1. Introduction
Fermentation processing of traditional Chinese medicine refers to the method of foaming and undressing the medicine after purification or treatment under certain temperature and humidity conditions through the catalytic decomposition of microorganisms and enzymes [
1] (p. 416). The fermentation method is different from the traditional heating and processing methods such as frying, steaming, boiling, simmering, frying, refining, etc. It has a mild effect and mainly uses microorganisms and their secreted enzymes to change the chemical composition of medicines. Due to the variety of microorganisms and the different synergistic effects among them, the same drug fermented by different methods often has different therapeutic effects. Traditional Chinese medicine fermentation converts macromolecular substances that are difficult for the human body to absorb into small molecular components easier to absorb through microbial strains, which can improve the speed of drug absorption and promote the effectiveness of drugs. Moreover, a variety of secondary metabolites are generated during fermentation, which can enhance or change the efficacy. Drug components decompose after fermentation to a certain extent, thus bringing reduced toxicity and making it relatively safe to take in. The taste of Chinese medicine is usually tough for patients, while fermentation changes the original taste into something more acceptable, and the patient’s compliance might be better. It can be seen that the fermentation processing of traditional Chinese medicine can provide new research ideas for the development of traditional Chinese medicine and has a very broad application prospect.
Jianqu, also known as Fanzhiqu and Baicaoqu, has a yellowish-brown surface, white mildew, fragrant smell, and slightly bitter taste. It has a long history of application. In addition to the effect of divine comedy, it also has the effect of deciphering the surface and reconciling it [
2] (p. 385). It is produced all over the country, but Quanzhou, Fujian is the most famous, also known as Quanzhou Divine Comedy [
3] (pp. 31–32). Jianqu, as a processed product fermented from dozens of fine medicinal powders, has been used by physicians in various dynasties intending to different therapeutic efficacy, and its prescriptions have changed accordingly. Jianqu is now fermented from Chinese medicines such as Artemisia annua, mint, Angelica dahurica, wheat bran, and flour. At present, there are more than 20 Jianqu products used in clinical applications. According to the literature, Jianqu can be used in the following four categories: (1) cough caused by cold. (2) cold, fever, dizziness, and vomiting caused by the plague. (3) chest stuffiness and fullness caused by qi stagnation, (4) eating and abdominal pain and indigestion caused by water and grain accumulation. At present, the research of Jianqu mainly focuses on the clinical prescription application and case analysis, and there are few reports on the chemical composition analysis of Jianqu, and the material basis of its efficacy is still unclear, which limits the clinical application of Jianqu. Therefore, clarifying the chemical composition of Jianqu before and after fermentation can provide a scientific basis for further improving the fermentation process of Jianqu and studying its pharmacodynamic material basis.
