Positional Distribution of Fatty Acids in Processed Chinook Salmon Roe Lipids Determined by 13C Magnetic Resonance Spectroscopy (NMR)
Abstract
1. Introduction
2. Results
2.1. 13C NMR Analysis of Salmon Roe Extracted Lipids
2.1.1. 13C NMR Carbonyl Region (174.3–172.6 ppm)
2.1.2. 13C NMR of Olefinic Region (132.5–126.5 ppm)
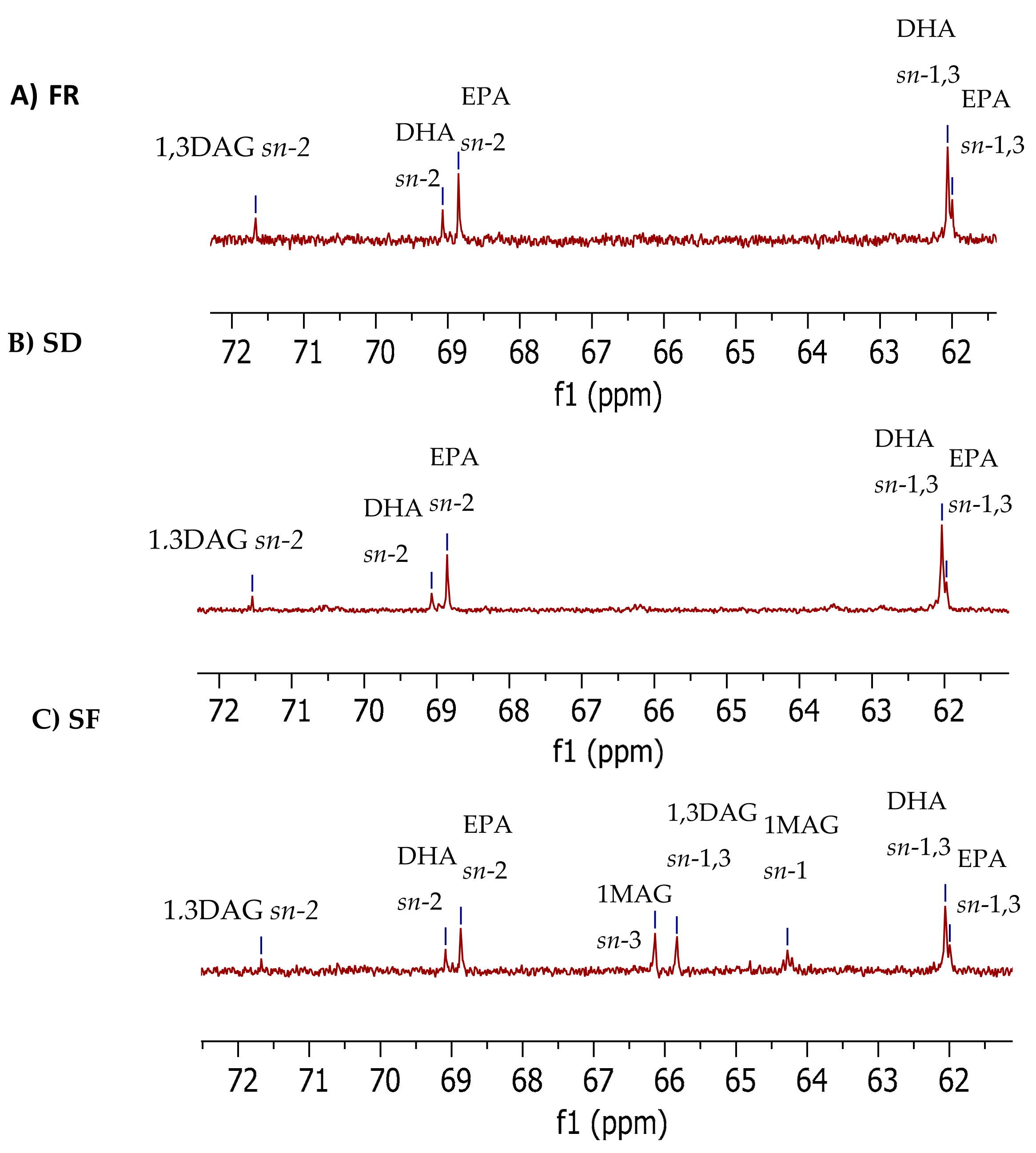
2.1.3. 13C NMR Glycerol Carbon Region (61.0–72.0 ppm)
2.1.4. 13C NMR Aliphatic Region (32.0–20.0 ppm) and Methyl End Chain Region (~14.0 ppm)
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Roe Lipid Extraction
3.3. 13C NMR
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Rogers, T.M.; Wenz, M.A.; Cavinato, A.G.; Mayes, D.M.; Bledsoe, G.E.; Rasco, B.A. Detection of sodium chloride in cured salmon roe by SW− NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 4161–4167. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Morton, J.D.; Dawson, C.O.; Zhao, J.H.; Lee, H.Y. Impact of maturity on the physicochemical and biochemical properties of chinook salmon roe. Food Chem. 2009, 117, 318–325. [Google Scholar] [CrossRef]
- Desantos, F.; Bechtel, P.; Smiley, S.; Brewer, M. Effect of inclusion of salmon roe on characteristics of salmon baby food products. J. Food Sci. 2010, 75, S231–S236. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and fish roe products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef] [PubMed]
- Bunga, S.J.; Ahmmed, M.K.; Lawley, B.; Carne, A.; Bekhit, A.E.D.A. Physicochemical, biochemical and microbiological changes of jeotgal-like fermented Chinook salmon (Oncorhynchus tshawytscha) roe. Food Chem. 2023, 398, 133880. [Google Scholar] [CrossRef]
- Bunga, S.; Ahmmed, M.K.; Carne, A.; Bekhit, A.E.-D.A. Effect of salted-drying on bioactive compounds and microbiological changes during the processing of karasumi-like Chinook salmon (Oncorhynchus tshawytscha) roe product. Food Chem. 2021, 357, 129780. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Carne, A.; Ahmmed, F.; Stewart, I.; Tian, H.S.; Bekhit, A.E.-D.A. Positional distribution of fatty acids and phospholipid composition in King salmon (Oncorhynchus tshawytscha) head, roe and skin using nuclear magnetic resonance spectroscopy. Food Chem. 2021, 363, 130302. [Google Scholar] [CrossRef]
- Taylor, L.A.; Pletschen, L.; Arends, J.; Unger, C.; Massing, U. Marine phospholipids—A promising new dietary approach to tumor-associated weight loss. Support. Care Cancer 2010, 18, 159–170. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Carne, A.; Tian, H.S.; Bekhit, A.E.D.A. Fish roe phospholipids and health: Composition, extraction, storage and brain health application. In Fish Roe; Elsevier: Amsterdam, The Netherlands, 2022; pp. 93–142. [Google Scholar]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Li, J.; Vosegaard, T.; Guo, Z. Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies. Prog. Lipid Res. 2017, 68, 37–56. [Google Scholar] [CrossRef]
