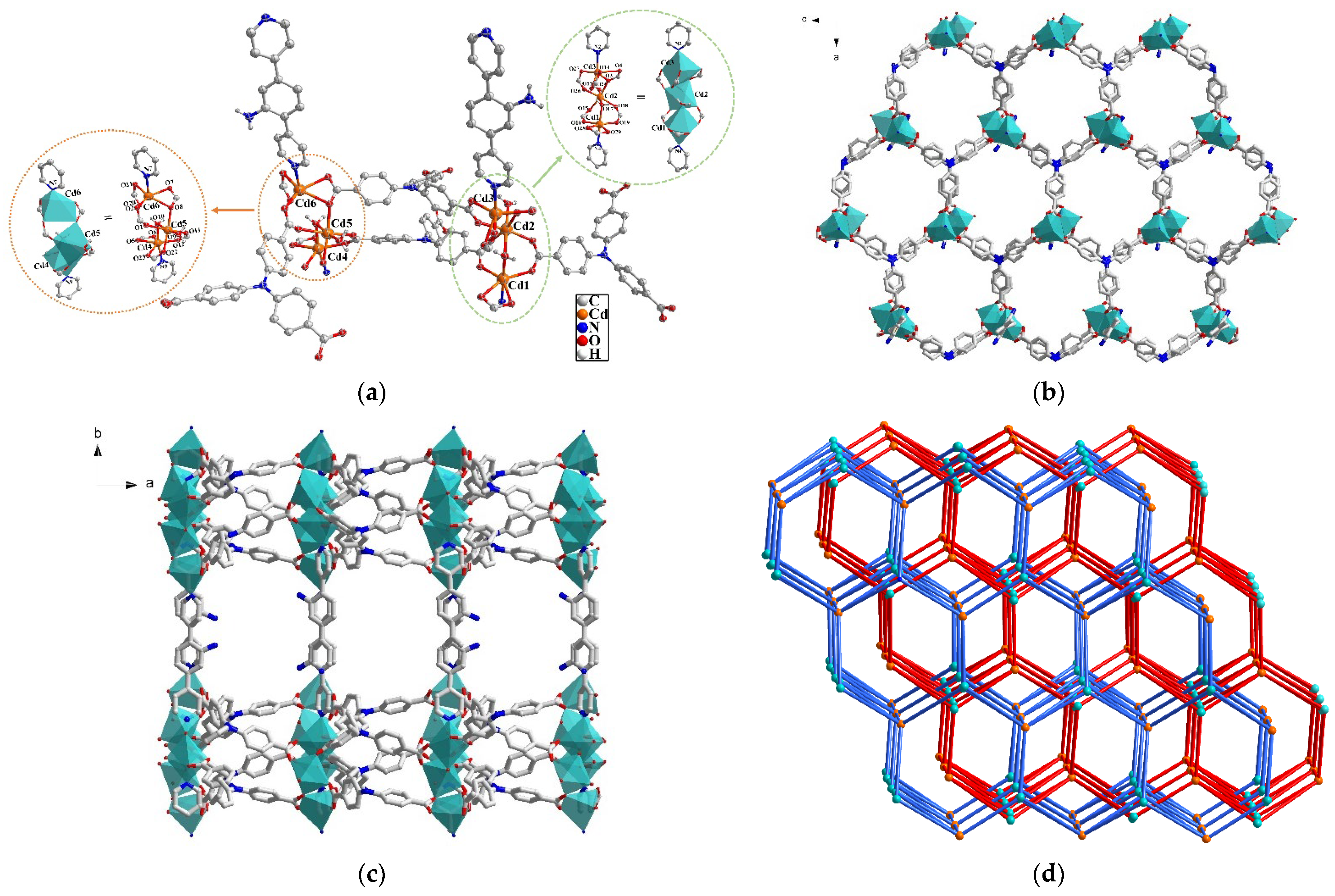
2.1. Crystal Structure Description of MOF 1
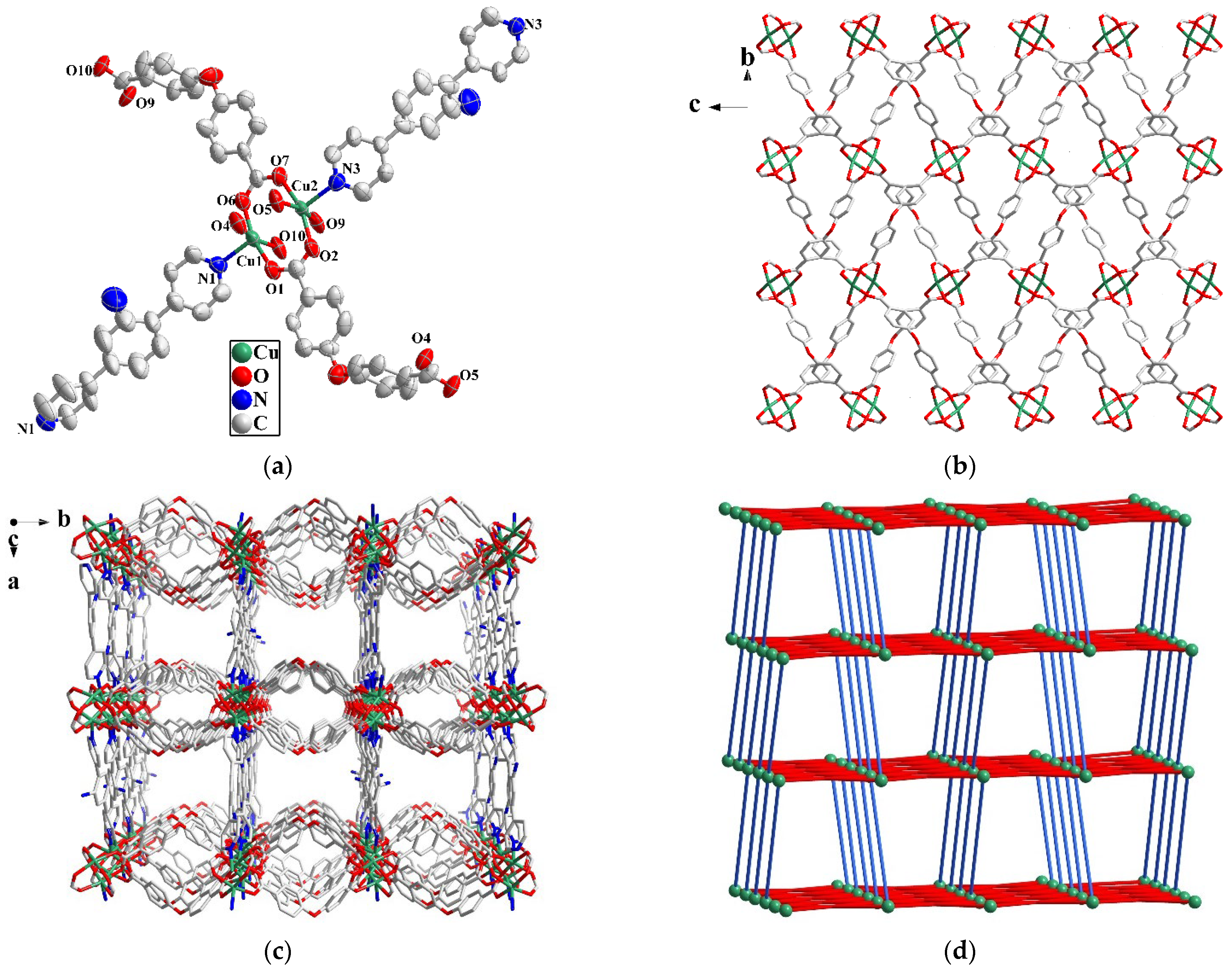
2.2. Crystal Structure Description of MOF 2
2.3. Powder X-ray Diffraction (PXRD) and Thermogravimetric Analyses (TGA)
2.4. Stability of MOF 1 in Different Solvent
2.5. Photoluminescence of MOF 1
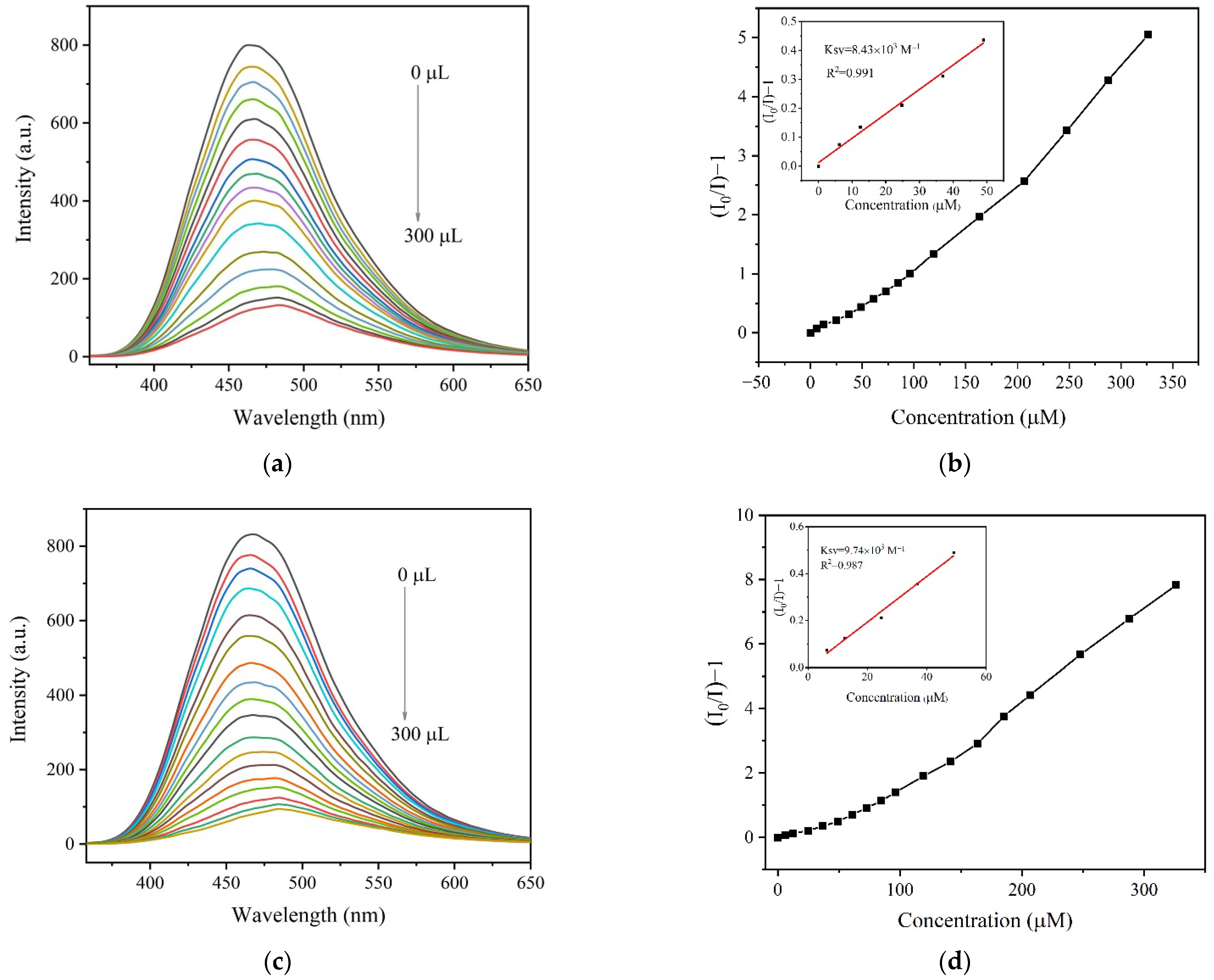
2.6. Fluorescence Sensing Specific AA by 1
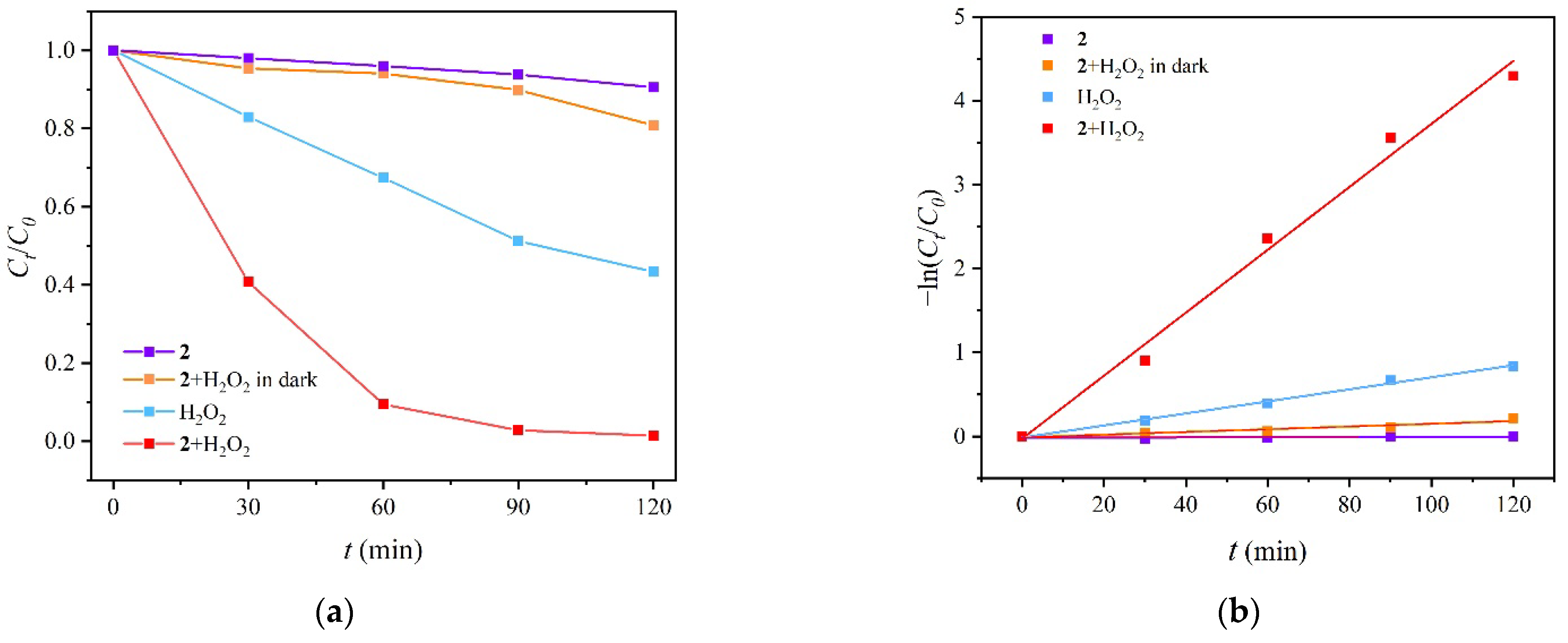
2.7. Photocatalytic Degradation of RhB by MOF 2
3. Materials and Methods
3.1. Synthesis of [Cd6(DPA)2(NTB)4(H2O)4]n·n(DPA·5DMA·H2O) (1)
3.2. Synthesis of [Cu2(DPA)(OBA)2]n·n(2.5DMF·H2O) (2)
3.3. Fluorescent Sensing AA by MOF 1
3.4. Photocatalyzing Degradation of RhB by MOF 2
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Matthew, M.; Hans, P.H.A.; Mine, B.T.; Annika, J. The Global Threat from Plastic Pollution. Science 2021, 373, 61–65. [Google Scholar]
- Zhao, S.M.; Qiu, Z.F.; Xu, Z.H.; Huang, Z.Q.; Zhao, Y.; Sun, W.Y. Fluorescent Zn(II) Frameworks with Multicarboxylate and Pyridyl N-donor Ligands for Sensing Specific Anions and Organic Molecules. Dalton Trans. 2022, 51, 3572–3580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhao, Y.; Zhang, X.D.; Kang, Y.S.; Lu, Q.Y.; Azam, M.; Al-Resayes, S.I.; Sun, W.Y. Metal–Organic Frameworks with 1,4-di(1H-imidazol-4-yl)benzene and Varied Carboxylate Ligands for Selectively Sensing Fe(III) Ions and Ketone Molecules. Dalton Trans. 2017, 46, 13943–13951. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q.; Chen, J.Q.; Zhao, S.M.; Qiu, Z.F.; Zhao, Y.; Sun, W.Y. Supramolecular Assemblies of Zn(II) Complexes with a D–π–A Ligand for Sensing Specific Organic Molecules. CrystEngComm 2022, 24, 3612–3620. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.D.; Xie, X.F.; Guo, F.; Sun, W.Y. Amino Group Dependent Sensing Properties of Metal–Organic Frameworks: Selective Turn-on Fluorescence Detection of Lysine and Arginine. RSC Adv. 2020, 10, 37449–37455. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.Y. Photoluminescent Metal–Organic Frameworks and Their Application for Sensing Biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, M.Y.; Bai, J.P.; Zeng, H.; Lu, W.; Li, D. pH-Modulated Luminescence Switching in a Eu-MOF: Rapid Detection of Acidic Amino Acids. J. Mater. Chem. A 2019, 7, 11127–11133. [Google Scholar] [CrossRef]
- White, T.L.; Monnig, M.A.; Walsh, E.G.; Nitenson, A.Z.; Harris, A.D.; Cohen, R.A.; Porges, E.C.; Woods, A.J.; Lamb, D.G.; Boyd, C.A.; et al. Psychostimulant Drug Effects on Glutamate, Glx, and Creatine in the Anterior Cingulate Cortex and Subjective Response in Healthy Humans. Neuropsychopharmacology 2018, 43, 1498–1509. [Google Scholar] [CrossRef]
- Mohammadi, L.; Khavasi, H.R. Anthracene-Tagged UiO-67-MOF as Highly Selective Aqueous Sensor for Nanoscale Detection of Arginine Amino Acid. Inorg. Chem. 2020, 59, 13091–13097. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, J.L.; Zheng, Q.M.; Chang, J.F.; Wu, L.Z.; Zhang, M.D.; Qin, L. The Ligand Effect Resulted in Different Fluorescence Responses of Two Similar Zinc-based MOFs to High-valence Metal Ions and Amino Acids. Micropor. Mesopor. Mat. 2021, 321, 111130. [Google Scholar] [CrossRef]
- Qiu, Z.F.; Zhao, S.M.; Xu, Z.H.; Zhao, Y.; Wang, Z.L.; Sun, W.Y. Crystal Structures and Luminescent Probe Behaviors of Three-Dimensional Zn(II) Frameworks with Multicarboxylate and Tetradentate Imidazole-Containing Ligands. Cryst. Growth Des. 2021, 21, 5306–5316. [Google Scholar] [CrossRef]
- Chen, Z.P.; Li, D.; Xu, L.; Jiang, Y.F.; Lin, K.; Zhao, Y.; Zhao, J. Cationic Metal–Organic Frameworks Constructed from a Trigonal Imidazole-containing Ligand for the Removal of Cr2O72− in Water. New J. Chem. 2022, 46, 12994–1300014. [Google Scholar] [CrossRef]
