Sarcoconvolutums F and G: Polyoxygenated Cembrane-Type Diterpenoids from Sarcophyton convolutum, a Red Sea Soft Coral
Abstract
:1. Introduction
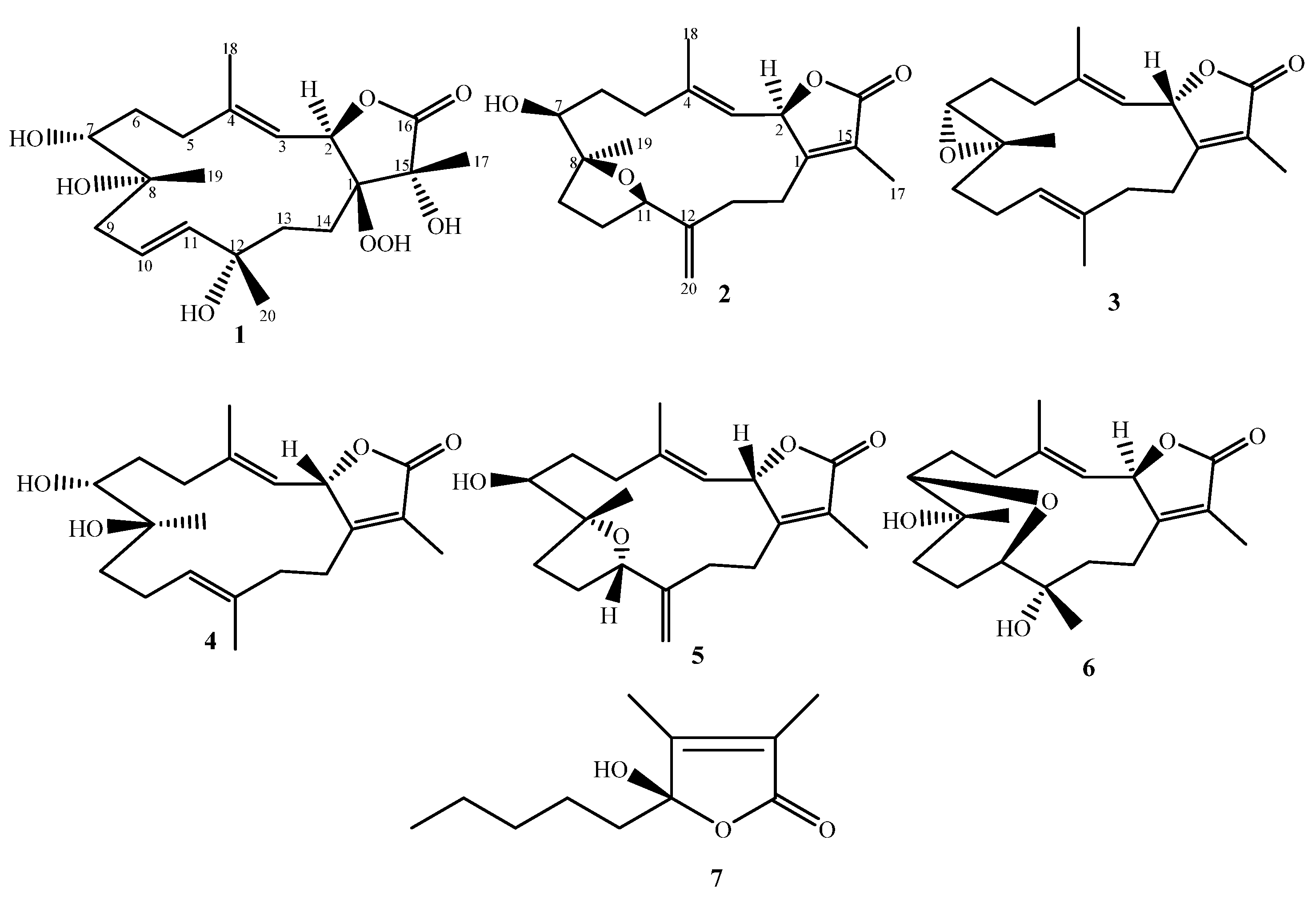
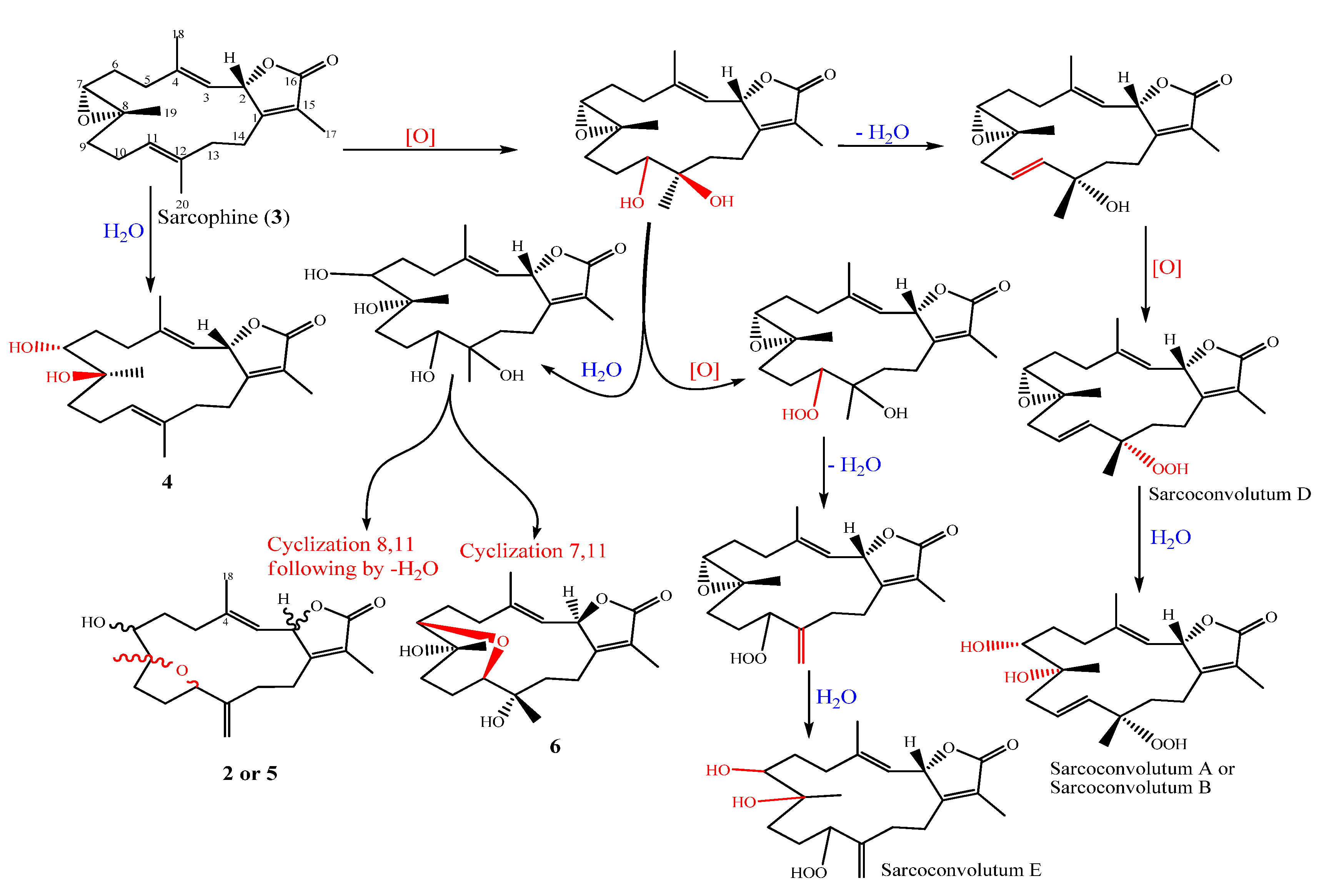
2. Results
Chemical Elucidation
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.4.1. Sarcoconvolutum F(1)
3.4.2. Sarcoconvolutum G (2)
3.5. Cell Culture and Treatment Conditions
3.5.1. Cytotoxicity Assay
3.5.2. Anti-Proliferation Quantitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mohamed, T.A.; Elshamy, A.I.; Abdel-Tawab, A.M.; AbdelMohsen, M.M.; Ohta, S.; Pare, P.W.; Hegazy, M.-E.F. Oxygenated Cembrene Diterpenes from Sarcophyton convolutum: Cytotoxic Sarcoconvolutum AE. Mar. Drugs 2021, 19, 519. [Google Scholar] [CrossRef] [PubMed]
- Edwards, F.J. Climate and oceanography. In Red Sea; Edwards, A., Head, S.M., Eds.; Key Environments Series; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Roethle, P.A.; Trauner, D. The chemistry of marine furanocembranoids, pseudopteranes, gersolanes, and related natural products. Nat. Prod. Rep. 2008, 25, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C.; Wallis, E.V. The function of surface sclerites in gorgonians (Coelenterata, Octocorallia). Biol. Bull. 1991, 181, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, S.; Raghunathan, C.; Chandra, K. New record of Sarcophyton cornispiculatum Verseveldt, 1971 (Octocorallia: Alcyonacea: Alcyoniidae) in India, from the Andaman Islands. Eur. Zool. J. 2017, 84, 167–171. [Google Scholar] [CrossRef]
- Verseveldt, J. A Revision of the Genus Sarcophyton Lesson (Octocorallia, Alcyonacea); Brill: Leiden, The Netherlands, 1982. [Google Scholar]
- Alderslade, P. A redescription of Alcyonium agaricum Stimpson with a generic placement in Sarcophyton (Coelenterata: Octocorallia). Precious Corals Octocorals Res. 1993, 1, 20–29. [Google Scholar]