- Lankhorst, P.P.; Chang, A.-N. The Application of NMR in Compositional and Quantitative Analysis of Oils and Lipids; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1743–1764. [Google Scholar]
- Viant, M.R.; Pincetich, C.A.; Tjeerdema, R.S. Metabolic effects of dinoseb, diazinon and esfenvalerate in eyed eggs and alevins of Chinook salmon (Oncorhynchus tshawytscha) determined by 1H NMR metabolomics. Aquat. Toxicol. 2006, 77, 359–371. [Google Scholar] [CrossRef]
- Tengku-Rozaina, T.M.; Birch, E.J. Positional distribution of fatty acids on hoki and tuna oil triglycerides by pancreatic lipase and 13C NMR analysis. Eur. J. Lipid Sci. Technol. 2014, 116, 272–281. [Google Scholar] [CrossRef]
- Standal, I.B.; Axelson, D.E.; Aursand, M. 13C NMR as a tool for authentication of different gadoid fish species with emphasis on phospholipid profiles. Food Chem. 2010, 121, 608–615. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Stewart, I.; Carne, A.; Tian, H.S.; Bekhit, A.E.-D.A. Omega-3 phospholipids in Pacific blue mackerel (Scomber australasicus) processing by-products. Food Chem. 2021, 353, 129451. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Carne, A.; Bunga, S.; Tian, H.S.; Bekhit, A.E.-D.A. Lipidomic signature of Pacific lean fish species head and skin using gas chromatography and nuclear magnetic resonance spectroscopy. Food Chem. 2021, 365, 130637. [Google Scholar] [CrossRef]
- Standal, I.B.; Axelson, D.E.; Aursand, M. Differentiation of fish oils according to species by 13C NMR regiospecific analyses of triacyglycerols. J. Am. Oil Chem. Soc. 2009, 86, 401–407. [Google Scholar] [CrossRef]
- Aursand, M.; Standal, I.B.; Praël, A.; McEvoy, L.; Irvine, J.; Axelson, D.E. 13C NMR pattern recognition techniques for the classification of Atlantic salmon (Salmo salar L.) according to their wild, farmed, and geographical origin. J. Agric. Food Chem. 2009, 57, 3444–3451. [Google Scholar] [CrossRef]
- Aursand, M.; Standal, I.B.; Axelson, D.E. High-resolution 13C nuclear magnetic resonance spectroscopy pattern recognition of fish oil capsules. J. Agric. Food Chem. 2007, 55, 38–47. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Vlahov, G. 13C Nuclear Magnetic Resonance Spectroscopy as a New Quantitative Method for Determining Fatty Acid Positional Distribution in Olive Oil Triacylglycerols: Applications to Olive Oil Authenticity. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 603–613. [Google Scholar]