- Dong, J.; Dao, X.Y.; Zhang, X.Y.; Zhang, X.D.; Sun, W.Y. Sensing Properties of NH2-MIL-101 Series for Specific Amino Acids via Turn-On Fluorescence. Molecules 2021, 26, 5336. [Google Scholar]
- Cheng, X.M.; Dao, X.Y.; Wang, S.Q.; Zhao, J.; Sun, W.Y. Enhanced Photocatalytic CO2 Reduction Activity over NH2-MIL-125(Ti) by Facet Regulation. ACS Catalysis 2021, 11, 650–658. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling Reaction between CO2 and Cyclohexene Oxide: Selective Control from Cyclic Carbonate to Polycarbonate by Ligand Design of Salen/Salalen Titanium Complexes. Catal. Sci. Technol. 2014, 4, 3964–3972. [Google Scholar] [CrossRef]
- Andrea, K.A.; Kerton, F.M. Triarylborane-Catalyzed Formation of Cyclic Organic Carbonates and Polycarbonates. ACS Catal. 2019, 9, 1799–1809. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and Photocatalysis by Metal-Organic Frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, H.; Zhang, K.; Hu, W.X.; Cheng, Z.Z.; Chen, H.P.; Feng, X.; Peng, T.; Kou, Z.K. Monodispersed Ruthenium Nanoparticles Interfacially Bonded with Defective Nitrogen-and-Phosphorus-Doped Carbon Nanosheets Enable pH-Universal Hydrogen Evolution Reaction. Appl. Catal. B. 2022, 306, 121095. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.Q.; Feng, X.; Zhu, L.; Fang, Q.Z.; Li, S.; Wang, L.Y.; Li, Z.J.; Kou, Z.K. A Chainmail Effect of Ultrathin N-Doped Carbon Shell on Ni2P Nanorod Arrays for Efficient Hydrogen Evolution Reaction Catalysis. J. Colloid Interface Sci. 2022, 607, 281–289. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of Water-Stable Metal-Organic Frameworks in the Removal of Water Pollutants: A Review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal Oxides and Metal-Organic Frameworks for the Photocatalytic Degradation: A Review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Liu, J.Q.; Kumar, A. Syntheses, Design Strategies, and Photocatalytic Charge Dynamics of Metal–Organic Frameworks (MOFs): A Catalyzed Photo-Degradation Approach Towards Organic Dyes. Catal. Sci. Technol. 2021, 11, 3946–3989. [Google Scholar] [CrossRef]