In this study, UPLC-QTOF-MS/MS technology was used to systematically analyze and study the chemical substances in Jianqu at different fermentation times, and HPLC technology was used to determine the content of the characteristic components.
3. Discussion
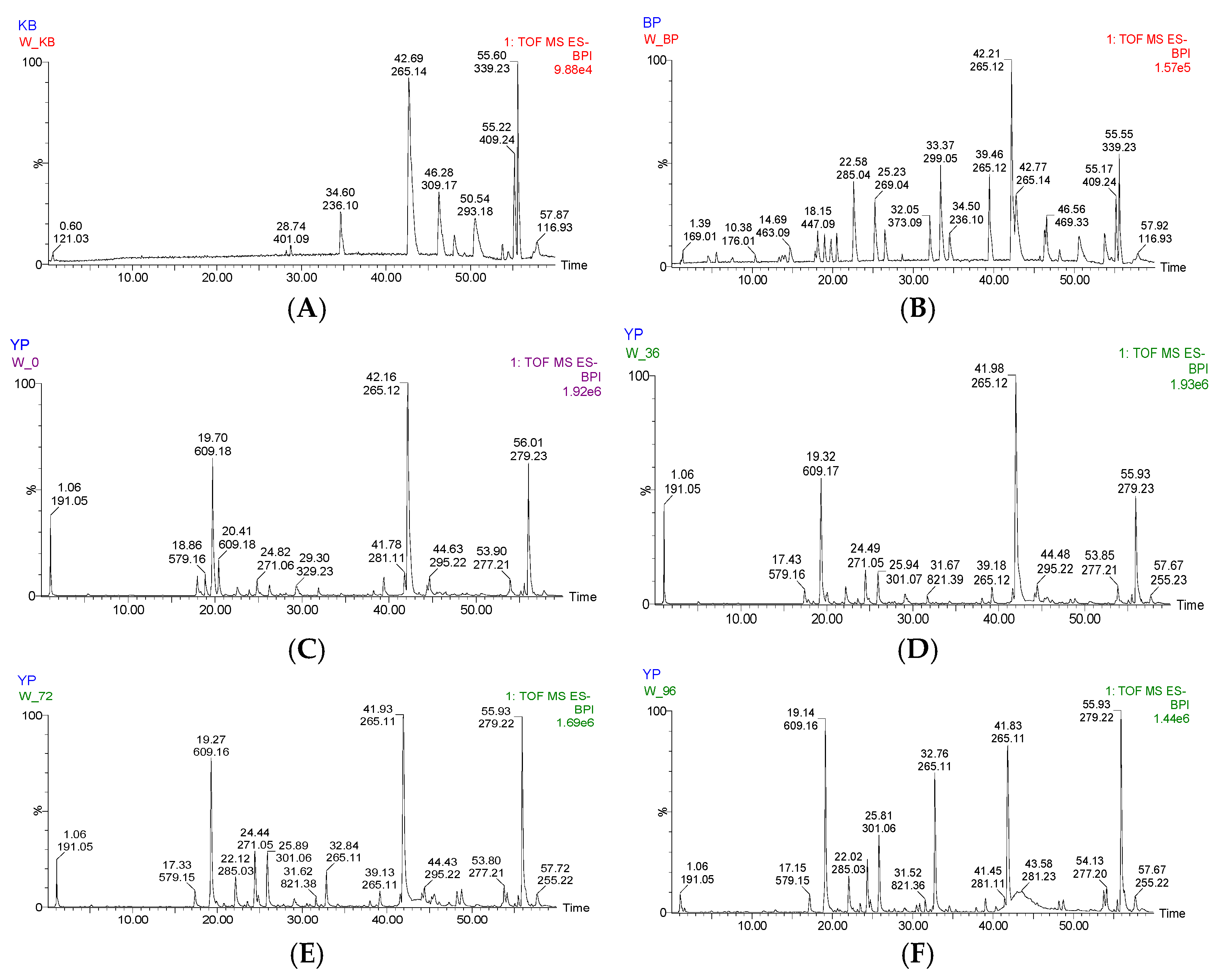
In this experiment, positive ion mode and negative ion mode were used to analyze the extract of Jianqu with different fermentation times, and the two scanning modes were compared. It was found that under the negative ion mode, the response signal of the chemical components in the sample was stronger, the peak number was more, and the mass spectrum information was clearer. Therefore, the selection is carried out in the negative ion mode.
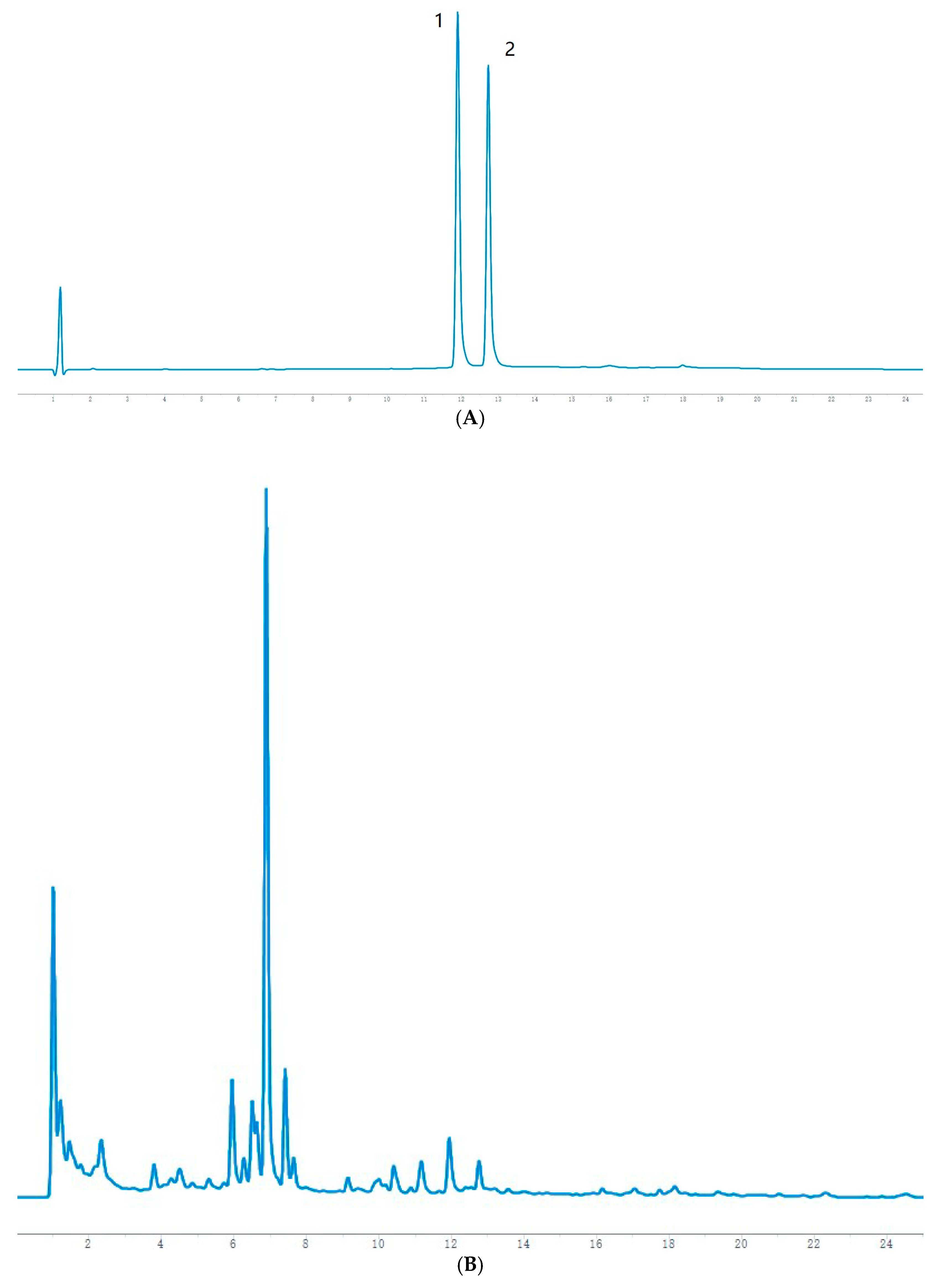
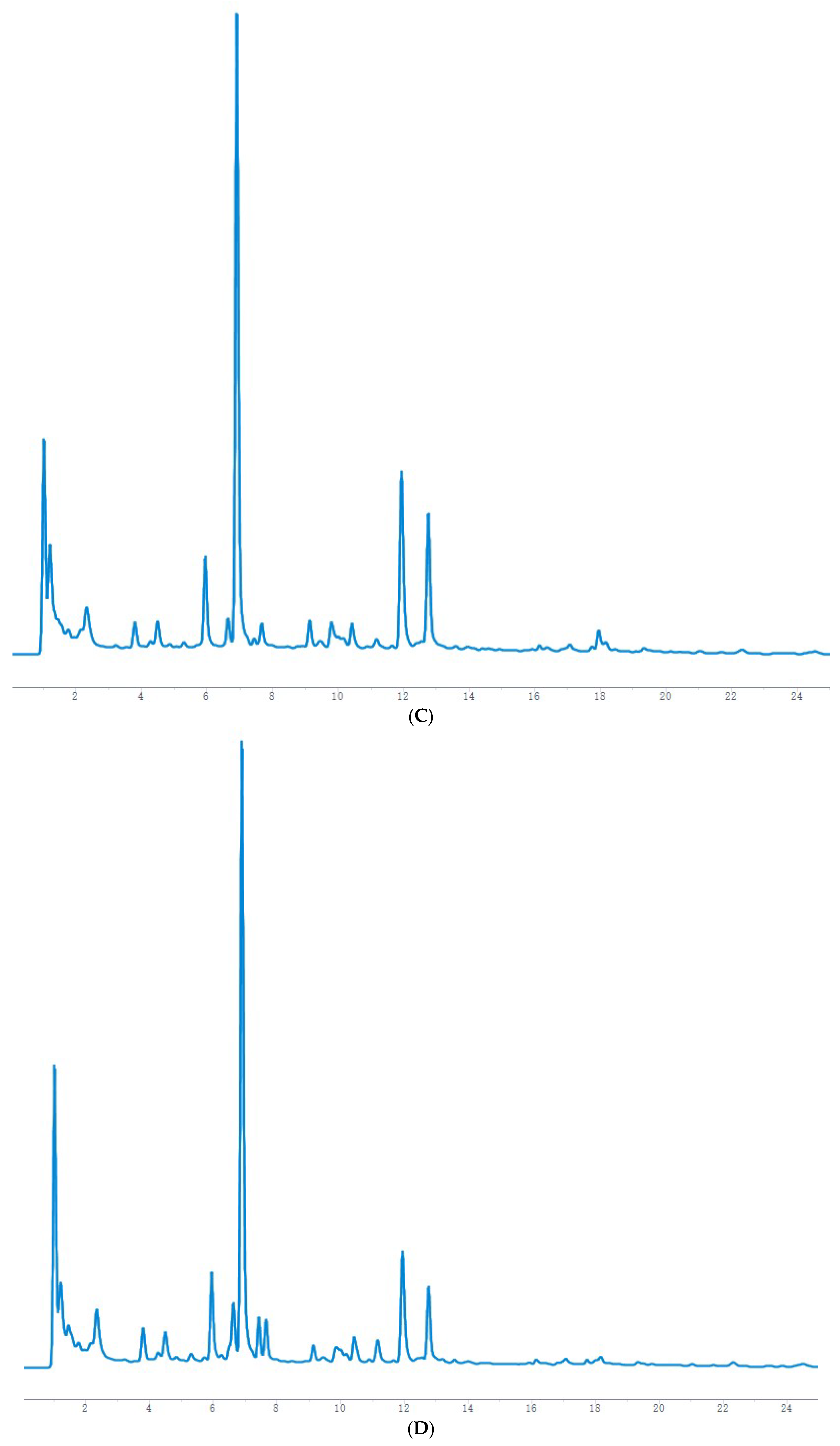
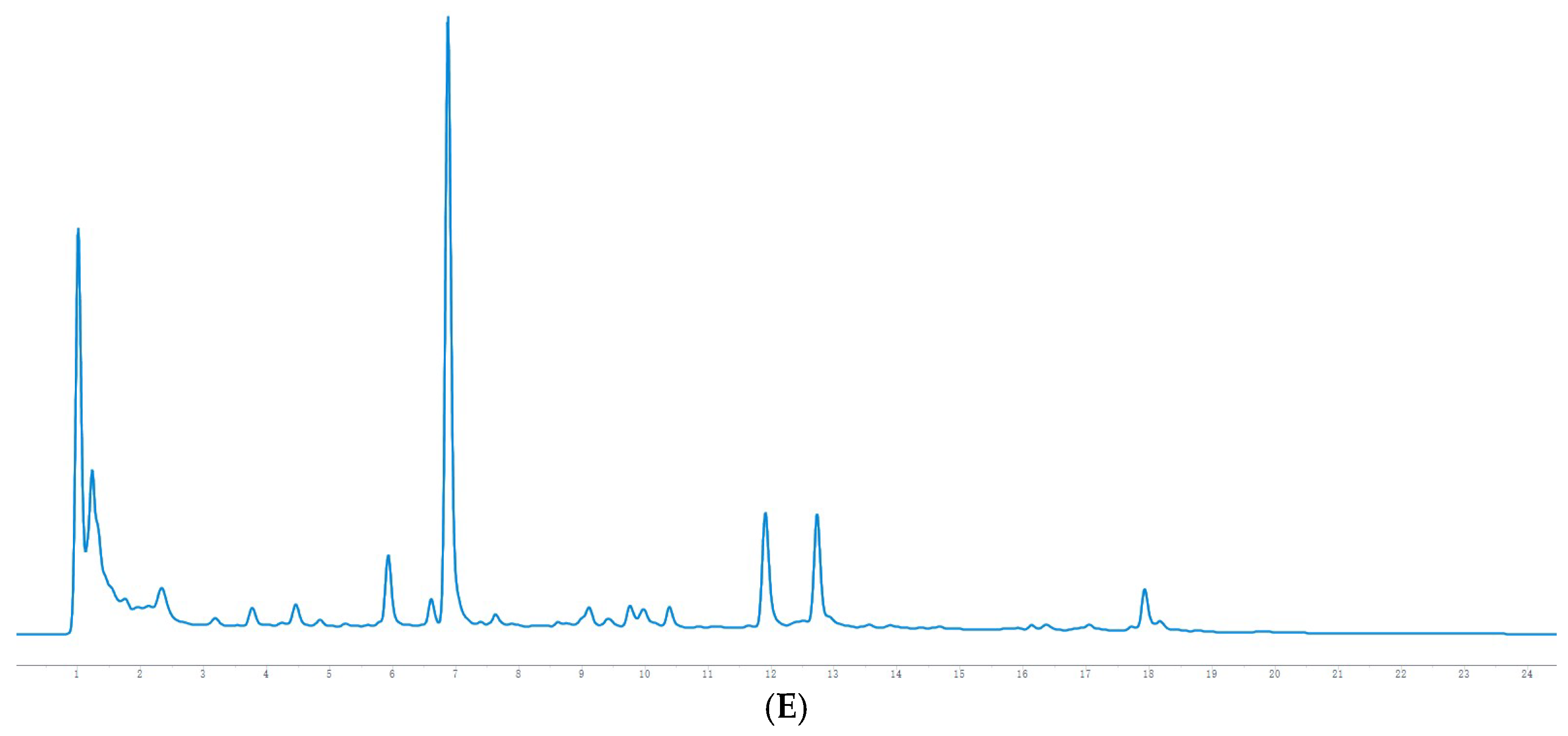
The HPLC chromatographic binding content determination results showed that the highest content of hesperidin and naringin reached 0.2353 mg/g and 0.2821 mg/g, respectively, at 36 h of fermentation. After 36 h, both are reduced to varying degrees and may be oxidized by microorganisms. It has been reported [
23] (pp. 136–142) that the optimal fermentation time of Jianqu is 30–36 h, indicating that the samples used in this experiment are qualified, and it can also be speculated that hesperidin and naringin may be the material basis for the curative effect of Jianqu. It can be clearly seen from the mass spectrum that the response values of hesperidin and neohesperidin are high. Because both are derived from dried tangerine peel and fructus aurantii, hesperidin accounts for more than 50% of the total in the dried tangerine peel [