- Xu, Y.; Zang, J.; Regenstein, J.M.; Xia, W. Technological roles of microorganisms in fish fermentation: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Carne, A.; Tian, H.S.; Bekhit, A.E.-D.A. The effect of pulsed electric fields on the extracted total lipid yield and the lipidomic profile of hoki roe. Food Chem. 2022, 384, 132476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Zhang, Y.; Shen, Y.; Su, H.; Jin, J.; Jin, Q.; Wang, X. Characterization of positional distribution of fatty acids and triacylglycerol molecular compositions of marine fish oils rich in omega-3 polyunsaturated fatty acids. BioMedical Res. Int. 2018, 2018, 3529682. [Google Scholar] [CrossRef] [PubMed]
- Yalagala, P.R.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef]
- Ando, Y.; Ota, T.; Matsuhira, Y.; Yazawa, K. Stereospecific analysis of triacyl-sn-glycerols in docosahexaenoic acid-rich fish oils. J. Am. Oil Chem. Soc. 1996, 73, 483–487. [Google Scholar] [CrossRef]
- Myher, J.; Kuksis, A.; Yang, L.-Y. Stereospecific analysis of menhaden oil triacylglycerols and resolution of complex polyunsaturated diacylglycerols by gas–liquid chromatography on polar capillary columns. Biochem. Cell Biol. 1990, 68, 336–344. [Google Scholar] [CrossRef]
- Myher, J.; Kuksis, A.; Geher, K.; Park, P.; Diersen-Schade, D. Stereospecific analysis of triacylglycerols rich in long-chain polyunsaturated fatty acids. Lipids 1996, 31, 207–215. [Google Scholar] [CrossRef]
- Ando, Y.; Satake, M.; Takahashi, Y. Reinvestigation of positional distribution of fatty acids in docosahexaenoic acid-rich fish oil triacyl-sn-glycerols. Lipids 2000, 35, 579–582. [Google Scholar] [CrossRef]
- Brockerhoff, H.; Hoyle, R.; Hwang, P.; Litchfield, C. Positional distribution of fatty acids in depot triglycerides of aquatic animals. Lipids 1968, 3, 24–29. [Google Scholar] [CrossRef]
- Ando, Y.; Nishimura, K.; Aoyanagi, N.; Takagi, T. Stereospecific analysis of fish oil triacyl-sn-glycerols. J. Am. Oil Chem. Soc. 1992, 69, 417–424. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Wang, W.; Barrow, C.J. Production of omega-3 triacylglycerol concentrates using a new food grade immobilized Candida antarctica lipase B. Aust. J. Chem. 2010, 63, 922–928. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Cong, P.; Chen, Q.; Li, H.; Fan, Y.; Xu, J.; Wang, J.; Wang, Y.; Xue, C. Lipid degradation during salt-fermented Antarctic krill paste processing and their relationship with lipase and phospholipase activities. Eur. J. Lipid Sci. Technol. 2018, 120, 1700443. [Google Scholar] [CrossRef]
- Gunstone, F. High resolution NMR studies of fish oils. Chem. Phys. Lipids 1991, 59, 83–89. [Google Scholar] [CrossRef]
- Aursand, M.; Grasdalen, H. Interpretation of the 13C NMR spectra of omega-3 fatty acids and lipid extracted from the white muscle of Atlantic salmon (Salmo salar). Chem. Phys. Lipids 1992, 62, 239–251. [Google Scholar] [CrossRef]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef]