- Wang, J.; Rao, C.Y.; Lu, L.; Zhang, S.L.; Muddassir, M.; Liu, J.Q. Efficient Photocatalytic Degradation of Methyl Violet Using Two New 3D MOFs Directed by Different Carboxylate Spacers. CrystEngComm 2021, 23, 741–747. [Google Scholar] [CrossRef]
- Dong, X.Y.; Li, Y.Y.; Li, D.Q.C.; Liao, D.H.; Qin, T.R.; Prakash, O.; Kumar, A.; Liu, J.Q. A New 3D 8-Connected Cd(II) MOF as a Potent Photocatalyst for Oxytetracycline Antibiotic Degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Feng, X.; Xu, C.; Wang, Z.Q.; Tang, S.F.; Fu, W.J.; Ji, B.M.; Wang, L.Y. Aerobic Oxidation of Alcohols and the Synthesis of Benzoxazoles Catalyzed by a Cuprocupric Coordination Polymer (Cu+-CP) Assisted by TEMPO. Inorg. Chem. 2015, 54, 2088–2090. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Chen, Z.; Qiu, M.; Hu, B.; Wang, X. Bismuth Oxychloride-Based Materials for the Removal of Organic Pollutants in Wastewater. Chemosphere 2021, 273, 128576. [Google Scholar] [CrossRef]
- Alesso, M.; Bondioli, G.; Talío, M.C.; Luconi, M.O.; Fernández, L.P. Micelles Mediated Separation Fluorimetric Methodology for Rhodamine B Determination in Condiments, Snacks and Candies. Food Chem. 2012, 134, 513–517. [Google Scholar] [CrossRef]
- Qi, P.; Lin, Z.; Li, J.; Wang, C.; Meng, W.; Hong, H.; Zhang, X. Development of a Rapid, Simple and Sensitive HPLC-FLD Method for Determination of Rhodamine B in Chili-Containing Products. Food Chem. 2014, 164, 98–103. [Google Scholar] [CrossRef]
- Ai, L.H.; Zhang, C.H.; Li, L.L.; Jiang, J. Iron Terephthalate Metal–Organic Framework: Revealing the Effective Activation of Hydrogen Peroxide for the Degradation of Organic Dye under Visible Light Irradiation. Appl. Catal. B 2014, 148, 191–200. [Google Scholar] [CrossRef]