24] (pp. 36–39,52). The response value of flavonoid glycosides, such as hesperidin, neophesperidin, and naringin, gradually decreases with the continuous fermentation, and it can be inferred that their content is decreasing. Combined with the response value changes and content determination results of hesperidin and naringin, it may be because they are used as raw materials in the fermentation process to synthesize other substances or are oxidized and degraded to form other substances. At present, only the content of two index components has been detected, and the optimal fermentation processing endpoint of Jianqu cannot be comprehensively judged according to the content of these two ingredients, and follow-up research is required.
After the fermentation of Jianqu, there is a slight aroma of wine. Relevant research on wine [
25] (pp. 46–50) shows that the aroma of wine is mostly the smell of acid and ester compounds. Acid esters such as linoleic acid, palmitic acid, methyl palmioleate, and ethyl linoleate were detected before and after fermentation of Jianqu liquor, which may be the source of the aroma of Jianqu liquor. Linoleic acid and linolenic acid are both unsaturated fatty acids. With the fermentation process, the response of these substances to mass spectrometry first decreased and then increased. The main reason may be that some bacteria or fungi produced at the beginning of fermentation oxidized the unsaturated fatty acids. Related research shows that [
26] (pp. 82–87) the content of oleic acid and linoleic acid in some plants was significantly negatively correlated. It is speculated that enzymes may be produced later in fermentation to convert oleic acid into linoleic acid and increase its content. The response values of some small molecular organic acids, such as quinic acid and caffeic acid, decrease continuously with the progress of fermentation, and some even cannot be detected in the late fermentation, which may be because other substances are synthesized as raw materials in the fermentation process or oxidized and degraded to produce other substances.
In addition, some amino acids, such as tyrosine, begin to appear at the later stage of fermentation, which may come from the decomposition of certain substances. Glucose has been decreasing since fermentation, because as fermentation progresses, the yeast and other microorganisms produced in the fermentation system continuously consume the reducing sugar. Triterpenoids were detected 72 h later, which may be due to the decomposition of enzymes that hydrolyze triterpenoid saponins during fermentation.
In this experiment, the chemical constituents of the different fermentation times of Jianqu were analyzed efficiently, accurately, and quickly. A total of 54 compounds were identified, including 15 flavonoids, 3 amino acids, 14 organic acids, 5 terpenes, 4 coumarins, 2 lignans, and 11 others. The main components were flavonoids and organic acids. The HPLC method was used to determine the content of characteristic components, and it was found that the contents of hesperidin and naringin were the highest after 36 h fermentation. It laid a foundation for further research on the pharmacodynamic material basis of Jianqu, the action mechanism of microorganisms in the fermentation process, and the fermentation process. After that, we will further study the role of the main components in Jianqu.