- Segura-Cardona, A.; Cardona-Arango, D.; Segura-Cardona, Á.; Garzón-Duque, M. Riesgo de depresión y factores asociados en adultos mayores. Antioquia, Colombia. 2012. Rev. Salud Pública 2015, 17, 184–194. [Google Scholar] [CrossRef]
- Baiocchi, C.; Medana, C.; Dal Bello, F.; Giancotti, V.; Aigotti, R.; Gastaldi, D. Analysis of regioisomers of polyunsaturated triacylglycerols in marine matrices by HPLC/HRMS. Food Chem. 2015, 166, 551–560. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Watkins, P.; Fagan, P.; Fraser, B.; Thienthong, N.; Perlmutter, P. Docosahexaenoic acid is more stable to oxidation when located at the sn-2 position of triacylglycerol compared to sn-1 (3). J. Am. Oil Chem. Soc. 2008, 85, 543–548. [Google Scholar] [CrossRef]
- Standal, I.B.; Carvajal, A.K.; Mozuraityte, R.; Storrø, I.; Størseth, T.; Abbasi, E.; Aursand, M. High-Resolution NMR as Tool to Study Enzyme-Catalyzed Production of Fatty Acid Ethyl Esters from Marine Oils. In Modern Magnetic Resonance; Springer: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar]
- Aursand, M.; Jørgensen, L.; Grasdalen, H. Positional distribution of ω3 Fatty acids in marine lipid triacylglycerols by high-resolution13C nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 1995, 72, 293–297. [Google Scholar] [CrossRef]
| Fatty Acids | δ (ppm) | FR Roe Lipids Integrated Area (%) | SD Roe Lipids Integrated Area (%) | SF Roe Lipids Integrated Area (%) | ||||
|---|---|---|---|---|---|---|---|---|
| sn-2 | sn-1,3 | sn-2 | sn-1,3 | sn-2 | sn-1,3 | sn-2 | sn-1,3 | |
| 22:6n-3 (DHA) | 172.08 | 172.27 | 6.8 ± 0.4 B | 1.0 ± 0.1 A | 7.1 ± 1.2 B | 0.8 ± 0.1 AB | 10.1 ± 1.1 A | 0.7 ± 0.1 B |
| 22:5n-3 (EPA) | 172.37 | 172.77 | 1.3 ± 0.23 A | 14.7 ± 1.5 | 0.4 ± 0.1 B | 18.5 ± 2.8 | 0.7 ± 0.1 B | 15.7 ± 0.8 |
| C18:4n-3 (SDA) | 172.39 | 172.92 | 0.9 ± 0.1 A | 2.7 ± 0.3 B | 0.6 ± 0.1 B | 4.6 ± 1.3 AB | 1.1 ± 0.1 A | 4.9 ± 0.7 A |
| SFA | 172.48 | 173.20 | 2.8 ± 0.2 | 25.3 ± 1.4 A | 3.0 ± 1.3 | 15.7 ± 1.6 B | 3.0 ± 0.3 | 19.1 ± 1.6 B |
| MUFA | 174.46 | 173.18 | 7.2 ± 1.1 A | 37.3 ± 1.0 | 4.5 ± 1.2 B | 44.9 ± 5.7 | 5.7 ± 0.3 AB | 39.2 ± 1.4 |
| Assignment | δ | FR Roe Lipids | SD Roe Lipids | SF Roe Lipids |
|---|---|---|---|---|
| (ppm) | Integrated Area % | Integrated Area % | Integrated Area % | |
| C4, n-3 | 126.98 | 7.0 ± 0.2 A | 5.9 ± 0.4 B | 4.0 ± 0.01 C |
| C4, 22:6n-3 | 127.59 | 4.1 ± 0.2 B | 7.8 ± 0.7 A | 2.5 ± 0.2 C |
|
22:5n-3 20:5n-3 20:4n-3 18:4n-3 | 128.03 | 17.2 ± 0.6 B | 20.0 ± 0.4 A | 9.2 ± 0.2 C |
|
C6, 20:5n-3 20:4n6 | 129.52 | 17.5 ± 0.7 A | 6.7 ± 0.4 B | 5.3 ± 0.2 C |
|
C5, 20:5n-3 20:4n6 | 128.78 | 1.1 ± 0.2 B | 2.2 ± 0.03 B | 9.1 ± 0.1 A |
|
C5, 22:6n-3 | 129.42 | 3.7 ± 0.2 B | 5.5 ± 0.4 A | 2.6 ± 0.02 C |
| 16:1 | 129.77 | 1.9 ± 0.1 B | 3.9 ± 0.3 A | 1.6 ± 0.1 B |
| 18:1 | 129.66 | 10.1 ± 0.4 B | 12.8 ± 0.5 A | 5.9 ± 0.1 C |