- Guo, X.G.; Zhang, Z.Y.; Qiu, S.; Su, X.; Wang, Y.B.; Sun, X.Q. Versatile Tailoring of NH2-Containing Metal–Organic Frameworks with Paddle-Wheel Units. Chem. Eur. J. 2017, 23, 17727–17733. [Google Scholar] [CrossRef]
- Gao, X.J.; Zheng, H.G. The Difference in the CO2 Adsorption Capacities of Different Functionalized Pillar-Layered Metal–Organic Frameworks (MOFs). Dalton Trans. 2021, 50, 9310. [Google Scholar] [CrossRef]
- Chen, K.; Wu, C.D. Transformation of Metal-Organic Frameworks into Stable Organic Frameworks with Inherited Skeletons and Catalytic Properties. Angew. Chem. 2019, 58, 8119–8123. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.H.; Zhu, C.D.; Kang, Y.S.; Wang, P.; Shi, Z.Z.; Lu, Y.; Sun, W.Y. Efficient and Reusable Metal–Organic Framework Catalysts for Carboxylative Cyclization of Propargylamines with Carbon Dioxide. ChemCatChem 2017, 9, 4598–4606. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Xu, Z.H.; Liu, X.H.; Zhao, Y.; Wang, P.; Liu, Z.Q.; Sun, W.Y. A Novel Copper Framework with Amino Tridentate N-donor Ligand as Heterogeneous Catalyst for Ring Opening of Epoxides. Appl. Organomet. Chem. 2021, 35, e6262. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhao, Y.; Chen, K.; Guo, J.H.; Wang, P.; Wu, H.; Sun, W.Y. Cucurbit[6]uril-Based Supramolecular Assemblies Incorporating Metal Complexes with Multiaromatic Ligands as Structure-Directing Agent for Detection of Aromatic Amines and Nitroaromatic Compounds. Sens. Actuators B Chem. 2019, 282, 844–853. [Google Scholar] [CrossRef]
- Azadbakht, R.; Koolivand, M.; Khanabadi, J. A New Fluorescence Chemosensor for Zn2+ with a Remarkable Red Shift in Emission Spectra. Anal. Methods 2017, 9, 4688–4694. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, X.; Zhang, X.Y.; Cheng, X.M.; Ma, J.; Sun, W.Y. Effect of the Defect Modulator and Ligand Length of Metal–Organic Frameworks on Carbon Dioxide Photoreduction. ACS Appl. Mater. Interfaces 2021, 13, 61578–61586. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, L.; Wang, Y.; Hao, J. Stimuli-Responsive Fluorescent Nanoswitches: Solvent-Induced Emission Enhancement of Copper Nanoclusters. Chemistry 2020, 26, 3545–3554. [Google Scholar] [CrossRef]