- Alderslade, P.; Shirwaiker, P. New species of soft corals (Coelenterata: Octocorallia) from the Laccadive Archipelago. Beagle Rec. Mus. Art Galleries North. Territ. 1991, 8, 189–233. [Google Scholar] [CrossRef]
- Benayahu, Y.; Perkol-Finkel, S. Soft Corals (Octocorallia: Alcyonacea) from southern Taiwan: I. Sarcophyton nanwanensis sp. nov. (Octocorallia: Alcyonacea). Zool. Stud. Taipei 2004, 43, 537–547. [Google Scholar]
- Benayahu, Y.; van Ofwegen, L. New species of Sarcophyton and Lobophytum (Octocorallia: Alcyonacea) from Hong Kong. Zool. Meded. 2009, 83, 863–876. [Google Scholar]
- Li, C. Studies on the Alcyonacea of the south China Sea II. Genera Lobophytum and Sarcophyton from the Xisha Islands, Guangdong province. Nanhai Studia Mar. Sin. 1984, 6, 103–119. [Google Scholar]
- Nurrachma, M.Y.; Sakaraga, D.; Nugraha, A.Y.; Rahmawati, S.I.; Bayu, A.; Sukmarini, L.; Atikana, A.; Prasetyoputri, A.; Izzati, F.; Warsito, M.F. Cembranoids of soft corals: Recent updates and their biological activities. Nat. Prod. Bioprospecting 2021, 11, 243–306. [Google Scholar] [CrossRef]
- Liang, L.F.; Guo, Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Eldeen, A.M.G.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Paré, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Lei, H. Diterpenoids of gorgonian corals: Chemistry and bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.-F.; Lin, X.-P.; Liu, J.; Peng, Y.; Yang, X.-W.; Liu, Y. Cembrane diterpenes chemistry and biological properties. Curr. Org. Chem. 2012, 16, 1512–1539. [Google Scholar] [CrossRef]