| 20:1 | 129.96 | 14.5 ± 3.0 AB | 17.6 ± 1.5 A | 10.0 ± 0.5 B |
| 22:1 | 129.87 | 3.7 ± 0.2 B | 4.4 ± 0.13 A | 1.6 ± 0.3 C |
| 18:2n6 | 130.05 | 1.7 ± 0.1 C | 5.0 ± 0.1 A | 2.0 ± 0.1 B |
| 18:2n-3 | 130.30 | 4.0 ± 1.08 | 4.9 ± 1.7 | 4.2 ± 1.4 |
| 20:4n6 | 130.42 | 2.2 ± 0.3 A | 1.3 ± 0.2 B | 0.9 ± 0. 1 B |
| β-eleostearic acid | 130.84 | ND | ND | 17.9 ± 0.9 |
| C3, n-3 | 131.96 | 11.1 ± 1.2 A | 5.9 ± 0.2 B | 4.3 ± 0.4 B |
| Assignment | Position | FR Roe Lipids | SD Roe Lipids | SF Roe Lipids | |
|---|---|---|---|---|---|
| δ (ppm) | Integrated Area % | Integrated Area % | Integrated Area % | ||
| 1,3-DAG | sn-2 | 71.60 | 6.0 ± 0.6 A | 6.3 ± 0.3 A | 2.3 ± 0.2 B |
| sn-1,3 | 64.80 | ND | ND | 5.5 ± 0.2 | |
| 22:6n-3 (DHA) | sn-2 | 69.07 | 10.4 ± 0.4 A | 8. 5 ± 0.4 B | 6.3 ± 0.2 C |
| sn-1,3 | 62.05 | 46.6 ± 0.8 | 44.7 ± 1.3 | 44.4 ± 0.7 | |
| 22:5n-3 (EPA) | sn-2 | 68.86 | 19.5 ± 0.6 B | 22.0 ± 0.7 A | 15.7 ± 1.4 C |
| sn-1,3 | 62.00 | 14.7 ± 1.5 | 18.5 ± 2.8 | 15.7 ± 0.8 | |
| 1-MAG | sn-1 | 64.28 | ND | ND | 10.2 ± 0.5 |
| 3-MAG | sn-3 | 65.83 | ND | ND | 7.9 ± 0.4 |
| Assignment | Chemical Shift | FR Roe Lipids | SD Roe Lipids | SF Roe Lipids |
|---|---|---|---|---|
| δ (ppm) | Integrated Area % | Integrated Area % | Integrated Area % | |
| (a). Aliphatic region | ||||
| C3, n-6 | 31.49 | 15.3 ± 0.9 B | 9.8 ± 0.6 C | 18.9 ± 0.8 A |
| C3, n-7 | 31.75 | 9.6 ± 0.3 | 8.3 ± 0.7 | 9.0 ± 0.9 |
| C3, n-9 | 31.88 | 49.7 ± 0.6 B | 56.0 ± 0.4 A | 44.7 ± 0.4 C |
| SFA | 31.90 | 25.4 ± 1.5 | 25.9 ± 0.5 | 27.5 ± 1.4 |
| (b). Methyl end chain region | ||||
| C1 | 14.08 | 80.2 ± 0.8 | 81.2 ± 0.7 | 80.5 ± 0.8 |
| C1, n-3 | 14.24 | 19.8 ± 0.8 | 18.8 ± 0.7 | 19.5 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunga, S.; Ahmmed, M.K.; Carne, A.; Bekhit, A.E.-D.A. Positional Distribution of Fatty Acids in Processed Chinook Salmon Roe Lipids Determined by 13C Magnetic Resonance Spectroscopy (NMR). Molecules 2023, 28, 454. https://doi.org/10.3390/molecules28010454
Bunga S, Ahmmed MK, Carne A, Bekhit AE-DA. Positional Distribution of Fatty Acids in Processed Chinook Salmon Roe Lipids Determined by 13C Magnetic Resonance Spectroscopy (NMR). Molecules. 2023; 28(1):454. https://doi.org/10.3390/molecules28010454
Chicago/Turabian StyleBunga, Senni, Mirja Kaizer Ahmmed, Alan Carne, and Alaa El-Din A. Bekhit. 2023. "Positional Distribution of Fatty Acids in Processed Chinook Salmon Roe Lipids Determined by 13C Magnetic Resonance Spectroscopy (NMR)" Molecules 28, no. 1: 454. https://doi.org/10.3390/molecules28010454
APA StyleBunga, S., Ahmmed, M. K., Carne, A., & Bekhit, A. E.-D. A. (2023). Positional Distribution of Fatty Acids in Processed Chinook Salmon Roe Lipids Determined by 13C Magnetic Resonance Spectroscopy (NMR). Molecules, 28(1), 454. https://doi.org/10.3390/molecules28010454