- Cai, Y.; Hua, Y.; Yin, M.; Liu, H.; Li, S.; Wang, F.; Wang, H. Fabrication of Test Strips with Gold-Silver Nanospheres and Metal–Organic Frameworks: A Fluorimetric Method for Sensing Trace Cysteine in Hela Cells. Sens. Actuators B Chem. 2020, 302, 127198. [Google Scholar] [CrossRef]
- Sun, D.; Yan, Z.H.; Liu, M.J.; Xie, H.Y.; Yuan, S.A.; Lu, H.F.; Feng, S.Y.; Sun, D.F. Three- and Eight-Fold Interpenetrated ThSi2 Metal–Organic Frameworks Fine-Tuned by the Length of Ligand. Cryst. Growth Des. 2012, 12, 2902–2907. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Cesare, K.; Di Dumontel, B.; Garino, N.; Canavese, G.; Hernandez, S.; Cauda, V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye via Radicals Generation by Micro- and Nano-Particles of ZnO. Appl. Catal. B 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yu, X.; Zhao, H.; Wang, S.; Wang, H.; Guo, Z.; Xing, H. Syntheses of Novel Lanthanide Metal–Organic Frameworks for Highly Efficient Visible-Light-Driven Dye Degradation. Cryst. Growth Des. 2017, 17, 4189–4195. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Ho, H.L.; Nguyen, H.V.; Tran, B.T.; Nguyen, T.T.; Bui, P.Q.T.; Bach, L.G. Photocatalytic Degradation of Rhodamine B in Aqueous Phase by Bimetallic Metal-Organic Framework M/Fe-MOF (M = Co, Cu, and Mg). Open Chem. 2022, 20, 52–60. [Google Scholar] [CrossRef]