- Dauben, W.G.; Thiessen, W.E.; Resnick, P.R. Cembrene, a Fourteen-Membered Ring Diterpene Hydrocarbon*, 1, 2. J. Org. Chem. 1965, 30, 1693–1698. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakagawa, T.; Mitsuhashi, H. Marine terpenes and terpenoids. I. Structures of four cembrane-type diterpenes: Sarcophytol-A, sarcophytol-A acetate sarcophytol-B, and sarcophytonin-A, from the soft coral, Sarcophyton glaucum. Chem. Pharm. Bull. 1979, 27, 2382–2387. [Google Scholar] [CrossRef]
- Xu, X.H.; Kong, C.H.; Lin, C.J.; Wang, X.; Zhu, Y.D.; Yang, H.S. A novel diterpenoid from the soft coral Sarcophyton crassocaule. Chin. J. Chem. 2003, 21, 1506–1509. [Google Scholar] [CrossRef]
- Kashman, Y.; Zadock, E.; Néeman, L. Some new cembrane derivatives of marine origin. Tetrahedron 1974, 30, 3615–3620. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; El-Beih, A.A.; Moustafa, A.Y.; Hamdy, A.A.; Alhammady, M.A.; Selim, R.M.; Abdel-Rehim, M.; Paré, P.W. Cytotoxic cembranoids from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Commun. 2011, 6, 1809–1812. [Google Scholar] [CrossRef]
- Cuong, N.X.; Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Thuy, D.T.T.; Song, S.B.; Nam, N.H.; van Kiem, P.; Kim, Y.H.; van Minh, C. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014, 62, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Liao, X.-J.; Deng, Z.; Xu, S.-H. Isolation of a new butenolide from the South China Sea gorgonian coral Subergorgia suberosa. Nat. Prod. Res. 2014, 28, 150–155. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Paré, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drugs 2017, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Mohamed, T.A.; Abdel-Latif, F.F.; Alsaid, M.S.; Shahat, A.A.; Pare, P.W. Trochelioid A and B, new cembranoid diterpenes from the Red Sea soft coral Sarcophyton trocheliophorum. Phytochem. Lett. 2013, 6, 383–386. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; Elshamy, A.I.; Hamed, A.R.; Ibrahim, M.A.A.; Ohta, S.; Umeyama, A.; Paré, P.W.; Efferth, T. Sarcoehrenbergilides D–F: Cytotoxic cembrene diterpenoids from the soft coral Sarcophyton ehrenbergi. RSC Adv. 2019, 9, 27183–27189. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Paré, P.W. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Ahmed, M.M.A.; Albadry, M.A.; Ragab, E.A.; El-Ghaly, E.M.; Ismail, S.K.; Ali, Z.; Khan, S.I.; Chittiboyina, A.G.; Khan, I.A. Sarcoroseolides AD, four undescribed cembranoids from the Red Sea soft coral Sarcophyton roseum. Nat. Prod. Res. 2020, 36, 1842–1850. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Song, S.B.; Cuong, N.X.; Nam, N.H.; van Kiem, P.; Kim, Y.H.; van Minh, C. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorganic Med. Chem. Lett. 2014, 24, 228–232. [Google Scholar] [CrossRef]
- Swapana, N.; Tominaga, T.; Elshamy, A.I.; Ibrahim, M.A.A.; Hegazy, M.-E.F.; Singh, C.B.; Suenaga, M.; Imagawa, H.; Noji, M.; Umeyama, A. Kaemgalangol A: Unusual seco-isopimarane diterpenoid from aromatic ginger Kaempferia galanga. Fitoterapia 2018, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]




| No | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | --- | 86.9 | ------ | 162.0 |
| 2 | 5.04 br d (10.4) | 80.9 | 5.47 d (9.8) | 79.5 |
| 3 | 5.22 br d (10.4) | 117.5 | 5.09 d (9.8) | 120.4 |
| 4 | --- | 143.5 | ------ | 145.9 |
| 5 | 2.17 m 2.42 ddd (13.3, 13.3, 3.7) | 36.1 | 2.08 ddd (13.7, 13.7, 2.4) 2.44 ddd (13.7, 3.7, 3.7) | 32.9 |
| 6 | 1.42 m 1.74 m | 24.9 | 1.58 overlap 1.99 ddd (13.7,13.7,4.0) | 33.4 |
| 7 | 3.64 br d (10.9) | 69.7 | 3.22 brd (10.1) | 74.6 |
| 8 | --- | 73.9 | ----- | 85.1 |
| 9 | 2.12 dd (14.5, 2.5) 2.52 dd (14.5, 9.1) | 43.3 | 2.17 m, 1.72 m | 36.1 |
| 10 | 5.64 dd (15.7, 3.7) | 126.6 | 1.90 m 1.90 m | 30.8 |
| 11 | 5.61 br d (15.7) | 136.3 | 4.50 t (6.9) | 83.1 |
| 12 | --- | 79.9 | ---- | 148.6 |
| 13 | 1.92 ddd (17.2, 17.2, 3.7) 2.08 br d (17.2) | 26.7 | 2.18 ddd (15.4, 13.1, 6.0) 2.26 ddd (15.4, 13.1, 4.0) | 26.8 |