- Sarkar, A.; Adhikary, A.; Mandal, A.; Chakraborty, T.; Das, D. Zn-BTC MOF as an Adsorbent for Iodine Uptake and Organic Dye Degradation. Cryst. Growth Des. 2020, 20, 7833–7839. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, Q.; Xing, H.; Chen, D.; Wang, H. Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation. ACS Sustain. Chem. Eng. 2017, 5, 4449–4456. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Lu, R.; Yu, A. Interaction between Triethanolamine and Singlet or Triplet Excited State of Xanthene Dyes in Aqueous Solution. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2017, 184, 204–210. [Google Scholar] [CrossRef]
- Drtinova, B.; Pospisil, M.; Cuba, V. Products of Radiation Removal of Lead from Aqueous Solutions. Appl. Radiat. Isot. 2010, 68, 672–675. [Google Scholar] [CrossRef]
- Toshifumi, N.; Setsuko, T.; Toshiki, M.; Ikuo, S.; Shu, F.; Zeji, D.; Shoshi, K. Levels of Active Oxygen Species Are Controlled by Ascorbic Acid and Anthocyanin in Arabidopsis. J. Agric. Food Chem. 2003, 51, 2992–2999. [Google Scholar]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface Oxygen Vacancy Modified Bi2MoO6/MIL-88B(Fe) Heterostructure with Enhanced Spatial Charge Separation at the Bulk & Interface. Appl. Catal. B 2020, 268, 118740. [Google Scholar]
- Zhao, C.; Meng, L.H.; Chu, H.Y.; Wang, J.F.; Wang, T.Y.; Ma, Y.H.; Wang, C.C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@N-C-x: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B 2022, 321, 122034. [Google Scholar] [CrossRef]
- Watts, R.J.; Teel, A.L. Hydroxyl Radical and Non-Hydroxyl Radical Pathways for Trichloroethylene and Perchloroethylene Degradation in Catalyzed H2O2 Propagation Systems. Water Res. 2019, 159, 46–54. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Wang, H.; Bao, Z.; Zhao, J.C.-G.; Chen, B. Highly Interpenetrat-ed Robust Microporous Hydrogen-Bonded Organic Framework for Gas Separation. Cryst. Growth Des. 2017, 17, 6132–6137. [Google Scholar] [CrossRef]
- SAINT. Program for Data Extraction and Reduction; Bruker AXS, Inc: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Adsorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXT-2014, Program for the Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXL-2018, Program for the Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Sluis, P.V.D.; Spek, A.L. BYPASS: An Effective Method for the Refinement of Crystal Structures Containing Disordered Solvent Regions. Acta Cryst. 1990, 46, 194–201. [Google Scholar] [CrossRef]



| Compound | 1 | 2 |
|---|---|---|
| Formula | C152H142N18O34Cd6 | C51.5H48.5N5.5O13.5Cu2 |
| Formula weight | 3439.23 | 1087.17 |
| Crystal system | monoclinic | orthorhombic |
| Space group | P21/c | Pbcn |
| a (Å) | 24.6399(11) | 28.208(3) |
| b (Å) | 22.0378(13) | 23.453(2) |
| c (Å) | 28.1864(13) | 15.7977(16) |
| β (°) | 90.107(2) | 90 |
| V (Å3) | 15,305.4(13) | 10,451.2(18) |
| Z | 4 | 2 |
| Dcalc (g·cm−3) | 1.493 | 1.127 |
| μ (mm−1) | 0.898 | 0.863 |
| F (000) | 6960.0 | 3616.0 |
| Reflections collected | 120,401 | 78,324 |
| Unique reflections | 27,987 | 9561 |
| Goodness-of-fit on F2 | 1.049 | 1.016 |
| R1a, wR2b [I > 2σ(I)] | 0.0517/0.1351 | 0.1066/0.2760 |
| R1, wR2 [all data] | 0.0783/0.1613 | 0.1435/0.3023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.-Q.; Zhao, S.-M.; Chen, J.-Q.; Zhao, Y.; Sun, W.-Y. Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation. Molecules 2022, 27, 7551. https://doi.org/10.3390/molecules27217551
Huang Z-Q, Zhao S-M, Chen J-Q, Zhao Y, Sun W-Y. Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation. Molecules. 2022; 27(21):7551. https://doi.org/10.3390/molecules27217551
Chicago/Turabian StyleHuang, Zi-Qing, Shu-Man Zhao, Jia-Qi Chen, Yue Zhao, and Wei-Yin Sun. 2022. "Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation" Molecules 27, no. 21: 7551. https://doi.org/10.3390/molecules27217551
APA StyleHuang, Z.-Q., Zhao, S.-M., Chen, J.-Q., Zhao, Y., & Sun, W.-Y. (2022). Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation. Molecules, 27(21), 7551. https://doi.org/10.3390/molecules27217551