| 14 | 1.66 m 2.16 m | 19.0 | 2.32 ddd (13.1, 13.1, 4.0) 2.66 ddd (13.1, 13.1, 6.0) | 27.2 |
| 15 | --- | 77.5 | ---- | 123.5 |
| 16 | --- | 175.2 | ---- | 174.8 |
| 17 | 1.80 s | 22.9 | 1.85 s | 8.8 |
| 18 | 1.69 s | 15.9 | 1.96 br s | 20.7 |
| 19 | 1.21 s | 22.7 | 1.16 s | 19.8 |
| 20 | 1.16 s | 27.3 | 4.88 br s 5.04 br s | 111.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, T.A.; Elshamy, A.I.; Abd El-Razek, M.H.; Abdel-Tawab, A.M.; Ali, S.K.; Aboelmagd, M.; Suenaga, M.; Pare, P.W.; Umeyama, A.; Hegazy, M.-E.F. Sarcoconvolutums F and G: Polyoxygenated Cembrane-Type Diterpenoids from Sarcophyton convolutum, a Red Sea Soft Coral. Molecules 2022, 27, 5835. https://doi.org/10.3390/molecules27185835
Mohamed TA, Elshamy AI, Abd El-Razek MH, Abdel-Tawab AM, Ali SK, Aboelmagd M, Suenaga M, Pare PW, Umeyama A, Hegazy M-EF. Sarcoconvolutums F and G: Polyoxygenated Cembrane-Type Diterpenoids from Sarcophyton convolutum, a Red Sea Soft Coral. Molecules. 2022; 27(18):5835. https://doi.org/10.3390/molecules27185835
Chicago/Turabian StyleMohamed, Tarik A., Abdelsamed I. Elshamy, Mohamed H. Abd El-Razek, Asmaa M. Abdel-Tawab, Sherin K. Ali, Mohamed Aboelmagd, Midori Suenaga, Paul W. Pare, Akemi Umeyama, and Mohamed-Elamir F. Hegazy. 2022. "Sarcoconvolutums F and G: Polyoxygenated Cembrane-Type Diterpenoids from Sarcophyton convolutum, a Red Sea Soft Coral" Molecules 27, no. 18: 5835. https://doi.org/10.3390/molecules27185835
APA StyleMohamed, T. A., Elshamy, A. I., Abd El-Razek, M. H., Abdel-Tawab, A. M., Ali, S. K., Aboelmagd, M., Suenaga, M., Pare, P. W., Umeyama, A., & Hegazy, M.-E. F. (2022). Sarcoconvolutums F and G: Polyoxygenated Cembrane-Type Diterpenoids from Sarcophyton convolutum, a Red Sea Soft Coral. Molecules, 27(18), 5835. https://doi.org/10.3390/molecules27185835










